Abstract
Summary
The aim of this study was to examine the gender-specific association between sarcopenia and bone geometry/metabolic parameters. Low muscle mass was associated with greater deterioration of bone than in deterioration of glucose or lipid profiles. This bone–muscle relationship was more prominent in men than in women.
Introduction
There are few studies that report on gender differences in the effects of low muscle mass on bone and metabolic parameters in elderly subjects. This study aimed to assess the gender-specific influence of muscle mass on bone and metabolic parameters.
Methods
A total of 2,264 participants (940 men and 1,324 women) whose age ranged from 65 to 92 years were analyzed using data from The Fourth Korea National Health and Nutrition Examination Surveys (2008–2009). We measured bone mineral density (BMD) and appendicular muscle mass using the dual-energy X-ray absorptiometry and also measured metabolic profiles.
Results
The age-related trend in bone and muscle coincided in men but not in women. Femoral neck (FN) and total hip (TH) BMD were highly correlated with muscle mass in both genders. However, in women, this correlation was not significant in the lumbar spine (LS). In addition, this positive correlation was stronger in the FN or TH than in the LS and was stronger in men than in women. Subjects with sarcopenia were at a higher risk for osteoporosis in the FN, TH, and LS in men, and in the TH and FN in women. The degree of association between muscle mass and metabolic profiles was relatively very weak.
Conclusion
Bone–muscle relationship was more prominent in men than in women. The gender differences in bone–muscle relationship may be helpful for the development of gender-specific preventive strategies in the elderly, especially in men.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcopenia is a syndrome characterized by the progressive loss of skeletal muscle mass and strength during the aging process. It is an emerging health problem, especially in the elderly population, as sarcopenia can lead to disability, increased risk of fracture, restriction of activity, gait and balance problem, and substantial healthcare costs [1–3]. Osteoporosis is also the most important health problem among the elderly, leading to a variety of morbidities and mortalities [4]. These two different diseases have the aging process as a commonality. Accordingly, there is now a considerable amount of evidence suggesting that the muscle and the bone have common genetic, nutritional, lifestyle, and hormonal determinants. Along with the cross-talk between bones and muscles, there are also complex pleiotropic bone–muscle relationships [5]. Skeletal structure adapts to the long-term loads exerted on the skeleton as a result of physical activity, and the most powerful loading forces are conferred by muscles, which must exert enough force to move the bones [6]. Thus, it is widely believed that the decline in function and muscle mass with age is concurrent with a decline in bone mineral density (BMD), followed by increased fragility fractures. Recently, Monaco et al. reported a high prevalence of sarcopenia and a significant association between sarcopenia and osteoporosis in a large sample of women with hip fractures [7]. However, Sylvia et al. found a significant relationship between muscle mass and bone density only in elderly men, not in elderly women [8]. Taken together, there are still many questions and discordant results regarding gender differences in the bone–muscle relationship, especially in elderly subjects.
Recent studies have shown that both muscles and bones are endocrine organs. Interleukin-6 and a host of other muscle-derived factors with systemic effects have been identified as factors that regulate glucose metabolism and have become potential targets for the treatment of obesity-induced metabolic disease [9]. Osteocalcin (OCN) is produced by osteoblasts and is released into the circulation during bone resorption [10]. Karsenty et al. have demonstrated that OCN exerts endocrine regulation on the islets, fat, and male fertility [11]. Therefore, we speculated that the bone and the muscle may influence each other to coordinate the regulation of glucose and lipid metabolism. We further supposed that these endocrine factors may also contribute direct gender-specific interactions with the muscle tissues, altering muscle mass and strength, and affecting the loads placed on the bone. Taken together, we hypothesized that gender differences might exist in bone–muscle relationship. In a previous study analyzing data from the Nationwide Survey in Korea (KNHANES IV), we reported that a relatively later onset with a greater acceleration with aging was observed in women [12]. With this in mind, we speculated that this dissimilarity of femoral bone loss can be explained by the dissimilar change of muscle mass in men and women during the aging process.
Therefore, the aim of our study was to assess the associations between sarcopenia and BMD, femoral geometric parameters, and glucose/lipid metabolic profiles with special reference to gender specificity. In addition, we further analyzed gender difference in terms of age-related trends in muscle mass and compared these with age-related trends in bone density in elderly populations. Based on the results, we have attempted to clarify the nature of bone–muscle interactions and their relationship with sarcopenia and osteoporosis.
Methods
Subjects
We recruited participants from the second (2008) and third (2009) years of the Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV). The KNHANES has been performed periodically since 1998 by the Division of Chronic Disease Surveillance of the Korean Centers for Disease Control and Prevention in order to assess the health and nutritional status of the civilian, non-institutionalized population of Korea. The KNHANES IV was a cross-sectional and nationally representative survey conducted from 2007 to 2008. The survey was composed of a health interview survey, a nutrition survey, and a health examination survey. The data were collected by household interviews and by direct, standardized physical examinations conducted in mobile examination centers. Nutritional status and medical history were evaluated using a 24-h recall method. Exercise was indicated as “yes” when the subject exercised for more than 20 min at a time and more than three times per week. Subjects with any pathological disorders (such as cancer, hyperthyroidism, malabsorption, renal failure, or hepatic failure) or subjects using medications (such as corticosteroids, heparin, or anticonvulsants) known to alter calcium and bone metabolism were excluded from the analysis. Subjects who used testosterone, anabolic steroids, and anti-resorptive agents, such as raloxifene and bisphosphonate, were also excluded. Among those who participated in the survey and met the inclusion criteria, 2,264 subjects were 65 years or older (940 men and 1,324 women).
Measurements of dual-energy X-ray absorptiometry and definitions of sarcopenia
A total of 5,003 men and 6,500 women aged 10 and older who completed the body composition examination using dual-energy X-ray absorptiometry (DXA) were included. The total body fat mass and appendicular skeletal muscle mass (ASM) as well as BMD at the lumbar spine (LS; L1–4) and hip region were measured using DXA (QDR 4500A; Hologic Inc., Waltham, MA). We analyzed the results from DXA using industry standard techniques at the Korean Society of Osteoporosis and performed the analyses using Hologic Discovery software (version 13.1) in its default configuration. We further analyzed geometric bone structure properties using the Hip Structure Analysis program included in the APEX software (Hologic Inc.), as previously described [13]. In addition to the hip axis length (millimeters), the Hip Structure Analysis program yielded data for mean cortical thickness (centimeters) and the cross-sectional area (CSA, square centimeters) in each of the narrow neck regions. Relative appendicular skeletal muscle mass (RASM) was calculated as the sum of the mass of skeletal muscle in the arms and legs, divided by the square of height (ASM/ht2 in kilograms per square meter). A subject was classified as having sarcopenia when he or she had a R less than one standard deviation (SD) below the sex-specific normal mean for the young reference group (healthy men and women aged 20–39 years) [1]. Relative total fat mass was calculated by dividing total body fat mass into the square of the height (kilograms per square meter).
Biochemical analysis
Collected blood samples were immediately refrigerated, transported to the Central Testing Institute in Seoul, Korea, and analyzed within 24 h. Fasting plasma glucose, total cholesterol, triglycerides, and high-density lipoprotein (HDL)-cholesterol levels were measured with a Hitachi 700–110 chemistry analyzer (Hitachi, Tokyo, Japan). Serum 25(OH)D concentrations were measured by radioimmunoassay (DiaSorin Inc., Stillwater, MN, USA) using a γ-counter (1470 Wizard; PerkinElmer, Turku, Finland). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: fasting (plasma glucose (milligrams per deciliter) × fasting insulin (milliinternational units per milliliter))/22.5 [14].
Statistical analysis
Statistical analyses were conducted using IBM’s SPSS version 20.0 for Windows (IBM Corp., Armonk, NY, USA). An independent, two-sample t test was used to compare differences in the mean values of baseline parameters among the groups. For categorical variables, chi-square test was used to compare the frequencies among the groups. Pearson’s correlation analyses were used to evaluate the association between muscle mass and bone and metabolic parameters with adjustments made for age, relative total fat mass, current smoking, regular exercise, vitamin–mineral supplement use, and estrogen replacement therapy (in women). Although the effect of body mass index (BMI) on BMD is well known, we adopted relative total fat mass as an adjusting variable instead of BMI because a collinearity between relative total fat mass and BMI was observed. Multiple linear regression analysis was then used to determine the association between RASM and the outcome (total hip, femoral neck, and lumbar spine BMD) adjusted for confounding factors. Multiple logistic regression analysis was used to examine the association between sarcopenia and osteoporosis, with the results expressed as odds ratios (OR) and 95 % confidence intervals (CI).
Results
Clinical characteristics of the study population
The baseline characteristics of all the participants are presented in Table 1. The mean age of the sarcopenia group was older in both genders than the non-sarcopenia group. The sarcopenic group also had lower body weight, BMI, waist circumference, RASM, and relative total fat mass. BMD at the total hip (TH), femoral neck (FN), and LS was significantly lower in those with sarcopenia than in those without sarcopenia in both genders. Femoral bone geometric indices, including cortical thickness and CSA, were also significantly lower in the sarcopenia group, but hip axis length was significantly lower in the sarcopenia group only in women. In both genders, there were no significant difference in 25(OH)D, HbA1c, fasting glucose, or total cholesterol between the two groups, except that triglycerides and HOMA-IR were lower and HDL-C was higher in men with sarcopenia. Female sarcopenic subjects were less likely to exercise regularly, and male sarcopenic subjects also tended to less likely to exercise regularly than non-sarcopenic patients (P = 0.079). The overall prevalence of sarcopenia was 46.81 % in men and 7.6 % in women.
Gender-specific age-related changes in muscle mass and TH BMD
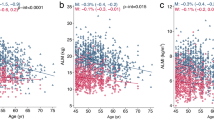
Figure 1 demonstrates changes in RASM and TH BMD according to age. Whereas peak muscle mass was achieved by age 20 to 39 years (7.95 ± 0.91 kg/m2) in men, it was not achieved until age 40 to 59 years (6.05 ± 0.72 kg/m2) in women. Interestingly, for elderly men, age-related changes in muscle mass showed a trend similar to that of TH BMD (Fig. 1a, c). However, for elderly women, age-related changes in muscle mass showed trends that are distinct from age-related change in TH BMD (Fig. 1b, d): while bone loss in TH accelerated after the sixth decade of life, near menopausal age, the loss of muscle mass progressed slowly and gradually after reaching peak muscle mass. Therefore, even the muscle mass of women in their seventh decade of life was comparable to that of women in their third decade of life.
Gender-specific differences in relationships between muscle mass and bone density
As shown in Table 2, there was a positive correlation between RASM and BMD at TH, FN, and LS in elderly men (R = 0.331, P < 0.001 at TH; R = 0.278, P < 0.001 at FN; R = 0.205, P < 0.001 at LS). In elderly women, there was a relatively weak positive correlation between muscle mass and BMD at TH and FN (R = 0.177, P < 0.001 at TH; R = 0.139, P < 0.001 at FN), but not at LS. This positive association between RASM and BMD at each site was more prominent in men than in women. There was also a weak negative correlation between RASM and fasting glucose (R = −0.096, P = 0.007) and HDL (R = −0.096, P = 0.007) in men, and total cholesterol (R = −0.101, P = 0.001) and HDL (R = −0.060, P = 0.044) in women. Figure 2a–c and Supplement Table 1 present the independent contribution of RASM to BMD at each site using multiple linear regression analysis. The increase in RASM significantly contributed to the increase of BMD at TH, FN, and LS in men and also to the increase of BMD at TH and FN in women. However, RASM did not correlate with BMD at LS in women (P = 0.358). To investigate the influence of RASM on femoral bone geometry, we also analyzed the association between RASM and cortical thickness at FN and at the intertrochanteric and femoral shafts (Fig. 2d–f, and Supplemental Table 2). Cortical thickness at the intertrochanteric and femoral shafts increased with increasing RASM in both genders. But gender differences in the association between muscle mass and cortical thickness at the femur were not observed.
Scatter plot analysis of RASM with the predicted value of BMD (a–c) and cortical thickness (d–f) using multiple linear regression. Data was adjusted for age, relative total fat mass, HOMA-IR, current smoking status, regular exercise, total cholesterol, triglyceride, vitamin–mineral supplement use, and estrogen replacement therapy (in women)
Gender-specific prevalence of osteoporosis according to muscle mass
After adjusting for confounding factors, including age, sarcopenic men (RASM at <7.04 kg/m2) had a significantly elevated OR for the development of osteoporosis at FN (OR = 3.44, 95 % CI = 1.73 − 6.83) and at LS (OR = 1.83, 95 % CI = 1.04 − 3.21; Table 3). Similarly, women with sarcopenia (RASM at <5.04 kg/m2) were at significantly increased risk for development of osteoporosis at TH (OR = 2.46, 95 % CI = 1.20 − 5.04) and at FN (OR = 1.76; 95 % CI = 1.02 − 3.04). Each SD increase in RASM was associated with a 59.9 % reduction in the likelihood of osteoporosis at TH, a 54 % reduction at FN, and a 35.9 % reduction at LS (OR = 0.411, 95 % CI = 0.188 − 0.900 at TH; OR = 0.460, 95 % CI = 0.327 − 0.648 at FN; OR = 0.651, 95 % CI = 0.483 − 0.877 at LS). Each SD increase in RASM was associated with a 39.3 % reduction in the likelihood of osteoporosis at TH and a 19.1 % reduction at FN (OR = 0.607, 95 % CI = 0.452 − 0.816 at TH, OR = 0.809, 95 % CI = 0.693 − 0.943 at FN) in women (Fig. 3). However, sarcopenia was not associated with development of osteoporosis at the LS in women.
Adjusted odds ratios with 95 % confidence interval for the development of osteoporosis for each standard deviation (SD) increase in RASM. Data was adjusted for age, relative total fat mass, HOMA-IR, current smoking status, regular exercise, total cholesterol, triglyceride, vitamin–mineral supplement use, and estrogen replacement therapy (in women)
Discussion
We have found that skeletal muscle mass is significantly associated with bone density and femoral bone geometry in the elderly, regardless of age, fat mass, behaviors, or metabolic parameters. This association was more prominent in men than women. This discovery adds to the growing body of evidence supporting the highly integrated nature of the skeletal muscle and the bone. The gender specificity in the bone–muscle relationship may aid in developing a better understanding of gender differences in age-related bone loss and fracture risk in the elderly men.
In our study, there were significant gender differences in age-related change in muscle mass and the prevalence of sarcopenia. The extent and the speed of muscle loss differed depending on the gender. In men, peak muscle mass was obtained at 20 to 39 years of age (7.95 ± 0.91 kg/m2). However, in women, peak muscle mass was reached at 40 to 59 years of age (6.05 ± 0.72 kg/m2). These findings are consistent with the earlier report from Austrian populations, which found a significant decline in lean body mass with increasing age only among men [8]. However, this differed from the results of a New Mexico report, which stated that mean RASM in the elderly female was approximately 80 % of the mean in the young women [1]. The lack of physical activity and the changing body image among young Korean women have been posed as reasons for why peak muscle mass is obtained at middle age. The prevalence of sarcopenia was 46.81 % in men and 7.6 % in women. This difference in prevalence of sarcopenia between genders was still evident even after the peak muscle mass at age 40 to 59 years of age was applied in women (46.81 % in men and 17.6 % in women). In a French study, the prevalence of sarcopenia was 23.6 % in men and 18.6 % in women [15]. These findings suggest that the prevalence of sarcopenia and the age in which peak muscle mass is achieved differ between countries. Including ethnic factor, lack of consensus of sacopenia definition, nutritional factors, and environmental factors such as lifestyle also might be cause of this difference [16].
Our study demonstrated a strong association between muscle mass and bone density, especially in men. Furthermore, compared with our control group, cortical thickness was significantly lower in the subjects with low muscle mass. This bone–muscle relationship was more prominent in femurs than in LS in our study. This positive association between BMD and muscle mass can be explained by the fact that muscle size is associated with bone size [17], and the result in our study showing that an increase in muscle mass contributed to an increase in cortical thickness at the femur. These results were consistent with previous studies showing that appendicular skeletal muscle mass is relatively associated with parameters of cortical bone geometry and microstructure [18, 19]. However, a gender-specific difference in the association between muscle mass and cortical thickness was not clearly demonstrated in our study. The low sensitivity of the DXA in measuring cortical thickness may have contributed to this result. Moreover, the site-specific associations between RASM and femur BMD may be further explained by the mechanical loading on the bone [20], where the femur, rather than the spine, is the main region where the mechanical loading and weight bearing is exerted. This site-specific bone–muscle relationship could support a potential osteogenic role for muscle forces through muscle contraction. In contrast, the associations between sarcopenia and metabolic parameters, including HOMA-IR, glucose, and lipid profiles, were not remarkable in either gender, except for a weak negative correlation between RASM and HDL in both genders, fasting glucose in men, and total cholesterol in women. The negative correlation between muscle mass and HDL might be explained by the fact that lipoprotein lipase in muscle plays a role in HDL catabolism [21]. All of the above data strongly suggest that the loss of muscle mass exerts greater influence on bone loss than on the deterioration of metabolic parameters in Korea’s elderly population.
The contribution of sarcopenia on the risk for development of osteoporosis was assessed in order to study the clinical impact of low muscle mass on bone density. Compared to the non-sarcopenic group, the risk for osteoporosis was significantly higher in sarcopenic men and women. One SD increase in muscle mass was associated with a significant reduction in occurrence of osteoporosis at TH (59 and 39.3 %) and FN (54 and 19.1 %) in men and women, respectively; however, the reduction effect at LS was only observed in men. Furthermore, the reduction effect was also more prominent at TH and FN than at LS in both genders. These results were valid even after adjusting for confounding factors. Our observation may support the previous notion that osteoporosis and sarcopenia are simply two different manifestations of the same process dictated by common gene/protein modifications [22]. Intriguing new data on the effects of factors secreted from osteocytes on muscle cells and vice versa have been reported [23]. Osteocytes secrete several factors that promote myogenic differentiation, while muscle factors may stimulate the Akt-signaling pathway in the neighboring cortical bone for protecting osteocytes from apoptosis [24].
The major interests of this study are gender differences in the age at which peak muscle mass is reached, the prevalence of sarcopenia, the extent and the speed of muscle loss, the degree of contribution of low muscle mass to BMD, and the sites prone to the consequences of low muscle mass. The strong association between RASM and BMD in men might be explained by gender-specific effects of sex hormones on muscle and bone. Testosterone increases both skeletal muscle and bone mass, especially cortical bone, while estrogen only affects the bone by inhibiting endocortical and trabecular bone resorption by binding to its estrogen receptor (ER) [25]. Moreover, mechanical strain and estrogen share a common transduction pathway involving activation of ER-α in in vivo study [26] and a decline in ER-α number reduce the ability of mechanical loading to induce an osteogenic response [25]. This resetting of the “mechanostat” due to estrogen deficiency may explain the decoupling of RASM and BMD in elderly women. Furthermore, since the absolute level and degree of decline in testosterone with age are much lower in women than in men, muscle mass can be relatively preserved with age in women. Additionally, IGF-1, a shared signal between bone and muscle and a proven anabolic factor in both organs, is known to be regulated by testosterone [27]. Therefore, age-related decreases in IGF-1 levels may lead to a decrease in both muscle mass and bone mass especially in elderly men. Fat mass might alter the bone–muscle relationship in women. Conversion of androgens to estrogens in adipose tissue may have a positive effect on the bone in post-menopausal women [28]. However, adipose tissue is not an important sex hormone source in men [29]. Gender differences in free radical homeostasis during aging may contribute to the gender differences in the prevalence of sarcopenia [30]. There is evidence supporting mitochondrial dysfunction as one of the causes of sarcopenia, and mitochondrial superoxide production is low in female mice [31]. All these emerging new studies promise to advance our knowledge regarding gender specificity in bone–muscle interaction. In addition, this study suggests that assessment of sarcopenia, identification of preventable contributors, and development of preventive strategies and treatment options for sarcopenia could be more emphasized in men.
The major strength of this study lies in that the data were collected from a nationwide survey that included 2,264 participants from ages 65 to 92 years throughout Korea. This is the first observational study that extensively investigated the gender-specific effects of low muscle mass on bone and metabolic profiles, while at the same time focusing on Korea’s elderly population. However, this study also has some limitations. First, the muscle mass was only measured for the evaluation of sarcopenia. Additional muscle strength and walk speed were not evaluated. Second, as the present study was a cross-sectional study and not a longitudinal study, our aging trends in body composition are only estimates, not observed changes. Furthermore, a causal relationship could not be definitely established. Third, bone geometry and body fat mass was measured by DXA, which cannot distinguish cortical bone from trabecular bone and cannot measure intramuscular fat mass. Finally, we could not measure hormones, such as estradiol, testosterone, growth hormone, or IGF-1.
In conclusion, our study is the largest population-based study to examine the gender-specific association between sarcopenia and bone geometry. Low muscle mass resulted in greater deterioration of bone, especially cortical bone-rich FN and TH, than in deterioration of glucose or lipid metabolic parameters in both genders. This bone–muscle relationship was stronger in men than in women. Evaluation of gender differences in acquisition and age-related loss in bone and muscle may be helpful to the development of gender-specific strategies for the treatment and prevention of sarcopenia and osteoporosis in elderly populations.
References
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR et al (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763
Leveille SG (2004) Musculoskeletal aging. Curr Opin Rheumatol 16:114–118
Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R (2004) The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 52:80–85
Riggs BL, Melton LJ 3rd, Robb RA, Camp JJ, Atkinson EJ, Peterson JM et al (2004) Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res 19:1945–1954
Mo C, Romero-Suarez S, Bonewald L, Johnson M, Brotto M (2012) Prostaglandin e2: from clinical applications to its potential role in bone–muscle crosstalk and myogenic differentiation. Recent Patents Biotechnol 6:223–229
Frost HM (2003) Bone's mechanostat: a 2003 update. Anat Rec A: Discov Mol Cell Evol Biol 275:1081–1101
Di Monaco M, Castiglioni C, Vallero F, Di Monaco R, Tappero R (2012) Sarcopenia is more prevalent in men than in women after hip fracture: a cross-sectional study of 591 inpatients. Arch Gerontol Geriatr 55:e48–e52
Kirchengast S, Huber J (2012) Sex-specific associations between soft tissue body composition and bone mineral density among older adults. Ann Hum Biol 39:206–213
Jiang LQ, Duque-Guimaraes DE, Machado UF, Zierath JR, Krook A (2013) Altered response of skeletal muscle to IL-6 in type 2 diabetic patients. Diabetes 62:355–361
Neve A, Corrado A, Cantatore FP (2011) Osteoblast physiology in normal and pathological conditions. Cell Tissue Res 343:289–302
Karsenty G (2011) Bone endocrine regulation of energy metabolism and male reproduction. C R Biol 334:720–724
Kim KM, Lim JS, Kim KJ, Choi HS, Rhee Y, Oh HJ et al (2013) Dissimilarity of femur aging in men and women from a Nationwide Survey in Korea (KNHANES IV). J Bone Miner Metab 31:144–152
Beck TJ, Looker AC, Ruff CB, Sievanen H, Wahner HW (2000) Structural trends in the aging femoral neck and proximal shaft: analysis of the Third National Health and Nutrition Examination Survey dual-energy X-ray absorptiometry data. J Bone Miner Res 15:2297–2304
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Tichet J, Vol S, Goxe D, Salle A, Berrut G, Ritz P (2008) Prevalence of sarcopenia in the French senior population. J Nutr Health Aging 12:202–206
Chin SO, Rhee SY, Chon S, Hwang YC, Jeong IK, Oh S et al (2013) Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS One 8:e60119
Edwards M, Gregson C, Patel H, Jameson K, Harvey N, Sayer AA et al (2013) Muscle size, strength and physical performance and their associations with bone structure in the Hertfordshire Cohort Study. J Bone Miner Res. doi:10.1002/jbmr.1972
Lebrasseur NK, Achenbach SJ, Melton LJ 3rd, Amin S, Khosla S (2012) Skeletal muscle mass is associated with bone geometry and microstructure and serum insulin-like growth factor binding protein-2 levels in adult women and men. J Bone Miner Res 27:2159–2169
Heinonen A, McKay HA, Whittall KP, Forster BB, Khan KM (2001) Muscle cross-sectional area is associated with specific site of bone in prepubertal girls: a quantitative magnetic resonance imaging study. Bone 29:388–392
Ellman R, Spatz J, Cloutier A, Palme R, Christiansen BA, Bouxsein ML (2013) Partial reductions in mechanical loading yield proportional changes in bone density, bone architecture, and muscle mass. J Bone Miner Res 28:875–885
Pietrobelli A, Lee RC, Capristo E, Deckelbaum RJ, Heymsfield SB (1999) An independent, inverse association of high-density-lipoprotein-cholesterol concentration with nonadipose body mass. Am J Clin Nutr 69:614–620
Seeman E, Hopper JL, Young NR, Formica C, Goss P, Tsalamandris C (1996) Do genetic factors explain associations between muscle strength, lean mass, and bone density? A twin study. Am J Physiol 270:E320–E327
Hamrick MW, McNeil PL, Patterson SL (2010) Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact 10:64–70
Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F (2010) The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol 205:201–210
Lee KC, Lanyon LE (2004) Mechanical loading influences bone mass through estrogen receptor alpha. Exerc Sport Sci Rev 32:64–68
Jessop HL, Sjoberg M, Cheng MZ, Zaman G, Wheeler-Jones CP, Lanyon LE (2001) Mechanical strain and estrogen activate estrogen receptor alpha in bone cells. J Bone Miner Res 16:1045–1055
Kanbur-Oksuz N, Derman O, Kinik E (2004) Correlation of sex steroids with IGF-1 and IGFBP-3 during different pubertal stages. Turk J Pediatr 46:315–321
Thomas T, Burguera B (2002) Is leptin the link between fat and bone mass? J Bone Miner Res 17:1563–1569
Mudali S, Dobs AS (2004) Effects of testosterone on body composition of the aging male. Mech Ageing Dev 125:297–304
Calvani R, Joseph AM, Adhihetty PJ, Miccheli A, Bossola M, Leeuwenburgh C et al (2013) Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem 394:393–414
Ali SS, Xiong C, Lucero J, Behrens MM, Dugan LL, Quick KL (2006) Gender differences in free radical homeostasis during aging: shorter-lived female C57BL6 mice have increased oxidative stress. Aging Cell 5:565–574
Acknowledgments
This work was supported by the National Research Foundation (NRF) of Korea grant funded by the Korea government (MEST) (No. 20110001024).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huh, J.H., Song, M.K., Park, K.H. et al. Gender-specific pleiotropic bone–muscle relationship in the elderly from a nationwide survey (KNHANES IV). Osteoporos Int 25, 1053–1061 (2014). https://doi.org/10.1007/s00198-013-2531-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2531-2