Abstract
Summary
This study showed that the prevalence of sarcopenia (low muscle mass and performance) among 70–80-year-old home-dwelling Finnish women is very low, while every third woman has WHO-based osteopenia (low bone mass). Muscle mass and derived indices of sarcopenia were not significantly related to measures of functional ability.
Introduction
This study aims to determine the prevalence of sarcopenia and osteopenia among four hundred nine 70–80-year-old independently living Finnish women. The study compared consensus diagnostic criteria for age-related sarcopenia recently published by the European Working Group on Sarcopenia in Older People (EWGSOP) and the International Working Group on Sarcopenia (IWG) and assessed their associations with functional ability.
Methods
Femoral bone mineral density and body composition were measured with dual-energy X-ray absorptiometry. Skeletal muscle mass index (SMI), gait speed, and handgrip strength were used for sarcopenia diagnosis. Independent samples t tests determined group differences in body composition and functional ability according to recommended diagnostic cutpoints. Scatter plots were used to illustrate the correlations between the outcome measures used for diagnosis.
Results
Prevalence of sarcopenia was 0.9 and 2.7 % according to the EWGSOP and IWG, respectively. Thirty-six percent of the women had WHO-based osteopenia. Women with higher gait speed had significantly lower body weight and fat mass percentage, higher lean mass percentage, and better functional ability. Women with a low SMI weighed significantly less, with no significant differences in other outcome measures. SMI, gait speed, and grip strength were significantly correlated.
Conclusions
Our study suggests that when using consensus definitions, sarcopenia is infrequent among older home-dwelling women while every third woman has osteopenia. In clinical practice, attention should be paid to the decline in functional ability rather than focusing on low muscle mass alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is accompanied by changes in body composition, including a decrease in both muscle and bone mass [1, 2]. After middle age, fat mass gradually increases while lean tissue mass decreases [3, 4], and the quantitative loss in muscle cross-sectional area contributes to muscle weakness in older adults [1]. This age-related loss of skeletal muscle mass, resulting in loss of strength and function, is defined as sarcopenia [5, 6]. Sarcopenia has also been used to describe the clinical condition of having exceptionally low levels of muscle mass [7] and is associated with a risk of adverse outcomes such as physical disability, poor quality of life, and death [8]. Sarcopenia may thus be an important and potentially reversible cause of morbidity and mortality in older persons [9].
There have been numerous attempts to diagnose sarcopenia based on the measurements of muscle mass alone or in combination with muscle function. Several studies have provided specific skeletal muscle cutpoints for diagnosis; some associated with a high risk of physical disability [10] and others as height-adjusted appendicular muscle mass of two or more standard deviations below the mean of young adults [6], as muscle mass relative to body weight [9] or as lean mass adjusted for body fat mass and height with the 20th percentile of the distribution of residuals of regression as the cutpoint for sarcopenia [7].
Recently, consensus diagnostic criteria for age-related sarcopenia have been published by the European Working Group on Sarcopenia in Older People (EWGSOP) [11] and the International Working Group on Sarcopenia (IWG) [5]. The EWGSOP recommends using the presence of both low muscle mass and low muscle function (strength or performance) for diagnosis [11]. It is rationalized that both these criteria must be used for diagnosis since muscle strength does not depend solely on muscle mass, and the relationship between strength and mass is not linear [10, 12]. Similarly, the IWG also emphasizes that muscle function as measured by gait speed, should be considered for diagnosis, besides muscle mass. The IWG further elaborates that sarcopenia should be considered in all older patients who present with observed declines in physical function, strength, or overall health and more specifically in patients who are bedridden, cannot rise independently from a chair, or who have a measured gait speed less than 1 m/s [5].
The WHO provided an operational definition of osteopenia and osteoporosis in 1994. A postmenopausal woman with a bone mineral density (BMD) of 2.5 standard deviations (SDs) or more below the young adult mean (i.e., T-score, ≤ –2.5) at the lumbar spine or hip is considered to have osteoporosis, and a woman with a BMD between −2.5 and −1.0 is considered to have osteopenia or low bone mass [13, 14].
Obviously, the genesis of both sarcopenia and osteoporosis is multifactorial. A common etiology may account for a positive association between osteoporosis and sarcopenia, the two conditions that may act together in the development of disability [2, 15, 16]. It is also well-known that muscle and bone strengths are strongly related to each other [17]. Older persons with early sarcopenia and osteopenia are probably those who are most likely to benefit from interventions targeted to increasing functional independence [18–20]. It is therefore important to identify such persons and intervene before substantial functional deterioration begins. However, the diagnosis of sarcopenia is complicated due to the lack of agreement on the precise diagnostic criteria and unavailability of standard reference data for establishment of diagnostic cutpoints.
The aim of this study was therefore to determine the prevalence of sarcopenia and osteopenia in a prospective cohort of 70–80-year-old Finnish women using data from the baseline examination of the randomized vitamin D vs. exercise (DEX) study [21]. In addition, the purpose was to compare the consensus diagnostic criteria for sarcopenia and assess their associations with functional ability.
Methods
Study population
All 70- to 80-year-old women living in the city of Tampere, Finland (n = 9,370) were invited to participate in the trial. Four hundred nine community-dwelling, independently living women were ultimately included in the study group after determining eligibility according to the inclusion criteria and medical screening by a physician. These women had a history of at least one fall during the previous year, had no contraindications to exercise, and understood the procedures of the DEX study.
Exclusion criteria were: moderate-to-vigorous exercise more than 2 h per week, regular use of vitamin D supplements, recent fractures (during the preceding 12 months), marked decline in the basic activities of daily living, cognitive impairments, and degenerative conditions, such as Parkinson’s disease.
The DEX study protocol was approved by the Ethics Committee of the Tampere University Hospital, Tampere, Finland, and all participants gave written informed consent. The DEX study is described in detail in the study protocol [21].
Outcome measures
Sarcopenia was separately diagnosed according to recommendations given by the EWGSOP [11] and the IWG [5]. The two diagnostic methods were compared, and the criterion variables used for each method were separately analyzed to assess their associations with functional ability.
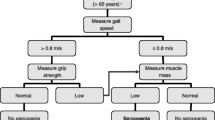
For intervention trials, the EWGSOP recommends three primary outcome variables (muscle mass, muscle strength, and physical performance) and suggests a number of possible measurement methods for each of these. For this study, we considered the skeletal muscle mass index (SMI) as a measure of muscle mass, handgrip strength as a measure of muscle strength, and gait speed as a measure of muscle performance. The EWGSOP diagnostic algorithm is shown in Fig. 1.
According to the IWG, diagnosis of sarcopenia is based on gait speed of less than 1 m/s and an objectively measured low muscle mass [5]; SMI was again used as a measure of muscle mass to ease comparison between the two methods. An algorithm to illustrate sarcopenia diagnosis according to the IWG is shown in Fig. 2.
Anthropometry and body composition (muscle mass)
Body height was measured to the nearest 0.1 cm and body weight to the nearest 0.1 kg with a high-precision scale. Body composition (fat mass and lean mass) was assessed with dual-energy X-ray absorptiometry (DXA, Lunar Prodigy Advance, GE Lunar, Madison, WI, USA). In our laboratory, the in vivo precision (coefficient of variation, CV %) based on repeated scans of 27 subjects with repositioning is 1.3 % for fat mass and 0.8 % for lean mass [22]. For the purpose of this study, appendicular skeletal muscle mass (ASM) in kilograms relative to height squared in meters was calculated as an index of relative skeletal muscle mass (SMI) as suggested by Baumgartner and colleagues [6]. ASM was measured as the sum of the lean soft tissue masses for the arms and the legs as described by Heymsfield and colleagues [23], assuming that all nonfat and nonbone tissue is skeletal muscle.
Bone mass and density
Bone mineral content (BMC, in grams) of the total body and areal BMD (in grams per square centimeter) of the left proximal femur were assessed with DXA (Lunar Prodigy Advance). The latter was used for the T-score calculation. In our laboratory, the in vivo precision (CV %) is 1.4 % for the total body BMC and 0.7 % for the femur BMD.
Muscle strength
The grip strength of the dominant forearms was measured with a standard grip strength meter (Lafayette, LA, USA). The maximal isometric lower limb extensor force at the knee ankle 110° was measured by a strain gauge dynamometer (Tamtron, Tampere, Finland). The participants were verbally encouraged to perform to their maximum, and the best performance from three trials was recorded and proportioned to body weight (in kilograms per kilogram).
Physical performance
The short physical performance test battery (SPPB) [24] and the timed up and go test (TUG) [25] were used in assessing physical functioning. The SPPB includes tests of static balance, gait speed (4 m), and five times repeated chair stands.
Reference groups and cutpoints
The EWGSOP devised an algorithm (Fig. 1) to define sarcopenia on the basis of specific cutpoints for gait speed (<0.8 m/s), handgrip strength (<20 kg), and SMI (<5.5 kg/m2) (in women). The cutpoints for gait speed and handgrip strength are as suggested by Lauretani et al. [26], and that for SMI by Baumgartner et al. [6].
The IWG suggested cutpoints of 1 m/s for gait speed and ≤5.67 kg/m² (in women) for SMI [5, 7]. Both these criteria should be present for the diagnosis of sarcopenia.
Since the suggested cutpoints may not be accurate for the Finnish population, we also used population-based data from 296 apparently healthy premenopausal Finnish women from our previous study [27] to calculate the SMI cutpoint. These women had a mean (SD) age of 40.1 (4.8) years, height of 1.71 (0.89) m, weight of 68.2 (12.7) kg, body mass index (BMI) 24.7 (4.6) kg/m², and SMI of 6.5 (0.8) kg/m². Their body composition was determined by the same DXA system and anthropometry by methods used in the present study. As suggested by Baumgartner and colleagues [6], sarcopenia was defined as muscle mass more than 2 SD below the cohort mean value, giving a cutpoint of 4.9 kg/m². Prevalence of sarcopenia was compared using this cutpoint for SMI in addition to EWGSOP and IWG criteria.
Data analyses
Mean and SD were used as descriptive statistics for body composition, bone traits, and functional ability of the study participants. Independent samples t tests were used to determine differences in functional ability between those with a diagnosis of sarcopenia and the remaining participants. Scatter plots were used to illustrate the correlations between the outcome measures used for sarcopenia diagnosis.
Results
The descriptive characteristics of the 409 participants are given in Table 1. Eight women had bilateral hip prosthesis and 401 were analyzed for femoral BMD. The prevalence of osteopenia in the cohort was 36 % according to the standard WHO-based T-score criterion. Three (0.8 %) women had a T-score of less than −2.5 or had osteoporosis. As shown in Fig. 1, only four women (0.9 %) fulfilled the EWGSOP criteria for sarcopenia, i.e., they had a gait speed of less than 0.8 m/s or a handgrip of less than 20 kg and a skeletal muscle mass index less than 5.5 kg/m². In contrast, 11 women (2.7 %) fulfilled the IWG criteria for sarcopenia, i.e., a gait speed of less than 1 m/s and an SMI ≤ 5.67 kg/m² (Fig. 2). Three of the four EWGSOP-based sarcopenic women fulfilled the IWG criteria as well. Only one participant had an SMI below the Finnish cutpoint of 4.9 kg/m2.
Figures 3 and 4 illustrate the correlation between handgrip strength and gait speed among the 409 participants, with emphasis on the cases with low muscle mass.
Table 2 shows differences between the participant characteristics broken down by the EWGSOP cutpoints. Women with higher gait speed were somewhat younger and had a significantly lower body weight and fat mass percentage. They also had a higher total body lean mass percentage, scored better on the TUG test and the chair stand test, and had better leg extensor force. However, women with a low SMI weighed significantly less than those with a higher SMI, but they did not differ in terms of age, relative total body muscle mass, or functional ability. Similarly, no significant differences were found between those who had good grip strength and those who did not, excepting the finding that women with good grip strength also had better leg extensor force.
Table 3 shows differences between the participant characteristics broken down by the IWG cutpoints. Again, women with higher gait speed were significantly younger and had a significantly lower fat mass percentage, but weighed slightly less. As according to the EWGSOP cutpoints, they had significantly higher total body lean mass percentage and scored better on the TUG test and the chair stand test and had better leg extensor force. Women with a low SMI weighed significantly less than those with a higher SMI. They also had a higher total body lean mass percentage, lower fat mass percentage, and scored better on the TUG test. They did not, however, differ in terms of age or other measures of functional ability.
Discussion
Our study suggests that according to the EWGSOP and IWG criteria, sarcopenia is relatively rare among older home-dwelling Finnish women while WHO-based osteopenia is rather common. The prevalence of sarcopenia slightly differs as per consensus definitions suggested by the EWGSOP and the IWG. Two of the four EWGSOP sarcopenic women and 7 of the 11 IWG sarcopenic women also had osteopenia. The prevalence of osteopenia in our cohort was 36 % according to the standard WHO-based T-score criterion. In a study in the Swedish population, the prevalence of osteopenia was 56.1 % in women aged 70–74 years and 53.2 % in women aged 75–79 years [28]. This is much higher than our prevalence of 36 %. Another study reported a prevalence of 38.7 % in postmenopausal French women with a mean (SD) age of 64.1 (8.5) years [29].
To our knowledge, this is the first study to examine prevalence of sarcopenia based on these two recent consensus definitions. Our study sample was large and consisted the very age group most likely to be at risk for developing sarcopenia and related problems. The participants underwent a thorough and complete examination including an initial screening by a physician, body composition assessment, and functional ability tests. However, as regards to the generalizability of our findings, older men were not studied. Our study population consisted only of women living at home independently, who voluntarily participated in the DEX study and did not have limitations to moderate physical exercise. Also, women with marked decline in basic activities of daily living, cognitive impairments, or certain degenerative conditions were excluded according to study criteria [21]. This fact probably contributed to the observed low prevalence of sarcopenia, and it is likely that the prevalence of sarcopenia and functional disability in the unselected Finnish population of elderly women would have been higher than reported here. Our study cohort was restricted to a 10-year age range, and more substantial age-related differences in functional ability may have been observed in a sample representing a wider age range including the oldest. Finally, we did not investigate the mechanisms underlying the associations we described, and the cross-sectional design did not permit causal inferences about the relation between muscle mass and functional ability.
Obviously, the prevalence of a disease or symptom depends on the criteria used for diagnosis and the reference population used to establish normative data. The EWGSOP further proposes three conceptual stages of sarcopenia indicating the severity of the condition, namely, “presarcopenia,” “sarcopenia,” and “severe sarcopenia” in order to help guide clinical management of the condition [11]. These terms are based on presence/absence of the three criteria of the definition of sarcopenia, namely, low muscle mass, low muscle strength, and low physical performance. Those who have normal muscle strength and performance but have low muscle mass are termed presarcopenic. Accordingly, the cases identified by the EWGSOP algorithm in our study are “severely sarcopenic.” Most prevalence studies have used the presence of low muscle mass as the only criterion for diagnosis [6, 15, 30, 31], which may explain the large differences in prevalence between previous studies and the present study. We see that the diagnostic criteria need to be standardized and consistently applied before they can be deemed worthy of comparison. Unless this is done, diagnosis and prevalence rates do not hold credibility.
Early reliable identification of sarcopenia would seem to be a powerful step towards understanding the process of aging, improving physical functioning, preventing falls and disabilities, and consequently, improving the quality of life in the older population. Previous cross-sectional studies have reported that older adults with severe levels of sarcopenia (SMI ≤ 8.50 kg/m² in men and ≤5.75 kg/m² in women) are about two to five times as likely to have functional impairment or disability as older adults with normal muscle mass [6, 9, 10, 31]. However, Janssen reported in an 8-year longitudinal study that sarcopenia was only a modest predictor of disability [32], indicating that the effects of sarcopenia on the development of disability may not be as strong as hypothesized based on cross-sectional observations. Goodpaster and colleagues reported that initial lean mass and changes in lean mass could explain only a small proportion of variability in declined muscle strength [12]. It is implied, therefore, that the nature of the relationship between sarcopenia and disability may be bidirectional; it is plausible that physical disability itself could lead to sarcopenia through lower levels of physical activity and consequently decreased stimulus to skeletal muscle [32]. Consistent with these findings, another study [33] indicated that sarcopenia (SMI < 7.26 kg/m² in men and <5.45 kg/m² in women) in the absence of obesity (76 % of the sarcopenic group) was not a significant risk factor for disability; notably, most people with sarcopenia are not obese. A recent systematic review [34] concluded that while muscle and fat mass have been considered an important factor of age-related decline in physical function, studies examining the relationship between fat/muscle mass and functionality have shown inconsistent results.
Although older persons with early sarcopenia (or presarcopenia) are probably those who are most likely to benefit from interventions, those with symptomatic loss of muscle strength or function are more likely to seek treatment and be identified. Detection of functional limitations based on body composition obtained from expensive and less accessible procedures like DXA may not be feasible in clinical practice. There are a number of tests of functional ability currently in use that can be performed simply, safely, and cost-effectively and predict adverse outcomes such as physical disability, fairly accurately. Several studies have suggested that lower extremity function, specifically timed gait, provides a predictive value for the onset of disability [35–37] and even as a predictor of adverse health events [38]. These studies have shown that poor performance on other tests of lower extremity function, such as the chair stand and standing balance performance, is equally prognostic when gait speed is unavailable, and conversely, assessing gait speed alone is nearly as good as performing the full battery of performance tests in the prediction of incident disability. It has also been shown that hip abductor strength is a better predictor of poor physical function rather than muscle mass, and that muscle strength may be a useful screening tool to detect those at risk of functional decline [39]. Our study further confirmed that muscle mass and derived indices of sarcopenia were not significantly related to measures of functional ability. An appropriate and standardized functional ability test battery that includes balance, strength, and mobility performance measures might be better suited to detect changes in physical function and, consequently, the onset of disability in older adults. Declined functional ability is the true clinical problem that needs to be treated and prevented properly.
The aged population in the developed world is increasing rapidly. In the present scenario, it is imperative that cost-effective diagnostic tools are employed and appropriate preventive as well as curative measures are prescribed to combat the negative effects of aging and improve the quality of life of older adults. Treatments currently under investigation include physical activity, nutritional therapies, androgen therapy, and other behavioural and pharmacological strategies [5, 40]. Substantial improvements in both muscle mass and strength are seen with strength training in older persons, leading to improved functional ability. Life-long improvements in physical activity and diet are probably the most effective public health intervention for this condition [20, 41]. In contrast, pharmacological trials of sarcopenia have not yet shown any significant efficacy in the treatment of the condition [42]. The measurement of muscle mass alone as a diagnostic test, therefore, does not seem to be very advantageous in the prediction of disability. Our study corroborates this argument by showing that even a low skeletal muscle mass index was not related to gait speed or grip strength. Low muscle mass may just not reflect the decline in functional performance properly.
For this study, the diagnostic cutoff points were primarily set according to those obtained from a population in New Mexico [6] or the Health ABC study [7] data. These may not, however, be appropriate for the Finnish population. If sarcopenia were to be defined as SMI > 2 SD below the mean of our large population-based cohort of healthy young Finnish women, the cutpoint for low muscle mass would be set at 4.9 kg/m², and the prevalence in our study population would drop further to 0.2 %, meaning that only 1 out of about 400 home-dwelling older women was sarcopenic. Should this be the case, low muscle mass itself is not the problem of the elderly community-dwelling population at large.
Future prospective studies that critically examine the relevance of additional skeletal muscle determinants including total body percentage fat and lean masses to predict loss of lean muscle mass and their effect on the loss of physical function are required. Reduction in muscle mass occurs as part of normal aging, which is a universal phenomenon in human physiology. The actual relevance of muscle mass as compared to muscular function as a predictor of decline in functional ability needs to be scrutinized. Cooper and colleagues [43] suggest the term sarcopenic frailty, with a conceptual definition of the inability of active, autonomous, community-dwelling older people without current disabilities (but with low muscle mass) to cope with stressors. However, screening and identification of such a population pose a lot of difficulties. One may also question the need to use a more expensive method to measure only a modest predictor of functional decline, rather than considering a strong predictor that is easily measured. We propose that an appropriate and standardized functional ability test battery that includes balance, strength, and mobility performance measures might be better suited to detect changes in physical function and consequently the onset of disability in older adults.
In conclusion, our study suggests that when using the EWGSOP and IWG definitions, sarcopenia is infrequent among older home-dwelling women while every third woman has the WHO-based osteopenia. In future studies, strategies to detect early disability and improve function should be high priority, rather than focussing on low muscle mass.
References
Frontera W, Hughes V, Fielding R, Fiatarone M, Ewans W, Roubenoff R (2000) Aging of skeletal muscle: a 12-year longitudinal study. J Appl Physiol 88:1321–1326
Kanis J, McCloskey E, Johansson H, Oden A, Melton L 3rd, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42:467–475
Baumgartner R, Stauber P, McHugh D, Koehler K, Garry P (1995) Cross-sectional age differences in body composition in persons 60+ years of age. J Gerontol A Biol Sci Med Sci 50(6):M307–M316
Gallagher D, Visser M, DeMeersman R, Sepúlveda D, Baumgartner R, Pierson R, Harris T, Heymsfield S (1997) Appendicular skeletal muscle mass: effects of age, gender and ethnicity. J Appl Physiol 83(1):229–239
Fielding R, Vellas B, Evans W, Bhasin S, Morley J, Newman A, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 12:249–256
Baumgartner R, Koehler K, Gallagher D, Romero L, Heymsfield S, Ross R, Garry P, Lindeman R (1998) Am J Epidemiol 147(8):755–763
Newman A, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky S, Tylavsky F, Rubin S, Harris T (2003) Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 51:1602–1609
Delmonico M, Harris T, Lee J, Visser M, Nevitt M, Kritchevsky S, Tylavsky F, Newman A (2007) Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 55:769–774
Janssen I, Heymsfield S, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and disability. J Am Geriatr Soc 50:889–896
Janssen I, Baumgartner R, Ross R, Rosenberg I, Roubenof R (2004) Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 159:413–421
Cruz-Jentoft A, Baeyens J, Bauer J, Boirie Y, Cederholm T, Landi F, Martin F, Michel J, Rolland Y, Schneider S, Topinkova E, Vandewoude M, Zamboni M (2010) Sarcopenia: European consensus on definition and diagnosis. Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423
Goodpaster B, Park S, Harris T, Kritchevsky S, Nevitt M, Schwartz A, Simonsick E, Tylavsky F, Visser M, Newman A (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61:1059–1064
World Health Organization (WHO Scientific Group) (2003) Prevention and management of osteoporosis: report of a WHO scientific group. World Health Organ Tech Rep Ser 921:1–192
World Health Organization (WHO Study Group) (1993) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO Study Group. World Health Organ Tech Rep Ser 843:1–129
Di Monaco M, Vallero F, Di Monaco R, Tappero R (2011) Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch Gerontol Geriatr 52:71–74
Weigl M, Cieza A, Cantista P, Reinhardt J, Stucki G (2008) Determinants of disability in chronic musculoskeletal conditions: a literature review. Eur J Phys Rehabil Med 44:67–79
Frost H (1987) Bone “mass” and the “mechanostat”: a proposal. Anat Rec 219:1–9
Evans W (1997) Functional and metabolic consequences of sarcopenia. J Nutr 127:998S–1003S
Guralnik JM, Ferrucci L, Balfour JL, Volpato S, Di Iorio A (2001) Progressive versus catastrophic loss of the ability to walk: implications for the prevention of mobility loss. J Am Geriatr Soc 49:1463–1470
Roth SM, Ferrell RF, Hurley BF (2000) Strength training for the prevention and treatment of sarcopenia. J Nutr Health Aging 4:143–155
Uusi-Rasi K, Kannus P, Karinkanta S, Pasanen M, Patil R, Lamberg-Allardt C, Sievänen H (2012) Study protocol for prevention of falls: a randomized controlled trial of effects of vitamin D and exercise on falls prevention. BMC Geriatr 12:12. doi:10.1186/1471-2318-12-12
Uusi-Rasi K, Rauhio A, Kannus P, Pasanen M, Kukkonen-Harjula K, Fogelholm M, Sievänen H (2010) Three-month weight reduction does not compromise bone strength in obese premenopausal women. Bone 46:1286–1293
Heymsfield S, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, Pierson R (1990) Appendicular skeletal muscle mass: measurement by dual-photon absorptiometery. Am J Clin Nutr 52:214–218
Guralnik J, Simonsick E, Ferrucci L et al (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49(2):M85–M94
Podsiadlo D, Richardson S (1991) The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39(2):142–148
Lauretani F, Russo C, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi A, Rantanen T, Guralnik J, Ferrucci L (2003) Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 95:1851–1860
Stigman S, Rintala P, Kukkonen-Harjula K, Kujala U, Rinne M, Fogelholm M (2009) Eight-year-old children with high cardiorespiratory fitness have lower overall and abdominal fatness. Int J Pediatr Obes 4:98–105
Kanis J, Johnell O, Oden A, Jonsson B, De Laet C, Dawson A (2000) Risk of hip fracture according to the World Health Organisation criteria for osteopenia and osteoporosis. Bone 27:585–590
Guggenbuhl P, Dufour R, Liu-Léage SH, Cortet B (2011) Efficiency of bone density testing by dual-biophotonic X-rays absortiometry for diagnosis of osteoporosis according to French guideline recommendations: the PRESAGE study. Joint Bone Spine 78(5):493–498
Iannuzzi-Sucich M, Prestwood K, Kenny A (2002) Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci 57(12):M772–M777
Melton L, Khosla S, Crowson C, O’Connor MK, O’Fallon M, Riggs L (2000) Epidemiology of sarcopenia. J Am Geriatr Soc 48:625–630
Janssen I (2006) Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc 54:56–62
Baumgartner R, Wayne S, Waters D, Janssen I, Gallagher D, Morley J (2004) Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 12(12):1995–2004
Shin H, Panton L, Dutton G, Ilich J (2011) Relationship of physical performance with body composition and bone mineral density in individuals over 60 years of age: a systematic review. J Aging Res 2011:191896. doi:10.4061/2011/191896
Guralnik J, Ferrucci L, Pieper C, Leveille S, Markides K, Ostir G, Studenski S, Berkman L, Wallace R (2000) Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 55(4):M221–M231
Ostir G, Markides K, Black S, Goodwin J (1998) Lower body functioning as a predictor of subsequent disability among older Mexican Americans. J Gerontol Med Sci 53A:M491–M495
Guralnick J, Ferrucci L, Simonsick E, Salive M, Wallace R (1995) Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 332:556–561
Cesari M, Kritchevsky S, Newman A, Simonsick E, Harris T, Penninx B, Brach J, Tylavsky F, Satterfield S, Bauer D, Rubin S, Visser M, Pahor M (2009) Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging and Body Composition Study. JAGS 57:251–259
Woods J, Iuliano-Burns S, King S, Strauss B, Walker K (2011) Poor physical function in elderly women in low-level aged care is related to muscle strength rather than to measures of sarcopenia. Clin Interv Aging 6:67–76
Waters D, Baumgartner R, Garry P, Vellas B (2010) Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: an update. Clin Interv Aging 5:259–270
Roubennoff R, Hughes V (2000) Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci 55(12):M716–M724
Chumlea W, Cesari M, Evans W, Ferrucci L, Fielding R, Pahor M, Studenski S, Vellas B, The Task Force Members (2011) Sarcopenia: designing phase IIB trials. International Working Group on Sarcopenia. J Nutr Health Aging 15(6):450–455
Cooper C, Dere W, Evans W, Kanis J, Rizzoli R, Sayer A, Sieber C, Kaufman J, Abellan van Kan G, Boonen S, Adachi J, Mitlak B, Tsouderos Y, Rolland Y, Reginster J (2012) Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int. doi:10.1007/s00198-012-1913-1
Acknowledgments
The authors thank the Academy of Finland, the Medical Research Fund of Tampere University Hospital, the Finnish Ministry of Education, the Juho Vainio Foundation, and the National Doctoral Programme of Musculoskeletal Disorders and Biomaterials for their financial support.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patil, R., Uusi-Rasi, K., Pasanen, M. et al. Sarcopenia and osteopenia among 70–80-year-old home-dwelling Finnish women: prevalence and association with functional performance. Osteoporos Int 24, 787–796 (2013). https://doi.org/10.1007/s00198-012-2046-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-012-2046-2