Abstract
Summary
We compared the distribution of vertebral fractures in adults and children and found that fractures occurred in different locations in the two age groups. This likely relates to the different shape of the immature spine.
Introduction
We hypothesized that the anatomical distribution of vertebral fractures (VF) would be different in children compared to adults.
Methods
We compared the distribution of VF defined using the Genant semi-quantitative method (GSQ method) in adults (N = 221; 545 fractures) and in children early in the course of glucocorticoid therapy (N = 44; 94 fractures).
Results
The average age in the adult cohort was 62.9 years (standard deviation (SD), 13.4 years), 26% was male, the mean lumbar spine Z-score was −1.0 (SD, 1.5), and the corresponding T-score was −2.4 (SD, 1.4). The pediatric cohort median age was 7.7 years (range, 2.1–16.6 years), the mean lumbar spine Z-score was −1.7 (SD, 1.5), 52% was male, and disease categories were acute lymphoblastic leukemia (66%), rheumatological conditions (21%), and nephrotic syndrome (14%). The VF distribution was biphasic in both populations, but the peaks differed in location. In adults, the peaks were at T7/T8 and at T12/L1. In children, the focus was higher in the thoracic spine, at T6/T7, and lower in the lumbar spine, at L1/L2. When children were assessed in two age-defined sub-groups, a biphasic VF distribution was seen in both, but the upward shift of the thoracic focus to T6 was observed only in the older group, with the highest rates of fracture present between ages 7 and 10 years.
Conclusions
These results suggest that the anatomical distribution of VF differs between children and adults, perhaps relating to the different shape of the immature spine, notably the changing ratio of kyphosis to lordosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The propensity for a vertebra to fracture is proportional to a force applied to the bone and inversely proportional to the ability of the bone to resist that force [1]. While vertebrae are subjected to a variety of forces, the dominant one in the case of osteoporotic vertebral fractures (VF) is the axial compressive force that is transmitted through the spinal column [2–4]. The normal thoracolumbar spine has an S-shape, with a kyphosis in the thoracic region and a lordosis in the lumbar region [3–5]. This shape is a consequence of bipedalism, providing stability and reduced energy use when walking [2, 3]. This shape also leads to force dispersion at several points along the spinal curve, predisposing to clustering of compression fractures around these foci [6, 7]. There have been many studies in adults evaluating the anatomical distribution of vertebral compression fractures using the Genant semi-quantitative method (GSQ method) [8]. These studies consistently show two foci of VF: one focus in the mid-thoracic spine centered at T7/T8 and a second focus at the thoracolumbar junction centered at T12/L1 [8–16].
VF are being increasingly recognized as an important aspect of the bone health of children and adolescents, particularly in the setting of glucocorticoid exposure. Cross-sectional studies show VF prevalence rates of up to 50% in some pediatric glucocorticoid-treated disease groups, although the VF methodology has often not been standardized in these studies [17]. The Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research initiative, which involves 10 tertiary care children’s hospitals across Canada, is one of the largest prospective studies of glucocorticoid-related bone health in this age group. The STOPP study is also one of the first to systematically assess vertebral status of all participants using the GSQ method and has found that within the first month of glucocorticoid initiation, VF prevalences were 7% in rheumatic conditions, 8% in nephrotic syndrome, and 16% in acute lymphoblastic leukemia (ALL) [17–19]. In contrast to the situation in adults, there has been little study of VF distribution in children and adolescents. Given that spine shape and biomechanical forces are different in the immature spine, and that intrinsic properties of vertebral bodies may also differ, a distinct VF distribution might be anticipated in children [5, 20–22]. As information about the pediatric spinal fracture profile will provide insight into the role of biomechanics in such fractures and will assist clinical assessment of children at risk for VF, we compared the anatomical distribution of VF in children and adults using the same diagnostic criteria to define VF.
Methods
Study subjects
The pediatric subjects were all of the participants with prevalent VF (N = 44) enrolled in the STOPP research initiative, a natural history study examining the development of VF and other markers of bone health in children with diseases treated by glucocorticoids. Characteristics of the entire STOPP population have been described in detail in prior publications [17–19]. In brief, there were 400 total subjects across three disease categories. The prevalent VF rate at baseline was 16% (95% CI, 11–21%) in the ALL study population, 7% (95% CI, 4–12%) in the rheumatological disease population, and 8% (95% CI, 4–16%) in the subjects with nephrotic syndrome. BMD was related to the presence of VF in ALL, with an odds ratio of 1.8 (95% CI, 1.1–2.9; p < 0.001) per standard deviation (SD) reduction in BMD, while the relationship of BMD to VF was not statistically significant in the other two disease groups. The ALL patients were treated according to Children’s Oncology Group (9 sites) or the Dana Farber Cancer Institute (1 site) protocols, while rheumatological and nephrotic syndrome subjects were treated with individualized doses of glucocorticoids. Exposure to medications was brief, however, with spine radiographs done within 30 days of initiation of glucocorticoid treatment or other disease chemotherapy, as per study protocol [17–19]. The current investigation was undertaken as a post hoc analysis when it appeared as though the fracture distribution in the STOPP population was different from the distribution reported in adults [8–16].
Rather than match pediatric cases with an equal number of adult cases, we included all available adult cases with idiopathic osteoporosis and prevalent VF from a single research center (N = 221) evaluated by two experienced radiologists [23]. This was done to enhance the statistical comparisons with the relatively small number of pediatric fractures and to ensure that the VF methodology was comparable to that used for the children. This study was approved by the Research Ethics Board in each participating institution, and local consent processes were followed. The study was compliant with the Declaration of Helsinki. Height was determined as the average of three standing measurements using regularly calibrated stadiometers for adults and older children, while infants and children unable to stand were measured in the supine position. For children, height was transformed into age- and gender-matched Z-scores [24]. BMD in children was measured at the lumbar spine (L1-L4) by dual-energy x-ray absorptiometry using either Hologic machines (QDR 4500, 3 centers; Discovery, 2 centers; Delphi, 1 center) or Lunar Prodigy (4 centers). In vivo precision ranged from 0.003 to 0.017 g/cm2. Machines were cross-calibrated using a Hologic spine phantom, and data were converted to Hologic units. Z-scores were generated using the Hologic 12.4 normative database. Adults were measured on a single Hologic QDR 4500 (in vivo spine precision of 0.007 g/cm2) and T-scores were derived using the Hologic reference database [23].
Vertebral morphometry
Spine radiographs were done by standard methods [17–19, 23]. Individual vertebrae from T4 to L4 were evaluated by experienced radiologists and were graded according to the GSQ method, which makes use of radiological expertise in evaluating vertebral fractures [8]. Vertebral body height ratios were visually graded on a four-point scale: grade 0 (no fracture) = reduction in a height ratio ≤20%, grade 1 (called a mild VF) if the height ratio reduction was from >20% to 25%, grade 2 (moderate VF) if the decrement was >25% to 40%, and grade 3 (severe VF) if the height ratio reduction exceeded 40% [8]. Vertebral morphometry on adult radiographs was performed by either of two experienced radiologists (H.J, R.W.) with extensive experience in morphometry using the GSQ method [23]. The interobserver kappa was 0.69 for fracture defined as grades 1, 2, or 3. For the pediatric cohort, a specific paradigm was followed to assign fracture status. Vertebral fracture assessment was carried out on all spine radiographs independently by two experienced pediatric radiologists (N.S., M.M), also with research experience in applying the GSQ method [17–19]. Any discrepancies between the first two radiologists were adjudicated by a third expert radiologist (B.L.). The interobserver reliability for the first two readers by kappa was 0.44 for fracture defined as grades 1, 2, or 3 and 0.66 for fracture defined as grades 2 or 3.
Statistical methods
Analyses were conducted using SPSS 16.0 (SPSS Inc.). Baseline population characteristics are expressed as mean and SD or median and range for continuous variables, while discrete variables are expressed as the value and percentage frequency. Proportions were calculated along with 95% confidence intervals (CI). The chi-square, Mann–Whitney, or Fisher’s exact tests were used to compare clinical parameters. No corrections were made for multiple fractures within individual subjects. Presented p values are two-sided and were deemed significant at or below a 5% level.
To evaluate the influence of age on VF distribution within the pediatric cohort, the children were divided into two age groups (termed Young and Older) so that each contained an equal number of vertebral fractures (n = 47), and fracture distributions were compared between these two groups. VF distribution was also assessed using T6 as a marker of thoracic location and L2 or L1/L2 as markers of the lumber location. For comparison with changes in fracture distribution, kyphosis/lordosis ratios at different ages were derived from the data of Cil et al. [5].
The probability ratio for fracture at each vertebra was determined as the probability of fracture at a given vertebra divided by the average probability for the 13 vertebrae that were examined:
where VF x = number of VF at vertebra x and VFTotal = total number of VF from T4 to L4.
Probability ratios were expressed in a novel graphical format we have termed a spinunculus. In the spinunculus, the spine is shown schematically in lateral view with vertebral bodies from T4 to L4 represented by rectangles. To illustrate the distribution of fractures in a given population, the anteroposterior diameter of each vertebral body in the sagittal plane is adjusted to reflect the relative probability of fracture at that vertebra. If the probability ratio of a given vertebra fracturing is greater than 1.0, the anteroposterior diameter of that vertebra in the spinunculus is increased proportionately, and if the probability ratio is less than 1.0, the anteroposterior diameter in the sagittal plane is decreased. For example, if the probability ratio is 2.0, the anteroposterior diameter in the sagittal plane is doubled; if the probability ratio is 0.5, the anteroposterior diameter in the sagittal plane is halved.
Results
Study subjects
For the pediatric cohort (Table 1), median age was 7.7 years (range, 2.1–16.6 years), and 52% was male. The mean lumbar spine Z-score was −1.7 (SD, 1.5). Height Z-score was 0.07 (SD, 1.07), not different from the normal value for age. The underlying disease categories were 66% ALL, 21% rheumatological conditions, and 14% nephrotic syndrome. For the adult cohort, the average age was 62.9 years (SD, 13.4 years), and 26% was male. The mean lumbar spine Z-score was −1.0 (SD, 1.5) and the corresponding T-score was −2.4 (SD, 1.4). Historical height loss (tallest recalled height minus measured height) was 3.9 cm (SD, 4.4 cm). There was no history of significant back trauma in either pediatric or adult subjects.
Pediatric vertebral fracture characteristics
There was a total of 94 VF in the pediatric cohort (Table 1). Subjects had an average of 2.1 (SD, 2.1) fractures per person, with 68% having one fracture and 32% having two or more. Most of the fractures were in the thoracic region (72%), in proportion to the greater number of thoracic vertebrae (9/13 of the assessed vertebra in each spine were thoracic (69%)), with 28% in the lumbar spine. Fracture grade was mild (grade 1) in 63% and moderate (grade 2) or severe (grade 3) in 37%.
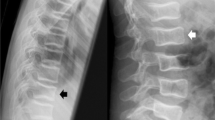
Two major peaks were present when the anatomic distribution was examined (Fig. 1): a peak centered at T6/T7 and a peak centered at L1/L2. For all fracture grades, 31% (95% CI, 22–41%) of VF were at T6/T7 and 21% (95% CI, 14–31%) were in L1/L2. The probability ratio of fracture at each vertebra is presented in graphical form as a spinunculus, where the adjusted anteroposterior diameter of each vertebral body in the sagittal plane reflects the probability ratio of fracture at that vertebra (Fig. 2). Fracture distributions were similar for males and females. Thirty-three percent (95% CI, 20–47%) of VF were present at T6/T7 in boys compared with 28% (95% CI, 15–41%) in girls, while boys had 19% (95% CI, 8–30%) of VF at L1/L2 and girls had 24% (95% CI, 12–36%) at this site. These did not statistically differ between the genders. Spinal distribution patterns were also similar for mild VF in comparison to moderate and severe fractures. Thirty-two percent (95% CI, 20–44%) of grade 1 fractures and 29% (95% CI, 14–44%) of grade 2/3 fractures were located at the thoracic focus of T6/T7, while 20% (95% CI, 10–31%) of grade 1 fractures and 23% (95% CI, 9–37%) of grade 2/3 fractures were found at L1/L2. These did not statistically differ between fracture grades.
Spinunculus. The probability ratio for the risk of fracture at each vertebra from T4 to L4 is expressed by the relative anteroposterior diameter of the schematic vertebral body in the sagittal plane. a Adults. b Children. c Young children (median age, 4.1 years; range, 2.1 to 6.4 years). d Older children (median age, 10.8 years; range, 7.2 to 16.6 years)
Adult vertebral fracture characteristics
There was a total of 545 VF in the adult cohort (Table 1). Subjects had an average of 2.5 (SD, 1.9) fractures per person, with 39% having one fracture and 61% having two or more. Seventy-six percent of fractures were thoracic and 24% were in the lumbar spine. Fractures were grade 1 in 45% and grade 2 or 3 in 55%.
As with the children, two major peaks were present when the anatomic distribution was examined, but the locations of the peaks differed from those in children (Figs. 1 and 2). In the thoracic spine, the peak was centered at T7/T8 and the second peak centered at the thoracolumbar junction, T12/L1. For all fracture grades, 27% (95% CI, 24–31%) of VF were at T7/T8 and 17% (95% CI, 14–20%) were in T12/L1. Fracture distributions were similar for males and females. Twenty-eight percent (95% CI, 20–34%) of VF were located at T7/T8 in men and 27% (95% CI, 23–32%) in women, while males had 15% (95% CI, 10–21%) at L1/L2 compared to 18% (95% CI, 14–22%) at this site in women. These were not statistically different between the genders. Similarly, spinal distribution patterns were similar for mild fractures and for moderate and severe fractures. For grade 1 fractures, 30% (95% CI, 25–35%) were found at T7/T8 while 24% (95% CI, 19–29%) of grade 2/3 fractures were located at this focus. The only statistically significant difference in distribution of fracture grade in adults was at T12/L1, where 12% (95% CI, 8–15%) of grade 1 fractures were present versus 23% (95% CI, 18–28%) of grade 2/3 fractures (p = 0.001).
Pediatric vertebral fracture distribution in relation to age
For the Young children (n = 20; 11 male), the median age was 4.1 years with a range from 2.1 to 6.4 years, while the Older sub-group of children (n = 24; 12 male) had a median age of 10.8 years and a range from 7.2 to 16.6 years. The VF distributions were biphasic in both age groups but showed different patterns (Figs. 2 and 3a). The Older sub-group of children showed a distribution similar to that described for the entire pediatric cohort, with a large peak at T6/T7 (45% of total VF in this age group; 95% CI, 31–59%) and a second peak at L1/L2 (21%; 95% CI, 12–35%). The Young sub-group of children, however, had a thoracic peak at T7/T8 (28%; 95% CI, 17–42%) with few fractures at T6 (4%; 95% CI, 1–14%), and although there were fractures at L1/L2, these were part of a broad peak with approximately equal percentages of total VF across T11 to L2 (40% of total fractures; 95% CI, 28–55%). The two age sub-groups differed in the proportion of fractures at T6/T7 (p = 0.004) but did not statistically differ for VF at L1/L2.
Vertebral fracture distribution in relation to age. a Young children (solid circles; median age, 4.1 years; range, 2.1 to 6.4 years) compared to Older children (open circles; median age, 10.8 years; range, 7.2 to 16.6 years). b Proportion of children at each age having fractures at T6 (circles). For comparison is shown the kyphosis/lordosis ratio at different ages derived from the publication of Cil et al. (squares) [5]
Within the Older sub-group, the median age for those with VF at T6 (n = 13) was 9.4 years and the median age for those without T6 fractures (n = 11) was 12.7 years. These ages were significantly different (p = 0.008). The proportion of children at each year of age (in the entire pediatric population) having a T6 VF was plotted against age (Fig. 3b). The highest T6 fracture rates were apparent between the ages of 7 and 10 years. Within the 7-to-10 age group (n = 12 subjects), 83% (95% CI, 55–95%) had T6 VF, while in all the remaining children (n = 32), only 16% (95% CI, 7–32%) had T6 fractures (p = 0.0001). On the graph are also plotted kyphosis/lordosis ratios at different ages. Cil and colleagues reported that in children of ages 3 to 6 years, the mean kyphosis angle (from T1 to T12) is 45° while the mean lordosis angle (from L1 to S1) is 44°, for a kyphosis/lordosis ratio of 1.01 (Fig. 3b) [5]. Older adolescents nearing skeletal maturation (age, 13 to 15 years) have a similar balanced ratio of 0.98, although both values have increased by this age (kyphosis 53°, lordosis 55°). In transitional age ranges, the ratio becomes unbalanced as the lordosis angle increases to mature values at an early age, while the kyphosis angle increases only much later. Children aged 7 to 9 years have a ratio of 0.92 as the lordosis is beginning to increase while the kyphosis does not (kyphosis 48°, lordosis 52°). Over the age range of 10 to 12 years, which encompasses the median age of our Older pediatric sub-group, the ratio is markedly lower still at 0.80 as a consequence of lordosis moving to a fully mature value at this age (57°) while kyphosis remains at an immature angle (46°). The downward shift of the lumbar VF focus in relation to age was similarly evaluated by examining the proportion of subjects with fracture at L2 or either L1 or L2. There was no age group at which the rate of VF at this location was statistically different from other ages (data not shown).
Discussion
In the normal adult, the body’s center of mass lies anterior to the spine and the resulting gravitational force creates torque (rotational moment of force) centered on the vertebral bodies that can be viewed as having a downward component and an anteriorly directed component (Fig. 4a) [3, 4, 25] In order to maintain balance, the posteriorly located paraspinal muscles and ligaments contract to produce a counterbalancing force [3, 4, 25]. This is also a downward torque; however, since it is posterior to the vertebral bodies, it has a posteriorly directed component in addition to the downward one [4]. The horizontal components of the two torques cancel while the downward components sum, leaving a net downward force centered on the vertebral body (Fig. 4b). With bending forward or lifting weight, especially with the arms outstretched, the anterior torque rises markedly, resulting in a greater compensatory contraction of the paraspinal muscles [3, 4, 26]. Again, the horizontal force vectors cancel, and the net downward force on the vertebral bodies rises markedly [4]. Similarly, when there is increased thoracic kyphosis, as occurs in association with aging or with the presence of vertebral fractures, the center of mass is also shifted anteriorly, once more resulting in amplified downward force on the vertebral bodies [25]. This phenomenon is felt to contribute to the “fracture cascade,” whereby the presence of VF increases the risk of further VF due to the increased stress on the vertebrae [27].
Forces on the vertebral body. a Body mass generates a torque with an anteriorly directed component and a downward component centered on the vertebral body. This is countered by the paraspinal muscles that produce a posteriorly directed force and a downward force. b The anteriorly and posteriorly directed forces cancel while the downward forces sum to create a net downward compressive force on the vertebral body
Each of the two curves of the thoracolumbar spine—the thoracic kyphosis and the lumbar lordosis—has a focus where forces are dissipated [9–16]. The reasons why fractures occur at the two foci are different for the two anatomical sites, but both relate at least in part to thoracolumbar spine shape [3, 4]. In our adult cohort, we found that the greatest number of thoracic fractures occurred at T7 and T8, as has been consistently demonstrated in the literature using the GSQ method [9–16]. These two vertebrae lie near the inflection point of the normal adult thoracic kyphotic curvature [3, 7]. When axial compressive forces reach this part of the thoracic spine, they dissipate here rather than move around the curve [7, 28]. Consistent with this concept is the fact that in adults, fractures subsequent to an initial fracture tend to cluster close to the original VF rather than at vertebrae farther away [27]. At T12/L1, the thoracolumbar junction, other variables come into play to make this a focus of fracturing. The downward force from the body’s center of mass passes directly through these vertebral bodies, and they are therefore subjected to greater compressive force on flexion than higher parts of the spinal column [3, 4]. A further factor occurring at the thoracolumbar junction that predisposes to fracture in this region is a change in orientation of the facet joints. Up to 25% of a compressive force is transmitted through the facets [28]. Higher in the spine, the facet joints are more horizontal and can participate to a greater degree in dispersing compressive force. Around T12, they become more vertical and so play a lesser role in force dissipation. A greater proportion of the force on these vertebrae is therefore borne by the vertebral bodies.
The ability of a vertebral body to resist compressive force is related to architectural strength and to the intrinsic strength of the structural material [26, 29]. Architectural strength can be viewed as consisting of macroarchitectural contributors, such as vertebral body diameter, vertebral body height, cortical thickness, and the degree of mineralization, and microarchitectural components like number and distribution of trabeculae as well as trabecular orientation and connectivity [27, 29]. Relevant aspects of intrinsic material strength include the type and amount of proteins and associated molecules, the density of mineral, and crystal size and number [1, 27, 29]. The importance of intrinsic vertebral body strength in resisting fracture is demonstrated by the fracture profile observed following vertebroplasty. This procedure involves filling fractured vertebral bodies with a substance such as methylmethacrylate that changes the intrinsic properties of the body [25]. Subsequent to vertebroplasty, forces pass through the treated vertebral body to adjacent vertebrae, which consequently have an increased risk of fracturing compared to vertebrae not adjacent to the treated bone [30]. There has not been sufficient study of all of the contributors to vertebral body strength and how they vary throughout the spine to assess their specific contributions to the occurrence of fracture foci. Clearly, though, some of these parameters are not prime determinants of fracture location in the untreated spine, or the observed fracture distributions would not occur. For example, smaller vertebrae are less intrinsically strong than larger vertebral bodies [27, 29]. Yet, despite the vertebrae immediately above T7 being smaller in size than T7 and T8, and the vertebrae above T12 being smaller than T12 and L1, their frequency of fracturing is less. This suggests that while these variables may contribute to vertebral body strength, spine shape is a key determinant of fracture location.
In the children we have studied, the thoracic fracture focus was shifted upward and the lumbar focus downward compared to the distribution in adults. We have developed the spinunculus as a way to visualize the difference in fracture distribution. The spinunculus expresses the relative probability of fracture at each vertebra compared to the average fracture rate for the entire spine through differences in the anteroposterior diameter in the sagittal plane of each schematic vertebral body (Fig. 2). Rather than peaks at T7/T8 and T12/L1, as in adults, we found fracture foci peaking at T6/T7 and L1/L2 in children. One published study has used the GSQ method to define fracture in pediatric patients with rheumatological disease and presented data that can be used to generate fracture distribution (Fig. 5) [31]. Their results similarly show a thoracic peak at T6/T7. There is also a lumbar peak that is actually even more caudal than our finding, peaking at L2/L3 rather than at L1/L2, as we found [31]. A third small peak may be present in their data at T11, but given the small numbers of fractures, it is not clear if this is real. Of greater importance, they found a low rate of fracture at T12, quite different from the adult profile and similar to our pediatric results. Another study of children with rheumatological disease assessed VF using a method related to the GSQ method, employing absolute vertebral height reduction rather than reduced height ratios [32]. The results from this study almost exactly parallel our data, with a biphasic distribution and peaks at T6/T7 and L1/L2. Our findings, together with this analysis of published data, suggest that fracture distribution in children differs from that of adults, perhaps resulting from the different shape of the immature spine. The juvenile spine is straighter with a lesser thoracic kyphosis and a reduced lumbar lordosis [5]. It also has a more anterior sagittal vertical axis, greater variability in the location of the apex of the thoracic kyphosis, and changing vertebral body shape during maturation in terms of the ratio of the vertical height to anteroposterior diameter measured in the sagittal plane [20–22]. Together, these features will lead to different locations of force maxima and thus differing tendencies of vertebrae at particular locations to fracture [3, 4].
Most adult osteoporotic VF are not related to known direct trauma [33]. The same is true in our cohort of glucocorticoid-treated children and adolescents, as none of the subjects in our study had sustained back trauma as a cause of the VF. When childhood VF are the result of substantial trauma, they tend to be more broadly distributed throughout the spine than was observed in our cohort, as would be expected, since the forces from trauma are variable in direction and are not purely compressive [34]. Even when due to trauma in childhood, however, there is a propensity for vertebral body compression fractures to occur above T7 and at L1/L2 or lower, just as in our subjects where fractures were non-traumatic [35, 36]. This again suggests that the observation of fracture foci at T6/T7 and L1/L2 in our pediatric cohort is related to the different manner in which forces are distributed in the developing spine compared to that of an adult.
When our pediatric cohort was divided into two sub-groups based on age, each group showed a biphasic VF distribution and had similar percentages of fractures at L1/L2. This suggests that the presence of the lower VF peak at L1/L2 is a characteristic of the pediatric spine. In the thoracic region, however, the increase in fractures at T6 was observed only in the Older sub-group of children (median age, 10.8 years), whereas the Young children (median age, 4.1 years) had few VF at T6 but, rather, a prominent peak at T7/T8. Within the Older sub-group, those with VF at T6 were younger than those without T6 fractures (9.4 vs. 12.7 years). To further explore the age at which the upward shift in thoracic VF occurs, we examined the proportion of children at each age that had fractures at T6 and found that the greatest rates were present from ages 7 to 10 years. This upward displacement of the thoracic focus around ages 7 to 10 years again likely relates to spine biomechanics. Studies have shown that in children around this age, spine shape is undergoing a dramatic alteration during which the thoracic kyphosis and lumbar lordosis become unbalanced [5, 19]. Using data on lordosis and kyphosis angles in different age groups from Cil and colleagues, we calculated kyphosis/lordosis ratios [5]. In young children and in those nearing skeletal maturity, the ratio was near 1.0, indicating a balance between kyphosis and lordosis. Over the age range of 7 to 10 years, in which we observed the highest rates of fractures at T6, the ratio was markedly below 1.0 as a consequence of lordosis moving to a fully mature value at an earlier age while kyphosis remains at an immature angle and only increases with skeletal maturation. It is likely that this imbalance changes the biomechanical forces in a way that contributes to the upward displacement in thoracic VF in the 7- to 10-year age range.
In both children and adults, we found that mild morphometric deformities (grade 1) were located in similar locations as moderate and severe fractures (grades 2 and 3), which suggests that mild deformities are true fractures rather than simply congenital variants of normal shape. There is always the concern that deformities classified as grade 1 fractures are actually normal shape variants, particularly in the upper thoracic region where vertebrae naturally have a greater posterior height than anterior height to conform to the spinal curvature [37]. This issue has been laid to rest in adults, in whom grade 1 fractures defined by the GSQ method correlate with various structural changes in cortical bone, with impaired quality of life, with non-bone clinical disorders, and with the risk of future vertebral fractures [38–41]. Further evidence comes from adult studies using the algorithm-based qualitative (ABQ) method, which employs qualitative structural alterations rather than quantitative changes in height ratios to define fracture [37, 42]. ABQ studies show an upper thoracic focus of fractures and a low rate of false positives at this location using the GSQ method [37, 42]. They also find false negatives in this region using the GSQ method, which suggests that the grade 1 definition may, in fact, be too stringent [42]. Normal vertebral height measurements on plain radiography are not available in children, but a recent study of the lower thoracic and lumbar spine in children using CT concluded that fracture should be suspected when the anterior/posterior height ratio is less than 0.893 [43]. An MRI study found that normal anterior/posterior height ratios throughout the pediatric thoracic spine average well over 0.90 [44]. This makes the GSQ method fracture height ratio definition of less than 0.80 a reasonable and conservative definition.
One possibility that we cannot assess in this study is that the VF distribution in our pediatric cohort, and in the two published datasets we highlight here for comparison, was a direct consequence of glucocorticoid exposure. Although the steroid exposure in our study was brief, glucocorticoids can have a rapid effect on bone [45]. There are no data in the literature comparing VF distribution differences between adults with and without steroid exposure, and no information on the distribution of VF defined by the GSQ method in children who have not received steroids. Another possibility is that the distribution we observed relates to ALL, as the majority of fractures in our study were among patients with this condition [20–22]. The data from children in published rheumatological studies showing similar distributions to our pediatric patients argue against this.
Conclusion
In this study, we have found that the apparent VF distribution in our pediatric cohort and in published pediatric studies differs from that of our adult cohort and of the adult distribution reported in the literature. We also found that the greatest shift in VF distribution takes place in the age range of 7 to 10 years. Further investigations will be necessary to determine whether similar fracture distributions are present in children not receiving glucocorticoids. The fact that mild VF occurred at the same location in the spine as moderate and severe fractures suggests that these deformities likely represent true fractures. Appreciation of the frequency of VF in children treated with glucocorticoids is rapidly evolving, and an understanding of the fracture distribution in the pediatric spine in various age groups will enhance clinical assessment.
References
Silva MJ (2007) Biomechanics of osteoporotic fractures. Injury 38S3:S69–S76
Zebaze R, Maalouf G, Maalouf N, Seeman E (2004) Loss of regularity in the curvature of the thoracolumbar spine: a measure of structural failure. J Bone Miner Res 19:1099–1104
Legaye J, Duval-Beaupere G (2008) Gravitational forces and sagittal shape of the spine. Clinical estimation of their relations. Int Orthop 32:809–816
Legaye J, Duval-Beaupere G (2005) Sagittal plane alignment of the spine and gravity. A radiological and clinical evaluation. Acta Orthop Belg 71:213–220
Cil A, Yazici M, Uzumcugil A et al (2004) The evolution of sagittal segmental alignment of the spine during childhood. Spine 30:93–100
Lunt M, O’Neill TW, Felsenberg D et al (2003) Characteristics of a prevalent vertebral deformity predict subsequent vertebral fracture: results from the European Prospective Osteoporosis Study (EPOS). Bone 33:505–513
Healy JH, Lane JM (1985) Structural scoliosis in osteoporotic women. Clinical Orthop Relat Res 195:216–223
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Chapurlat RD, Duboeuf F, Marion-Audibert HO, Kalpakcioglu B, Mitlak BH, Delmas PD (2006) Effectiveness of instant vertebral assessment to detect prevalent vertebral fracture. Osteoporos Int 17:1189–1195
Fechtenbaum J, Cropet C, Kolta S, Verdoncq B, Orcel P, Roux C (2005) Reporting of vertebral fracture on spine X-rays. Osteoporos Int 16:1823–1826
Fuerst T, Wu C, Genant HK et al (2009) Evaluation of vertebral fracture assessment by dual X-ray absorptiometry in a multicenter setting. Osteoporos Int 20:1199–1205
Genant HK, Jergas M, Palermo L et al (1996) Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. J Bone Miner Res 11:984–996
Leidig-Bruckner G, Genant HK, Minne HW et al (1994) Comparison of a semiquantitative and a quantitative method for assessing vertebral fractures in osteoporosis. Osteoporos Int 4:154–161
Rea JA, Li J, Blake GM, Steiger P, Genant HK, Fogelman I (2000) Visual assessment of vertebral deformity by X-ray absorptiometry: a highly predictive method to exclude vertebral deformity. Osteoporos Int 11:660–668
Vallarta-Ast N, Krueger D, Wrase C, Agrawal S, Binkley N (2007) An evaluation of densitometric vertebral fracture assessment in men. Osteoporos Int 18:1405–1410
Wu CY, Li J, Jergas M, Genant HK (1995) Comparison of semiquantitative and quantitative techniques for the assessment of prevalent and incident vertebral fractures. Osteoporos Int 5:354–370
Huber AM, Gaboury I, Cabral DA et al (2010) Prevalent vertebral fractures among children initiating glucocorticoid therapy for the treatment of rheumatic disorders. Arthritis Care Res 62:516–528
Halton J, Gaboury I, Grant R et al (2009) Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-associated Osteoporosis in the Pediatric Population (STOPP) Research Program. J Bone Miner Res 24:1326–1334
Feber J, Gaboury I, Ni A et al (2011) Skeletal findings in children initiating glucocorticoids for the treatment of nephrotic syndrome. Osteoporos Int (in press)
Willner S, Johnson B (1983) Thoracic kyphosis and lumbar lordosis during the growth period in children. Acta Paediatr Scand 72:873–878
Singer KP, Jones TJ, Breidahl PD (1990) A comparison of radiographic and computer-assisted measurements of thoracic and thoracolumbar sagittal curvature. Skeletal Radiol 19:21–26
Nissensen M (1995) Spinal posture during pubertal growth. Acta Paediatr 84:308–312
Siminoski K, Warshawski RS, Jen H, Lee K (2006) The accuracy of historical height loss for the detection of vertebral fractures in postmenopausal women. Osteoporos Int 17:290–296
Ogden CL, Kuczmarski RJ, Flegal KM et al (2000) Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:45–60
Yuan HA, Brown CW, Phillips FM (2004) Osteoporotic spinal deformity. A biomechanical rationale for the clinical consequences and treatment of vertebral body compression fractures. J Spinal Disord Tech 17:236–242
Myers ER, Wilson SE (1997) Biomechanics of osteoporosis and vertebral fracture. Spine 22(Suppl 2):25S–31S
Briggs AM, Greig AM, Wark JD (2007) The vertebral fracture cascade in osteoporosis: a review of aetiopathogenesis. Osteoporos Int 18:575–584
Andersson GBJ (1992) Biomechanics of the spine. In: Kirkadldy-Willis WH, Burton CV (eds) Managing low back pain, 3rd edn. Churchill Livingstone, New York, pp 27–37
Davison KS, Siminoski K, Adachi JD et al (2006) Bone strength the whole is greater than the sum of its part. Semin Arthritis Rheum 36:22–31
Trout AT, Kallmes DF, Layton KF, Thielen KR, Hentz JG (2006) Vertebral endplate fracture: an indicator of the abnormal forces generated in the spine after vertebroplasty. J Bone Miner Res 21:1797–1802
Nakhla M, Scuccimarri R, Duffy KN et al (2009) Prevalence of vertebral fractures in children with chronic rheumatic disease at risk for osteopenia. J Pediatr 154:438–443
Varonos S, Ansell BM, Reeve J (1987) Vertebral collapse in juvenile chronic arthritis: its relationship with glucocorticoid therapy. Calcif Tissue Int 41:75–78
Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ (1992) Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res 7:221–227
Reilly CW (2007) Pediatric spine trauma. J Bone Joint Surg 89-A(Suppl 1):98–107
Hegenbarth R, Ebel KD (1976) Roentgen findings in fractures of the vertebral column in childhood. Pediat Radiol 5:34–39
Leonard M, Sproule J, McCormack D (2007) Paediatric spinal trauma and associated injuries. Injury 38:188–193
Ferrar L, Jiang G, Clowes JA, Peel NF, Eastell R (2008) Comparison of densitometric and radiographic vertebral fracture assessment using the algorithm-based qualitative (ABQ) method in postmenopausal women at low and high risk of fracture. J Bone Miner Res 23:103–111
Ismail AA, Cooper C, Falsenberg D et al (1999) Number and type of vertebral deformities: epidemiological characteristics and relation to back pain and height loss. Osteoporos Int 9:206–213
Siris ES, Genant HK, Laster AJ, Chen P, Misurski DA, Krege JH (2007) Enhanced prediction of fracture risk combining vertebral fracture status and BMD. Osteoporos Int 18:761–770
Delmas PD, Genant HK, Crans GG et al (2003) Severity of prevalent vertebral fractures and the risk of subsequent vertebral and non-vertebral fractures: results from the MORE trial. Bone 33:522–532
Tanko LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR (2005) Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res 20:1912–1920
Ferrar L, Jiang G, Cawthon PM et al (2007) Identification of vertebral fracture and non-osteoporotic short vertebral height in men: the MrOS study. J Bone Miner Res 22:1434–1441
Gaca AM, Barnhart HX, Bisset GS (2010) Evaluation of wedging of lower thoracic and upper lumbar vertebral bodies in the pediatric population. Am J Roentgenol 194:516–520
Goh S, Price RI, Song S, Davis S, Singer KP (2000) Magnetic resonance-based vertebral morphometry of the thoracic spine: age, gender and level-specific influences. Clin Biomech 15:417–425
Natsui K, Tanaka K, Suda M et al (2006) High-dose glucocorticoid treatment induces rapid loss of trabecular bone mineral density and lean body mass. Osteoporos Int 17:105–108
Acknowledgments
The Canadian STOPP Consortium would like to thank the children and their families who participated in the study and without whom the STOPP research program would not have been possible.
STOPP would also like to thank the following:
The research associates who managed the study at the Children’s Hospital of Eastern Ontario: Steve Anderson (project management), Victor Konji (project management), Catherine Riddell (project management), Maya Scharke (data analysis, database management), Elizabeth Sykes (project management), Monica Tomiak (statistical analysis).
The research associates who took care of the patients: Claude Belleville, Ronda Blasco, Erika Bloomfield, Dan Catte, Heather Cosgrove, Susan Docherty-Skippen, Diane Laforte, Maritza Laprise, Josie MacLennan, Natacha Gaulin Marion, Leila MacBean, Amanda Mullins, Michele Petrovic, Eileen Pyra, Mala Ramu, Angelyne Sarmiento, Terry Viczko, and Aleasha Warner.
The research nurses and support staff from the various Divisions of Nephrology, Oncology, Rheumatology and Radiology who have contributed to the care of the children enrolled in the study.
The STeroid-associated Osteoporosis in the Pediatric Population (STOPP) Consortium:
Alberta Children’s Hospital, Calgary, Canada: Reinhard Kloiber, Victor Lewis, Julian Midgley, Paivi Miettunen, David Stephure; British Columbia Children’s Hospital, Vancouver, Canada: David Cabral, David B. Dix, Kristin Houghton, Helen R. Nadel; British Columbia Women’s Hospital and Health Sciences Center, Vancouver, Canada: Brian C. Lentle; Brock University, St. Catharines, Canada: John Hay; Children’s Hospital of Eastern Ontario, Ottawa, Canada: Janusz Feber, Jacqueline Halton, Roman Jurencak, MaryAnn Matzinger, Johannes Roth, Nazih Shenouda, Leanne M. Ward; IWK Health Center, Halifax, Canada: Elizabeth Cummings, Conrad Fernandez, Adam M. Huber, Bianca Lang, Kathy O’Brien; McMaster Children’s Hospital, Hamilton, Canada: Stephanie Atkinson, Steve Arora, Ronald Barr, Craig Coblentz, Peter B. Dent, Maggie Larche, Colin Webber; Montreal Children’s Hospital, Montreal, Canada: Sharon Abish, Lorraine Bell, Celia Rodd, Rosie Scuccimarri; Ottawa Hospital Research Institute, Ottawa, Canada: David Moher, Tim Ramsay; Shriners Hospital for Children, Montreal, Canada: Francis Glorieux, Frank Rauch; Ste. Justine Hospital, Montreal, Canada: Nathalie Alos, Caroline Laverdiere, Josee Dubois, Veronique Phan, Claire Saint-Cyr; Stollery Children’s Hospital, Edmonton, Canada: Robert Couch, Janet Ellsworth, Claire LeBlanc, Maury Pinsk, Kerry Siminoski, Beverly Wilson; Toronto Hospital for Sick Children, Toronto, Canada: Martin Charron, Ronald Grant, Diane Hebert; Universite de Sherbrooke, Sherbrooke, Canada: Isabelle Gaboury; University of Western Ontario, London, Canada: Elizabeth Cairney, Cheril Clarson, Guido Filler, Joanne Grimmer, Keith Sparrow, Robert Stein; Winnipeg Children’s Hospital, Winnipeg, Canada: Tom Blydt-Hansen, Sara Israels, Kiem Oen, Martin Reed, Shayne Taback
Conflicts of interest
H. Nadel received an honorarium from Siemens Canada for speaking at a radiology symposium. None of the other authors have any disclosures.
Funding
The primary funding source of this study was from The Canadian Institutes of Health Research. Additional funding sources were from The Canadian Child Health Clinician Scientist Program, The Children’s Hospital of Eastern Ontario Research Institute, and Women and Children’s Health Research Institute, University of Alberta.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Siminoski, K., Lee, KC., Jen, H. et al. Anatomical distribution of vertebral fractures: comparison of pediatric and adult spines. Osteoporos Int 23, 1999–2008 (2012). https://doi.org/10.1007/s00198-011-1837-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1837-1