Abstract
Summary
Simvastatin solution was injected subcutaneously to the site of fractured tibiae of ovariectomized rats. Afterwards healing quality was evaluated by morphologic, radiographic, biomechanical, histological and histomorphometric methods at 1, 2 and 4 weeks after fracture. Results showed that locally applied simvastatin improved fracture healing.
Introduction
Many studies have documented an anabolic effect of hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, statins, on undisturbed bone. Reports of their effects, however, on fractured skeletal sytems have been limited. A study was, therefore, conducted to check the effects of statins on fracture healing.
Methods
Simvastatin (10 mg/kg/day) was injected subcutaneously to tissue overlying the site of fractured tibiae of ovariectomized rats for a treatment period of 5 days. Vehicle reagent was used as a control. Healing quality was evaluated at 1, 2 and 4 weeks after fracture.
Results
Compared with that in the vehicle group, the callus cross-section area in simvastatin-treated rats was significantly enlarged by 21.3% (p < 0.05) at 1 week and by 21.5% (p < 0.05) at 2 weeks; new woven bone was relatively substantive and arranged more tightly and regularly at 2 and 4 weeks; and maximal load was increased by 57.5% (p < 0.05) at 2 weeks and by 31.4% (p < 0.05) at 4 weeks. Histomorphometrically, simvastatin was associated with a significant (p < 0.05) increase of mineralization width (MLW), mineralization volume (MLV) and mineral apposition rate (MAR).
Conclusion
The current study suggests that local application of simvastatin could promote fracture healing in ovariectomized rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current therapies for the treatment of osteoporosis, including estrogen and related compounds, bisphosphonates, calcitonin, vitamin D analogues and selective estrogen receptor modulators, are primarily based on blunting the resorption component of bone homeostasis -- a tightly coupled process of bone formation and bone resorption. Drugs that could stimulate new bone formation remain desirable. A study conducted several years ago showed that statins, the widely used lipid-lowering hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, could dramatically stimulate bone formation in cultured osteoblasts, neonatal murine calvaria, and the cortical bone of mice [1]. Then, a number of experimental and clinical studies sustained that statins had postive effects on bone turnover parameters [2–4], bone mineral density (BMD) [5–8], and fracture risk [9–12]. These exciting findings strongly indicated that statins would be a potential approach for eliciting anabolic effects in the skeleton.
In subjects with osteoporosis, susceptibility of fracture increases and healing quality is considered to be impaired in osteoporotic fracture [13–15]. Although most anti-osteoporosis drugs have the ability to decrease the risk of osteoporotic fractures, fractures still occur in patients under medical intervention. For this reason, the safety and efficacy of most anti-osteoporosis drugs for fracture healing have been evaluated in order to decide whether they should be ceased or continued after fracture [16–20]. Moreover, it is well expected that the anti-opsteporosis drugs have the ability to promote fracture healing at the same time.
In terms of statins, although no consensus has been made for their clinical application of osteoporosis, they have been widely used in hypercholesterolemic patients -- mostly in older populations who also have a high prevalence of osteoporosis and a high fracture rate. If statins can promote the healing quality of osteoporotic fractures, it must be favorable.
Till now, the extensive research on statins has, to a large extent, been carried out on undisturbed bone. Only a few studies have been carried out to investigate their effects on fracture healing. To the author’s knowledge, statins in those existing studies were administered systemically in extremely high doses [21, 22]. It should be noted that most gastrointestinal absorbed statins will be stored in the liver, resulting in a much lower concentration at the fracture site [23], and that exceedingly high dose of systemic applied statins will raise the risk of liver failure, kidney disease, and rhabdomyolysis [24]. This prompted us to investigate whether statins could improve fracture healing when applied locally.
In the present study, an osteoporotic fracture model was established in ovariectomized (OVX) rats to imitate fracture in postmenopausal women. In order not to interfere with the healing process by reduplicative injection, which might result in atrophic pseudoarthrosis, the local application in the present study does not mean the direct injection of drugs into the fracture site, rather a subcutaneous injection to tissue in close proximity of the fracture. The aim of the present study was to test the hypothesis that locally applied simvastatin has the ability to improve fracture healing.
Materials and methods
Experimental subjects
The study was carried out on 2-month-old female Sprague-Dawley rats (purchased from the Experimental Animal Center of Zhejiang University, Hangzhou, China) with initial body weight of 178–201 g. The rats were housed in conventionally controlled clean facilities with a temperature of 23°C and a 12-h light-dark cycle. The rats were fed on chow with 0.46% of calcium, 0.38% of phosphorus and tap water.
The study was approved by the local ethics committee for animal experiments in Zhejiang University, China and was conducted in compliance with national and international laws and guidelines for the Use of Animals in Biomedical Research [25].
Ovariectomy
Three days after arrival, all the rats were randomly subjected to either OVX (n = 120) or sham surgery (n = 10). For OVX, the animals were anesthetized with an intraperitoneal injection of 5% ketamine hydrochloride (2 ml/kg body weight). Bilateral dorsal incisions were made on the back; both of the ovaries were identified; the blood vessels were clamped and tied off; and the ovaries were removed. The muscle layer was tied and skin incision was closed with silk suture. Sham surgery was performed following the steps above with the visualization of the ovaries, but without clamping or removing of the tissue.
After the operation, the sham rats had free access to food throughout the experiment, while the food of OVX rats was restricted to that of sham group in order to minimize the increase of body weight associated with OVX.
Confirmation of osteopenia status
Twelve weeks after surgery, BMD of the right tibia was measured under anaesthetic condition (ketamine hydrochloride, same dosage as above) to document osteopenia status. Ten rats were sampled from OVX and sham group. Dual-energy X-ray absorptiometer (DEXA, LUNAR Radiation, USA) was employed using the small animal model (voltage:76 kv, current:150 μA.). The accuracy of this equipment is 0.3%, with an inter-assay coefficient of variation (CV) of 1.3%.
Fracture
All the OVX rats that survived were further used to establish open fracture models and fixed with intermedullary nails. Under sterile conditions after anesthetization by ketamine hydrochloride (same dosage as above), a simple transverse open fracture model was made in the proximal one-third of the right tibia. To stabilize the fracture, a small incision was made on the medial aspect of the knee, and the patella was deflected laterally to expose the tibial nod where a small hole was drilled. Then a sterile stainless steel wire (Ø0.5 mm, Shanghai Medical Needle Factory, China) was inserted through the hole to the distal end of the tibia. The muscle layer was tied and the skin incision was closed with silk suture. Each animal received antibiotics (penicillium, 0.80 million units, im, BID) for three days postoperatively.
The OVX rats were then randomly, but equally allocated to OVX + vehicle and OVX + SVS groups.
Pharmaceutical intervention
Simvastatin or vehicle drugs were injected subcutaneously to the fracture site once on the day of fracture and twice in 5 days thereafter. A solution of 5% simvastatin (Hisun Pharmaceutical Co. Ltd. China) was prepared according to literature [1], i.e., simvastatin was diffused in PBS with 2% dimethylsulfoxide and 0.1% BSA. The vehicle with no simvastatin was also prepared. Each injection contained 5 mg/kg simvastatin of the SVS group and an equal volume (about 30 μl–50 μl) of vehicle solution to the vehicle groups. In this study, simvastatin was injected via the method described by Mundy. In order not to interfere the healing process by reduplicative injection, drugs were injected not directly into the fracture site, but to subcutaneous tissue overlying the fracture site. Solutions were delivered through a 0.4 mm gauge pinhead connected to a micro-syringe with maximal scale of 250 μl and minimal scale of 10 μl (Shanghai Medical Laser Instrument Co. Ltd). Care was taken not to disturb or damage the healing callus.
In order to achieve undecalcified histomorphometry parameters, ten rats in each group were double fluorochrome labeled by tetracycline (BioBasic Inc. Canada), which was injected subcutaneously 14, 13, 4, and 3 days before sacrifice in a dosage of 25 mg/kg.
Evaluation of fracture healing
Rats were killed in 1, 2 and 4 weeks after fracture, and healing quality was evaluated as follows.
Radiography
On the day before sacrifice, the rats were generally anaesthetized (ketamine hydrochloride, same dosage as above) to get posterior-anterior radiographs of the fractured tibiae. X-rays were taken by DMR+ Mo target mammography machine (22 KV, 250 mAS, GE, USA). Fracture callus was described by two independent observers blinded to the treatment groups using following parameters: (a) no bridging, (b) unilateral bridging, or (c) complete bridging. The mean of duplicate scores was used for statistical analysis.
Biomechanical analysis
Ten rats in each group were scheduled to be killed for biomechanical analysis. Bilateral tibiae of each rat were harvested, wrapped in pair with saline-moistened gauze and reserved in −20°C circumstance.
The specimens were thawed at room temperature before the biomechanical test, and the intramedullary nails were removed. The tibia was then subjected to a three-point bending test on Zwick-Z010 testing systems (Zwick GmbH & Co. Ulm, Germany. CV: 0.5%), with a span of 30 mm, and at a displacement rate of 2 mm/min. The specimens were kept in a coincident position and were moist through examination. The load-displacement data and the failure load were recorded. The anteroposterior and transverse diameters of the callus zones were measured by a vernier caliper with a sensitivity of 0.02 mm.
Serum chemistry
Serum total cholesterol was measured to prove the lipid-lowering effect of locally delivered simvastatin, and serum alkaline phosphatase (ALP) was detected as a marker of bone formation. Blood samples were taken from the left ventricle after the rats were anaesthetised and stored at −80°C. Serum ALP and total cholesterol were analyzed by AU1000 automatic biochemical analyzer (Olympus, Japan) using the same kit (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The intra-assay and inter-assay CVs were as follows: ALP, 1.8% and 2.2%; total cholesterol, 0.5% and 0.58%, respectively.
Histological and immunohistochemical analysis
Five rats from each group were killed for histological and immunohistochemical analysis. The right tibia was harvested without damaging the periosteum. After the steel wire had been drawn out, the middle part of the tibia with the callus in it was cut. The tissue blockers were then fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 24 hours at room temperature, and decalcified in ethylene diaminetetra-acetic acid (EDTA, 0.5 mol/L, PH 7.4) for 3–5 weeks. EDTA were refreshed every 5–7 days until the bone could be easily inserted with a fine needle. Decalcified specimens were then washed in a phosphate buffer, dehydrated in a graded series of ethanol and embedded in paraffin wax. Tissue was sliced (5 μm) along the longitudinal plane, mounted on a 0.01% poly-L-lysine (Fuzhou Maixin Biotech Inc. China) coated glass slide and dried overnight at 60°C. Haematoxylin and eosin (HE) staining was performed. The slices were examined under a Olympus BH-2 light microscope.
Bone histomorphometry
Rats with tetracycline label were killed 4 weeks after fracture. The tibial mid-shaft (1 cm) was immerged in 100% acetone for 3 days and then embedded without decalcification in methylmethacrylate. A longitudinal section (5 μm) from the center of the callus block was cut with a Reichert-Jung microtome (Leica Instruments GmbH, Germany) and left unstained for dynamic measurement. An additional section was stained with toluidine blue in order to measure static parameters. The measurement was performed under light microscope and fluorescent microscope (BX51 systems, Olympus, Japan) with a CCD camera (DP11, Olympus, Japan) connected to an image analyzing system installed with Qwin and Qfish software (Leica Instruments GmbH, Germany).
For static measurement, mineralization width (MLW), mineralization volume (MLV), osteoid width (O.Wi), osteoid volume (OV), osteoid surface (OS) and active osteoblastic surface (AOS) were calculated in four fields of each section. For dynamic measurement, the mineral apposition rate (MAR) was calculated by dividing the inter-label distance by the time between the tetracycline labels.
Statistical analysis
All data are presented as mean and standard deviation. Evaluation was performed using the one-way ANOVA for paired and unpaired data. The radiological score was analyzed using the chi-square test. Statistical analysis was done using the SPSS 11.5 software program. p < 0.05 was considered to be statistically significant.
Results
During the experimental process after fracture, 4 animals died in the OVX + vehicle group and 3 in the OVX + SVS group. No infection occurred at the fracture site.
Tibial BMD turned out to be distinctly different between the two groups at 12 weeks after ovariectomy(p < 0.01), namely 0.241 ± 0.010 g/cm2 in the sham group and 0.219 ± 0.005 g/cm2 in the OVX group.
The subcutaneous injection was easily performed, with minimal leakage of the solution from the site of injection. No significant difference was found in body weight between the OVX + SVS and the OVX + vehicle rats.
Radiography
None of the fractures presented nonunion on X-ray radiograph. As expected, the amount of bridging bone formation increased with time in both groups. At 1 week, no difference could be noticed between the manifestations of the two groups. At 2 and 4 weeks, the OVX + SVS group presented enhanced consolidation of the fracture compared with the OVX + vehicle group (Fig. 1). This qualitative impression was confirmed by radiological score analysis, although these differences were not statistically significant (p = 0.077 at 2 weeks; and p = 0.062 at 4 weeks. Chi-square test, data omitted).
Callus dimension
Substantial histological differences between the OVX + vehicle and OVX + SVS callus zones were visible at 1 and 2 weeks after treatment. The callus cross-section area in simvastatin-treated rats was significantly enlarged by 21.3% (p < 0.05) at 1 week (20.22 ± 3.42 mm2 vs. 16.67 ± 4.02 mm2) and by 21.5% (p < 0.05) at 2 weeks (23.63 ± 3.25 mm2 vs. 19.45 ± 3.19 mm2) than the vehicle group (Fig. 2a).
Cross-sectional area (a) and biomechanical results (b) of callus at 1, 2, 4 weeks after fracture. (cross-sectional \( {\text{area = }}{\pi \times {\text{a}}} \mathord{\left/ {\vphantom {{\pi \times {\text{a}}} {\text{2}}}} \right. \kern-\nulldelimiterspace} {\text{2}} \times {\text{b}} \mathord{\left/ {\vphantom {{\text{b}} {\text{2}}}} \right. \kern-\nulldelimiterspace} {\text{2}} \). a: Anteroposterior diameter; b: Transverse diameter)
Biomechanical analysis
Values of biomechanical strength were presented as actual maximal load and relative maximal load (as percentage of nonfractured contralateral tibiae) (Fig. 2b). As expected, the mechanical strength increased with time during the evaluation period. Significantly higher mechanical strength was found in the OVX + SVS group compared with the OVX + vehicle group at 2 and 4 weeks. The tested tibiae of the OVX + SVS group demonstrated a mean actual maximum load of 24.87 ± 6.67 N (increased by 52.3% vs. 16.33 ± 6.32 N, p < 0.05) and relative maximal load of 49.88 ± 10.60% (increased by 57.5% vs. 31.66 ± 13.13%, p < 0.05) at 2 weeks. The mean actual maximum load at 4 weeks was 47.32 ± 9.59 N (increased by 24.4% vs. 38.05 ± 8.51 N p < 0.05), and relative maximal load was 87.25 ± 19.93% (increased by 31.4% vs. 66.41 ± 13.25% p < 0.05). No statistical difference was detected on mechanical strength at 1 week after fracture.
Serum chemistry
Changes of serum total cholesterol concentrations indicated that local application of simvastatin could also result in lowing of serum lipid. Simvastatin-treated rats presented a 23.0% (p < 0.01) reduction at 1 week (118.0 ± 11.58 mg/dL vs. 90.6 ± 11.57 mg/dL) and a 14.0% (p < 0.05) reduction at 2 weeks (103.6 ± 15.4 mg/dL vs. 89.1 ± 13.9 mg/dL) of total cholesterol over the OVX + vehicle animals (Fig. 3a).
Serum ALP in OVX + SVS rats showed a significant increase over that in OVX + vehicle animals. Namely, total ALP increased by 20.0% (p < 0.01) and 12.4% (p < 0.05) at 1 and 2 weeks, respectively. No difference presented at 4 weeks (Fig. 3b).
Callus histology
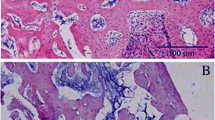
Light microscopy examination of H & E-stained histological sections showed callus formation at various stages of development, generally represented by a mixture of endochondral and intramembranous ossification. One week after fracture, cartilaginous callus formation was visible in the OVX + SVS group, whereas less cartilage formation was visible in the OVX + vehicle group. Relatively more periosteal bone formation was presented in OVX + SVS sections than in OVX + vehicle sections. At 2 and 4 weeks, callus zones of both groups showed periosteal bone formation at the edge and substantial cartilage formation in the central region. Compared with OVX + vehicle callus zones, new woven bone in OVX + SVS animals was relatively substantive and arranged more tightly and regularly (Fig. 4a–f).
Histological presentations of OVX + vehicle (a–c, g, i, j) and OVX + SVS (d–f, h,k, l) callus zones at 1 (a, d), 2 (b, e) and 4 (c, f, g–l) weeks after fracture. a–f: decalified sections stained with haematoxylin and eosin demonstrates that callus of the OVX + SVS group presents more cartilage at 1 week; osteoblast cells in OVX + SVS sections are more distinct; and woven bones are relatively substantive and arranged more tightly and regularly in 4 weeks. g, h: toluidine-blue-stained sections of undecalified tibiae at 4 weeks after fracture. i– l: single (i, k) and double (j, l) fluorochrome labeled micrographs of undecalified tibiae at 4 weeks after fracture. Original magnifications: g, h:×20; a, d:×40; c, f, i, k:×100; b, e, j, l:×200. Fb, fibrous tissure; Cg, cartilage callus; Wb, woven bone; OB, osteoblast; SL, single label; DL, double label
There was no evidence of malignant transformation in any of the cell types in the calluses in simvastatin-treated groups over the study period.
Bone histomorphometry
Histomorphometric measurement was performed on undecalcified sections. In coincident with HE-stained sections, the toluidine blue-stained section also indicated a more impaired histological manifestation in OVX + vehicle sections than in OVX + SVS sections (Fig. 4g, j). An unstained undecalcified section of the fracture callus showed a mixture of single and double labels of tetracycline. The single label line was mainly allocated in a newly formed woven region and double labels in relative mature trabecula. The intensity of tetracycline label indicated an increased osteogenic activity in simvastatin-treated animals (Fig. 4h ,i, k, l).
Histomorphometric parameters revealed that simvastatin was associated with a statistically significant increase of MLW, MLV and MAR compared to results in the OVX + vehicle group. A larger increase in O.Wi, OV, OS and AOSwas found in the OVX + SVS group compared to the OVX + vehicle group, although no statistical difference was detected (Table 1).
Discussion
The present study demonstrates the promotion of the healing process following subcutaneous injection of simvastatin to tissue in close proximity of the fracture. This is the first evidence indicating that locally applied simvastatin could promote the healing process in a fracture model.
After the discovery of Mundy et al., many studies have been conducted to evaluate the effects of statins on bone. Although in vitro and animal studies have shown positive effects of statins on bone mineralization [5, 26], clinical data on surrogate markers and fracture rates are conflicting. Most observational studies showed positive results on bone mass [6–8] and fracture risk [9–12], while some controversial data existed [27–31]. Reasons leading to those differences are complex. Residual confounding by matching variables, different definitions of the exposure time window, the lack of association in randomized trials, and the heterogeneity among observational studies may explain differences in those results. Besides, data suggested that statins affect bone, to a certain extent, in a dose-dependent manner. Animal testing by Frans et al. indicated that high-dose simvastatin(20 mg/kg/day) increases bone formation and resorption, while low-dose simvastatin(1 mg/kg/day) decreases bone formation and increases bone resorption [32]. For postmenopausal women, Tikiz et al. [30] found no change in BMD after a 1-year follow-up under 20 mg/day simvastatin medication, while Montagnani et al. [7] reported an increase of 2.8%, 1.0%, and 0.8% in lumber, femoral neck and total femur BMD after one year under 40 mg/day simvastatin medication. And also under 40 mg/day simvastatin intervention, Rejnmark et al. found no effect on biochemical bone markers and BMD at the hip or spine [31]. The mixed results may be due to two factors. First, most of the orally administered simvastatin is stored in the liver, and only 5% of simvastatin will be distributed in the circulation [23]; even less will be allocated in bone. Such a low concentration is easily disturbed, resulting in a variance of clinical manifestation. Second, in normally remodeling skeleton, the skeletal effects of compounds probably would be confined to the relatively rare and small remodeling sites. While in healing bone, the entire region is involved in bone formation, so the presentation of skeletal response to treatments will be magnified. As a matter of fact, most previous studies were conducted on undisturbed bones and statins were orally administrated; little literature was available regarding these two factors.
In this study, simvastatin was applied subcutaneously to tissue in close proximity of the fracture. This would yield high local concentration (much higher concentration than what could be obtained by oral administration) via simple diffusion.. The effect of locally applied drugs depends on an adequate delivery method. In this study, simvastatin was injected via a micro-injector. This technique requires reproducible placement of small volumes of factors or compounds adjacent to the bone. It is minimally invasive, and care should be taken not to disturb or damage the healing callus.
The history of statins as a therapeutic drug for hypercholesterolemia is long, and the toxicity of those drugs has been well examined. In this study, the dose of simvastatin was based on earlier safety and efficacy studies in rats [33] and with reference to previous studies conducted by Mundy in which simvastatin was also injected subcutaneously over the calvaria of mice [1]. Regarding pharmacokinetics, we considered a 10 mg/kg/day dose to rats about equivalent to 70 mg/day for humans, taking into account that metabolic processes in rodents are 10 times faster than in humans [34]. So, the current amount of simvastatin was higher than the routine dose in clinical applications (20–40 mg/day), while much lower than that administrated orally in previous animal testing on fractures (120 mg/kg/day) [21]. In accordance with Mundy’s study [1], no serious side effects were seen in the present study.
Fracture healing is a complex cascade of cellular events. The majority of fractures are healed by secondary fracture healing which involves a combination of osteoblast differentiation and ossification. In vitro studies have shown that simvastatin could promote osteoblast differentiation and mineralization in MC3T3-E1 cells and bone marrow stromal cells [35, 36]. Other studies have described a potential role of statins on chondrocytes and periosteum. For example, an in vitro study showed that mevastatin increased mRNA expression of BMP-2, aggrecan, type II collagen, and proteoglycan synthesized by cultured chondrocytes [37], and animal researches demonstrated a role of statins in cortical bone formation in ovariectomized rats [26, 38]. Those studies suggested that statins might play an important role in the variety mechanism of bone healing, including endochondral and intramembranous ossification. In current study, the increased area of calluses, together with the increased bone formation related histomorphometric parameters, proved that simvastatin had anabolic effect on bone formation. And the presentation of substantive new woven bone indicated an increased histological maturity of callus related to simvastatin treatment. Enhanced consolidation of the fracture healing was also found on X-ray radiography in this study.
In two previous experiments, the effects of statins on healing bone defects have been examined. The femur fracture model was made and stabilized with marrow-nailing in mature male BALB/c mice with normal bone quality, and simvastatin was administrated systemically in an extremely high dose (120 mg/kg/day) [21]. After 14 days of treatment, results showed that simvastatin was associated with a 53% increase of the callus area and a 63% increase of break force. Another histological study showed that orally administrated high-dose cerivastatin (1.0 mg/kg/day) induced the reconstruction of vascularized bone allograft in a rat tibia-fibula graft model [22]. In the present experiment, simvastatin was injected subcutaneously to the fracture site in a relatively lower dose, and increased callus area and enhanced fracture strength were found at 2 weeks post fracture. The value of fracture strength lies in the size and material properties of diaphysis. Interestingly, we also found increased fracture strength in the simvastatin-treated fractures at 4 weeks post fracture when the difference in callus size disappeared, thus pointing to an improved material property of fracture.
It is generally considered that the skeletal anabolic effect of statins is mainly to increase the gene expression of bone morphogenetic protein-2, which is an autocrine-paracrine factor for osteoblast differentiation. These agents stimulated BMP-2 transcription and also increased endogenous BMP-2 mRNA and protein expression in human osteoblastic cells [39, 40]. The exact mechanisms of statins on accelerating bone metabolism remain unclear. The reduction in mevalonate pathway intermediates with a subsequent inhibition of prenylation is responsible for a large proportion of the anabolic effects of these drugs [41]. Although these findings suggest direct effects on cellular function and acceleration of a normal physiological process, there was no evidence of malignant transformation in any of the calluses stimulated by simvastatin. The effect of statins on osteoclast is considered relatively unimportant compared to their effects on osteoblast. Their antiresorptive effect is thought to result from the enhanced production of OPG by osteoblast [42].
In conclusion, the present study clearly showed that simvastatin could promote fracture healing in OVX rats when injected subcutaneously into tissue in close proximity of the fracture. Limitations existed in our study. First, the doses used were higher than the normal dose in a clinical application. It is hard to predict the results in daily clinical practice, where much lower doses are used. Nevertheless, the current study showed an approach: it might be possible to use statins to improve healing of osteoporotic fractures. Second, simvastatin was delivered by local injection, an invasive approach which has the possibility of increasing the risk of infection. If it is administered via an adequate carrier (e.g., combined with fibrin glue, coated on an implant or mixed with poly D, L-lactide, etc.), we believe that local application of simvastatin could be a new option to improve the quality of fractures healing. Further studies need to be carried out to find an appropriate carrier and non-invasive delivery systems. Moreover, the potential side effects, i.e., carcinogenesis linked to rapid growth of bone cells, remain to be tested.
References
Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G (1999) Stimulation of bone formation in vitro and in rodents by statins. Science 286:1946–1949
Chan MH, Mak TW, Chiu RW, Chow C, Chan IH, Lam CW (2001) Simvastatin increases serum osteocalcin concentration in patients treated for hypercholesterolemia. J Clin Endocrinol Metab 86:4556–4559
Mostaza JM, De la Piedra C, Curiel MD, Pena R, Lahoz C (2001) Pravastatin therapy increases procollagen I N-terminal propeptide. Clin Chim Acta 308:133–137
Stein EA, Farnier M, Waldstreicher J, Mercuri M (2001) Effects of statins on biomarkers of bone metabolism: a randomized trial. Nutr Metab Cardiovasc Dis 11:84–87
Oxlund H, Dalstra M, Andreassen TT (2001) Statin given perorally to adult rats increases cancellous bone mass and compressive strength. Calcif Tissue Int 69:299–304
Sirola J, Sirola J, Honkanen R, Kroger H, Jurvelin JS, Maenpaa P, Saarikoski S (2002) Relation of statin use and bone loss: a prospective population-based cohort study in early postmenopausal women. Osteoporos Int 13:537–541
Montagnani A, Gonnelli S, Cepollaro C, Pacini S, Campagna MS, Franci MB, Lucani B, Gennari C (2003) Effect of simvastatin treatment on bone mineral density and bone turnover in hypercholesterolemic postmenopausal women: a 1-year longitudinal study. Bone 32:427–433
Lupattelli G, Scarponi AM, Vaudo G, Siepi D, Roscini AR, Gemelli F, Pirro M, Latini RA, Sinzinger H, Marchesi S, Mannarino E (2004) Simvastatin increases bone mineral density in hypercholesterolemic postmenopausal women. Metabolism 53:744–748
Pasco J, Kotowicz M, Henry M, Sanders K, Nicholson G (2002) Statin use, bone mineral density, and fracture risk: Geelong Osteoporosis Study. Arch Intern Med 162:537–540
Bauer D, Mundy G, Jamal S, Black DM, Cauley JA, Ensrud KE, van der Klift M, Pols HA (2004) Use of statins and fracture: results of four prospective studies and cumulative metaanalysis of observational studies and controlled trials. Arch Intern Med 164:146–152
Rejnmark L, Olsen ML, Johnsen SP, Vestergaard P, Sorensen HT, Mosekilde L (2004) Hip fracture risk in statin users-a population-based Danish case-control study. Osteoporos Int 15:452–458
Schoofs MW, Sturkenboom MC, van der Klift M, Hofman A, Pols HA, Stricker BH (2004) HMG-CoA reductase inhibitors and the risk of vertebral fracture. J Bone Miner Res 19:1525–1530
Ralph A, Meyer J, Paul JT, David FM, David MB, Matt EH, Gary MK (2001) Age and ovariectomy impair both the normalization of mechanical properties and the accretion of mineral by the fracture callus in rats. J Orthop Res 19:428–435
Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T (2001) Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 28:80–86
Wang JW, Li W, Xu SW, Yang DS, Wang Y, Lin M, Zhao GF (2005) Osteoporosis influences the middle and late periods of fracture healing in a rat osteoporostic model. Chinese Journal of Traumatology 8:111–116
Nakajima A, Shimoji N, Shiomi K, Shimizu S, Moriya H, Einhorn TA, Yamazaki M (2002) Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1–34). J Bone Miner Res 17:2038–2047
Delgado-Martinez AD, Martinez ME, Carrascal MT, Rodriguez-Avial M, Munuera L (1998) Effect of 25-OH-vitamin D on fracture healing in elderly rats. J Orthop Res 16:650–653
McCormack AP, Anderson PA, Tencer AF (1993) Effect of controlled local release of sodium fluoride on bone formation: filling a defect in the proximal femoral cortex. J Orthop Res 11:548–555
Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, Akiyama T, Shi L, Komatsubara S, Miyamoto K, Norimatsu H (2002) Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res 17:2237–2246
Paavolainen P, Taivainen T, Michelsson JE, Lalla M, Penttinen R (1989) Calcitonin and fracture healing. An experimental study on rats. J Orthop Res 7:100–106
Skoglund B, Forslund C, Aspenberg P (2002) Simvastatin improves fracture healing in mice. J Bone Miner Res 17:2004–2008
Ohno T, Shigetomi M, Ihara K, Matsunaga T, Hashimoto T, Kawano H, Sugiyama T, Kawai S (2003) Skeletal reconstruction by vascularized allogenic bone transplantation: effects of statin in rats. Transplantation 76:869–871
Blum CB (1994) Comparison of properties of four inhibitors of 3-hdroxy-3methyglutary-coenzyme A reductase. Am J Cardiol 73:3D–11D
Guyton JR (2006) Benefit versus risk in statin treatment. Am J Cardiol 97:95C–97C
Giles AR (1987) Guidelines for the use of animals in biomedical research. Thromb Haemost 58:1078–1084
Pytlik M, Janiec W, Misiarz-Myrta M, Gubala I (2003) Effects of simvastatin on the development of osteopenia caused by ovariectomy in rats. Pol J Pharmacol 55:63–71
LaCroix AZ, Cauley JA, Pettinger M, Hsia J, Bauer DC, McGowan J, Chen Z, Lewis CE, McNeeley SG, Passaro MD, Jackson RD (2003) Statin use, clinical fracture, and bone density in postmenopausal women: results from the women’s health initiative observation study. Ann Intern Med 139:97–104
Reid IR, Hague W, Emberson J, Baker J, Tonkin A, Hunt D, MacMahon S, Sharpe N (2001) Effect of pravastatin on frequency of fracture in the LIPID study: secondary analysis of a randomised controlled trial. Long-term intervention with pravastatin in ischaemic disease. Lancet 357:509–512
Reid IR, Tonkin A, Cannon CP (2005) Comparison of the effects of pravastatin and atorvastatin on fracture incidence in the PROVE IT-TIMI 22 trial-secondary analysis of a randomized controlled trial. Bone 37:190–191
Tikiz C, Tikiz H, Taneli F, Gumuser G, Tuzun C (2005) Effects of simvastatin on bone mineral density and remodeling parameters in postmenopausal osteopenic subjects: 1-year follow-up study. Clin Rheumatol 24:447–452
Rejnmark L, Buus NH, Vestergaard P, Heickendorff L, Andreasen F, Larsen ML, Mosekilde L (2004) Effects of simvastatin on bone turnover and BMD: a 1-year randomized controlled trial in postmenopausal osteopenic women. J Bone Miner Res 19:737–744
Maritz FJ, Conradie MM, Hulley PA, Gopal R, Hough S (2001) Effect of statins on bone mineral density and bone histomorphometry in rodents. Arterioscler Thromb Vasc Biol 21:1636–1641
Gerson RJ, MacDonald JS, Alberts AW, Kornbrust DJ, Majka JA, Stubbs RJ, Bokelman DL (1989) Animal safety and toxicology of simvastatin and related hydroxy-methylglutaryl-coenzyme A reductase inhibitors. Am J Med 87:28S–38S
Barnes CD, Eltherington LG (1964) Drug dosage in laboratory animals. University of California Press, Berkeley
Maeda T, Matsunuma A, Kawane T, Horiuchi N (2001) Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun 280:874–877
Song C, Guo Z, Ma Q, Chen Z, Liu Z, Jia H, Dang G (2003) Simvastatin induces osteoblastic differentiation and inhibits adipocytic differentiation in mouse bone marrow stromal cells. Biochem Biophys Res Commun 308:458–462
Hatano H, Maruo A, Bolander ME, Sarkar G (2003) Statin stimulates bone morphogenetic protein-2, aggrecan, and type 2 collagen gene expression and proteoglycan synthesis in rat chondrocytes. J Orthop Sci 8:842–848
Oxlund H, Andreassen TT (2004) Simvastatin treatment partially prevents ovariectomy-induced bone loss while increasing cortical bone formation. Bone 34:609–618
Sugiyama M, Kodama T, Konishi K, Abe K, Asami S, Oikawa S (2000) Compactin and simvastatin, but not pravastatin, induce bone morphogenetic protein-2 in human osteosarcoma cells. Biochem Biophys Res Commun 271:688–692
Ohnaka K, Shimoda S, Nawata H, Shimokawa H, Kaibuchi K, Iwamoto Y, Takayanagi R (2001) Pitavastatin enhanced BMP-2 and osteocalcin expression by inhibition of rho-associated kinase in human osteoblasts. Biochem Biophys Res Commun 287:337–342
Garrett IR, Mundy GR (2002) The role of statins as potential targets for bone formation. Arthritis Res 4:237–240
Viereck V, Grundker C, Blaschke S, Frosch KH, Schoppet M, Emons G, Hofbauer LC (2005) Atorvastatin stimulates the production of osteoprotegerin by human osteoblasts. J Cell Biochem 96:1244–1253
Acknowledgments
This study was supported by The Provincial Health Bureau of Zhejiang Province, China. Fund number: 491020-I 40206.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J.W., Xu, S.W., Yang, D.S. et al. Locally applied simvastatin promotes fracture healing in ovariectomized rat. Osteoporos Int 18, 1641–1650 (2007). https://doi.org/10.1007/s00198-007-0412-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-007-0412-2