Abstract
Introduction: Eroded or thin inferior cortex of the mandible detected on dental panoramic radiographs may be useful for identifying postmenopausal women with low bone mineral density (BMD) or osteoporosis. The purpose of this study was to evaluate whether these panoramic measurements are useful for identifying low BMD or osteoporosis in postmenopausal women younger than 65 years. Methods: We compared the diagnostic performances of panoramic measurements with those of the osteoporosis self-assessment tool (OST) for identifying women with low BMD (T-score of −2.0 or less at either the lumbar spine or the femoral neck) and osteoporosis (T score of −2.5 or less) in 158 healthy Japanese postmenopausal women aged 46 years to 64 years. Mandibular cortical shape (erosion) and width were evaluated on dental panoramic radiographs. Receiver operating characteristic curve analysis was used to determine the optimal cutoff thresholds for cortical width and OST index. Results: The sensitivity and specificity, respectively, for identifying women with low BMD were 82.3% and 55.2% for OST index, 79.0% and 50.0% for cortical width, and 72.6% and 74.0% for cortical shape. The sensitivity and specificity, respectively, for identifying women with osteoporosis were 86.7% and 46.9% for OST index, 90.0% and 45.3% for cortical width, and 86.7% and 65.6% for cortical shape. Likelihood ratio for identifying women with low BMD was 13.90 for thin cortical width (<3.0 mm) and 10.84 for severely eroded cortex. That for identifying women with osteoporosis was 6.40 for thin cortical width and 7.11 for severely eroded cortex. Conclusions: Dentists may be able to refer postmenopausal women younger than 65 years for bone densitometry on the basis of incidental findings on dental panoramic radiographs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporotic fractures are a health burden worldwide, resulting in the reduction of physical activity, increased risk of mortality and incremental medical costs. The Surgeon General in the United States of America warns that, by 2020, half of all American citizens older than 50 years will be at risk for fractures from osteoporosis and low bone mass if no immediate actions are taken by individuals at risk, doctors, health systems, and policy makers [1]. Dual-energy X-ray absorptiometry (DXA) is the most reliable way to determine bone mineral density (BMD), which is a major risk factor for fractures from osteoporosis. Since postmenopausal women have a high risk of osteoporosis, BMD testing for all postmenopausal women by DXA is considered one of the immediate actions to conquer osteoporotic fractures. However, BMD testing for all postmenopausal women is not practicable in many countries where bone assessment methods, especially DXA, are not widely available [2].
Several investigators have developed simple questionnaire-based screening tools to select postmenopausal women who should be referred for bone densitometry [3, 4]. The sensitivity of these tools for identifying postmenopausal women with low BMD or osteoporosis was reported to be approximately 90%, although the specificity was relatively low. In clinical practice, subjects are referred for DXA testing if their score of questionnaire-based screening indicates a risk of low BMD or osteoporosis. However, the response rate for questionnaires in the Canadian Multicentre Osteoporosis Study was only 42% [5]. This implies that a large segment of postmenopausal women may not respond to the questionnaire if they have no knowledge about, and interested in, osteoporosis. Furthermore, it is likely that the selection cutoff point of questionnaire-based screening tools may vary among different populations.
Dental panoramic radiographs are frequently taken for the examination of teeth and jaws in general dental practice over the world. In Japan, approximately 10 million dental panoramic radiographs are taken annually in about 65,000 dental offices. Approximately one-third of these radiographs are taken for patients in the range of 45 years to 65 years [6]; however, mandibular inferior cortical measurements, clearly detected on dental panoramic radiographs, are now rarely used as additional diagnostic information in general dental practice. Recent studies in Finland [7], Japan [8, 9, 10, 11], the USA [12, 13, 14], the United Kingdom [15, 16], Poland [17], and Sweden [18] offer differing opinions on the usefulness of mandibular inferior cortical measurements, cortical width and shape, detected on dental panoramic radiographs, for identifying postmenopausal women with low skeletal BMD or osteoporosis. If dentists can refer postmenopausal women with undetected low BMD or osteoporosis for bone densitometry by dental panoramic radiographs, it may comprise a possible action to identify postmenopausal women with asymptomatic osteoporosis who do not respond to questionnaire-based screening.
We recently demonstrated that the diagnostic performance of dental panoramic radiography measurements for identifying postmenopausal women with suspected spinal osteoporosis was similar to that of the osteoporosis self-assessment tool (OST) [11]. The OST was a questionnaire-based screening tool that was first developed for Asian postmenopausal women [3] and has been equally applied for Caucasian postmenopausal women [19, 20]. However, it is likely that high prevalence rates of osteoporosis in postmenopausal women aged 65 years and older might have influenced the overall diagnostic performance in our previous study. Mauck et al. recently reported that there remains uncertainty as to who should undergo BMD testing among postmenopausal women younger than 65 years, although there is less ambiguity for the clinician about BMD testing in all postmenopausal women aged 65 and older [21]. Identification of younger postmenopausal women with low BMD or osteoporosis might be the more important immediate action to prevent osteoporotic fractures.
The purpose of this study was therefore to evaluate whether dental panoramic radiography measurements are useful for identifying women with low BMD or osteoporosis in postmenopausal women younger than 65 years.
Methods
Study sample
There were 836 women who visited our clinic for BMD assessment between 1996 and 2005, of whom 141 were patients from the dental clinic in our hospital, 607 were from the gynecology clinic, 62 were from Internal Medicine, and 26 were from Surgery. At the BMD assessment all the women were asked whether they would give informed consent to dental panoramic radiographic examination for oral care. There were 138 that refused such examination. Forty-two were premenopausal or perimenopausal. Of the remainder (656 postmenopausal women), who had not menstruated for at least 1 year, 158, aged from 46 years to 64 years (mean ± SD 55.6±4.7) and with no previous diagnosis of osteoporosis, were recruited for this study. No subject had any metabolic bone disease (hyperparathyroidism, hypoparathyroidism, Paget’s disease, osteomalacia, renal osteodystrophy, or osteogenesis imperfecta), cancer with bone metastasis, significant renal impairment or took any medication that would affect bone metabolism, such as estrogen. None had undergone hysterectomy or oophorectomy. None had a history of smoking or had any bone destructive lesions (e.g., malignant tumors or osteomyelitis) in the mandible. No vertebral osteoporotic fractures were found on lateral spine digital X-rays at skeletal BMD assessment. Vertebral fracture was assessed semi-quantitatively [22]. Hiroshima University Institutional Human Subjects Committee approved our taking dental panoramic radiographs of subjects that had given informed consent.
BMD assessment, dental panoramic radiographic measurements and questionnaire-based screening
BMD at the lumbar spine and the femoral neck was determined by DXA (DPX-alpha, Lunar, Madison, Wis., USA.). Height and weight were measured at the time of DXA measurement. The in vivo short-term precision error for the lumbar spine and the femoral neck BMD in our clinic was 1.0% and 2.8%, respectively. Osteoporosis was defined as a BMD T-score of −2.5 or less at either the lumbar spine or the femoral neck, in accordance with the WHO classification. An alternative T-score of −2.0 or less was also explored to allow comparison with published results for risk indices that were based on this cutoff value [4, 5, 19]. This cutoff value has also been widely used in many communities [23].
Dental panoramic radiographs were obtained at the time of DXA measurement with an AZ-3000 (Asahi, Kyoto, Japan) at 12 mA and 15 s; the voltage varied between 70 kV and 80 kV. Screens of speed group 200 (HG-M, Fuji Photo Film Co., Tokyo, Japan) and film (UR-2, Fuji Photo Film Co.) were used. All dental panoramic radiographs used in this study were satisfactory for the measurements. Two dental panoramic radiographic measurements: mandibular cortical width and shape, were estimated on dental panoramic radiographs by one oral radiologist (A.T.) with 17 years of clinical experience.
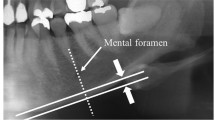
Measurement of mandibular cortical width was made bilaterally on the radiographs at the site of a mental foramen according to our previous study [8]. A line parallel to the long axis of the mandible and tangential to the inferior border of the mandible was drawn. A line perpendicular to this tangent, intersecting the inferior border of the mental foramen, was constructed, along which the mandibular cortical width was measured by a caliper (Fig. 1). Mean cortical width on both sides of the mandible was used in this study. The coefficient of variation due to positioning error and operator error in cortical width measurement was less than 2%. Intra-observer variation in cortical width measurement was 0.1 mm, which was similar to the inter-observer variation.
Measurement of mandibular cortical width. A line parallel to the long axis of the mandible and tangential to the inferior border of the mandible was drawn. A line (dotted line) perpendicular to this tangent and intersecting the inferior border of the mental foramen was constructed, along which the mandibular cortical width was measured. The distance between the two parallel solid lines is the cortical width. The white arrow shows a mental foramen
The mandibular cortical shape on the dental panoramic radiograph was determined by observing the mandible distally from the mental foramen, bilaterally, and categorized into one of three groups according to the method of Klemetti et al. [7] (Fig. 2), as follows:
-
1.
Normal cortex: the endosteal margin of the cortex is even and sharp on both sides.
-
2.
Mildly to moderately eroded cortex: the endosteal margin shows semi-lunar defects (lacunar resorption) or appears to form endosteal cortical residues.
-
3.
Severely eroded cortex: the cortical layer forms heavy endosteal cortical residues and is clearly porous.
Overall agreements for intra-observer and inter-observer performances were 92% and 82%, respectively.
To compare the diagnostic performances of questionnaire-based screening tools, mandibular inferior cortical width and cortical shape, we calculated the OST index in this study. The OST index was obtained from an integer of 0.2-times the weight minus the integer of 0.2-times the woman’s age [3].
Data analysis
For the mandibular cortical shape, the subjects were first divided into two groups based on the assessment of panoramic radiographs: women with and women without an eroded mandibular cortex. The subjects were also divided into two groups based on skeletal BMD: women with and women without low BMD (T score of −2.0 or less at either the lumbar spine or the femoral neck) or osteoporosis (T score of –2.5 or less). The sensitivity, specificity, positive predictive values (PPVs), negative predictive values (NPVs), and likelihood ratios for the positive (LR+) and negative (LR−) risk results for identifying women with low BMD or osteoporosis by an eroded mandibular cortex were calculated in dichotomous 2-times-2 tables. Likelihood ratio (LR) and 95% confidence interval (CI) were calculated according to the method described by Simel et al. [24].
Further, subjects were divided into three groups as follows: women with normal cortex (low osteoporosis risk group), women with mildly to moderately eroded cortex (intermediate osteoporosis risk group), and women with severely eroded cortex (high osteoporosis risk group). LR and 95% CI for identifying women with low BMD or osteoporosis were calculated for each group.
Receiver operating characteristics (ROCs) curve analyses were used to determine the optimal cutoff thresholds of OST index and cortical width for identifying women with osteoporosis. The risk index range corresponding to a sensitivity of approximately 90% was chosen to define the low-risk group. The sensitivity, specificity, PPV, NPV, LR+ and LR− for identifying women with low BMD or osteoporosis by optimal cutoff thresholds of OST index and cortical width determined on ROC analyses were calculated. Further, the risk index range corresponding to a specificity of approximately 90% was chosen to define the high-risk and intermediate-risk groups for cortical width. LR and 95% CI were calculated for the low-risk, intermediate-risk and high-risk groups.
Cohen kappa statistics were used to evaluate the agreements among OST index category, cortical width category and cortical shape category [25]. Interpretation of the kappa statistics was obtained from the guidelines of Landis and Koch [26]. Logistic regression analysis, adjusting for OST index, was also performed to calculate the odds ratio of having low BMD or osteoporosis in women identified by dental panoramic measurements. Data analyses were performed with the Statistical Package for the Social Sciences (SPSS; version 8.0; SPSS, Chicago, Ill., USA). P values less than 0.05 were considered statistically significant.
Results
Characteristics of study subjects are shown in Table 1. Based on BMD of the lumbar spine, 28.5% of women had low BMD (T-score of –2.0 or less) and 14.6% of women had osteoporosis (T-score of −2.5 or less). Based on BMD of the femoral neck, 24.7% of women had low BMD and 10.1% of women had osteoporosis. Based on BMD of both sites, 39.2% of women had low BMD and 19.0% of women had osteoporosis.
The area under the ROC curve for identifying women with low BMD was 0.761 (95% CI, 0.686 to 0.837) for OST index and 0.777 (95% CI, 0.701 to 0.852) for cortical width. The area under the ROC curve for identifying women with osteoporosis was 0.737 (95% CI, 0.637 to 0.839) for OST index and 0.802 (95% CI, 0.705 to 0.899) for cortical width. No significant differences in the area under the ROC curve were seen between OST index and cortical width for identifying women with both low BMD and osteoporosis, although the area under the ROC curve for cortical width tended to be higher than that for OST index.
Using the cutoff thresholds of 0 for OST index, 4.3 mm for cortical width and selecting any cortical erosion, we found that the sensitivity and specificity, respectively, for identifying women with osteoporosis were 86.7% and 46.9% for OST index, 90.0% and 45.3% for cortical width, and 86.7% and 65.6% for cortical shape (Table 2). Those for identifying women with low BMD were 82.3% and 55.2% for OST index, 79.0% and 50.0% for cortical width, and 72.6% and 74.0% for cortical shape, respectively. The specificity for cortical shape was significantly higher than that for OST index and cortical width; however, there were no significant differences in the sensitivity, PPV, NPV, LR+ and LR− among OST index, cortical width and cortical shape.
When the risk-index range corresponding to a specificity of approximately 90% was chosen to determine the optimal cutoff threshold, the cutoff thresholds for cortical width was 3.0 mm. Subjects with cortical widths >4.2 mm, 3.0 mm to 4.2 mm, and <3.0 mm belonged to low-risk, intermediate-risk, and high-risk groups, respectively.
The prevalence rate of women with low BMD and the likelihood ratio in the high-risk group were 90.0% and 13.90, respectively, for cortical width and 87.5% and 10.84, respectively, for cortical shape (Table 3). The prevalence rate of women with low BMD in the intermediate-risk group of cortical width was similar to the background prevalence; however, that in the intermediate-risk group of cortical shape was significantly higher than the background prevalence. Prevalence rates of women with low BMD in the low-risk group of both cortical width and cortical shape were significantly lower than the background prevalence.
The prevalence rate of women with osteoporosis and the likelihood ratio in the high-risk group were 60.0% and 6.40, respectively, for cortical width and 62.5% and 7.11, respectively, for cortical shape (Table 4). The prevalence rate of women with osteoporosis in the intermediate-risk group of cortical width was similar to the background prevalence; however, that in the intermediate-risk group of cortical shape was significantly higher than the background prevalence. The prevalence rates of women with osteoporosis in the low-risk group of both cortical width and cortical shape were significantly lower than the background prevalence
The Cohen kappa index was 0.246 (P=0.02) between OST index and cortical width, 0.331 (P<0.001) between OST index and cortical shape, and 0.321 (P<0.001) between cortical width and cortical shape. These indicated that the degree of agreement was fair among the three indices. After adjustment for OST index, the odds ratio of having low BMD was 2.92 (95% CI 1.32 to 6.46) in women identified by cortical width (<4.3 mm) and 5.18 (95% CI, 2.48 to 11.18) in women identified by cortical shape (erosion). The odds ratio of having osteoporosis was 5.90 (95% CI, 1.64 to 21.18) in women identified by cortical width (<4.3 mm) and 8.66 (95% CI, 2.75 to 27.27) in women identified by cortical shape (erosion).
Discussion
The sensitivities for identifying women with low BMD or osteoporosis by cortical shape detected on dental panoramic radiographs were similar to those by OST index and cortical width; however, the specificities of cortical shape were significantly higher than those of the other two indices. This tendency of diagnostic performance is similar to that for identifying women with spinal osteoporosis in our recent study [11]. These results suggest that panoramic measures, especially cortical shape, may be useful for identifying women with low BMD or osteoporosis in postmenopausal women younger than 65 years, in comparison with the OST index.
In our study the sensitivity and specificity for identifying women with osteoporosis by OST index were 86.7% and 46.9%, respectively. Koh et al. reported that the sensitivity and specificity for identifying women with osteoporosis (14% of women) in 797 Asian women aged 45 years to 88 years (mean age 62.3 years) were 90.9% and 44.8%, respectively [3]. Their cutoff threshold of the OST index was the same as ours. Richy et al. reported that the sensitivity and specificity was 89% and 40% for identifying women with low BMD (T-score of –2.0 or less, 29% of women) and 92% and 37% for identifying women with osteoporosis (19% of women) in 4,035 Caucasian women aged 45 years to 96 years (mean age 61.5 years), although their cutoff threshold was 2 [27]. Our results suggest that the diagnostic performance of the OST index used in our study is similar to that previously reported in both Asian and Caucasian populations, although our subjects are younger, healthy, postmenopausal women.
We divided our subjects into three groups by cortical shape; women with a normal cortex (low-risk group), women with a mildly to moderately eroded cortex (intermediate-risk group) and women with a severely eroded cortex (high-risk group). Halling et al. considered that subjects with both normal cortex and mildly to moderately eroded cortex belonged to a normal group based on heel DXA measurements [18]. However, after adjustment for potentially confounding factors, the odds ratio for an osteoporotic fracture associated with mildly to moderately eroded and severely eroded cortex was 2.0 (95% CI 1.2 to 3.3) and 8.0 (95% CI 2.0 to 28.9), respectively, in our previous study [12]. We also demonstrated that women with any eroded cortex had an elevated bone resorption and were at-risk of low BMD [10]. In the present study, 33.9% of women with mildly to moderately eroded cortex and 62.5% of women with severely eroded cortex had osteoporosis. In addition, 61.2% of women with mildly to moderately eroded cortex and 87.5% of women with severely eroded cortex had low BMD. These prevalence rates were significantly higher than the background prevalence rates. Our results suggest that postmenopausal women with an eroded cortex should be referred for bone densitometry.
Almost 90% of women with cortical width <3.0 mm had low BMD, and 60% of women with cortical width <3.0 mm had osteoporosis; however, there were no significant differences between prevalence rates of women with low BMD or osteoporosis in the intermediate-risk group of cortical width (3.2 mm to 4.2 mm) and background prevalence rates. This suggests that women in the high-risk group, defined by thin cortical width (<3.0 mm), should be identified. Devlin and Horner reported that a diagnostic threshold for cortical width of 3 mm (or less) was suggested as the most appropriate threshold for referral for bone densitometry in 74 Caucasian women [15].
Similar diagnostic performances among OST index, cortical width and cortical shape imply the possibility that the OST index might be the more useful index in clinical use, for identifying women, than other indices, because the OST index is simply calculated and is objective. However, the degree of agreement among the three indices was fair. This indicates that women identified by OST index may be different from those identified by dental panoramic measurements. In our previous study, women with an eroded cortex had higher bone turnover and high risk of low BMD (T score <−1.0) than women with a normal cortex, after adjustment for age and body mass index [10]. This suggests that younger postmenopausal women with an eroded cortex may still have a high risk of low BMD or osteoporosis, after adjustment for OST index. In fact, the odds ratio of having low BMD or osteoporosis in women with an eroded cortex was 5.18 and 8.66, respectively. Further, younger postmenopausal women with low BMD who were identified by eroded cortex may be more likely to have osteoporosis in the near future than those identified by OST index, because women with an eroded cortex have high bone turnover. It is also likely that younger postmenopausal women with asymptomatic osteoporosis who were identified by eroded cortex may have an osteoporotic fracture in the near future.
Previous studies suggest that cortical width may reflect peak bone mass obtained in younger age but not bone turnover after menopause [10, 16]. It is possible that cortical width may be a useful indicator of low BMD or osteoporosis, independent of cortical shape; however, how do the dentists estimate cortical width in general dental practice? First, the dentists determine the cortical width by simple visual estimation, such as “thin or not”, according to their clinical experience. In our recent study, the likelihood ratio for identifying postmenopausal women with low BMD (T score of –1.0 or less) by simple visual estimation of cortical width (thin or not) by four experienced dentists was 3.1 at the lumbar spine and 3.2 at the femoral neck [28]. Further training might improve the diagnostic efficacy of simple visual estimation of cortical width. Second is the computer-aided diagnosis (CAD) in the digital panoramic radiography system. We have already developed a CAD system with which we can semi-automatically measure the cortical width precisely [29]. A CAD system may help the dentists to identify women with low BMD or osteoporosis in the future.
Dentists should refer postmenopausal women with an eroded cortex for bone densitometry. However, can dentists actually identify women with an eroded cortex by their panoramic radiographs, whose image qualities may vary among dental clinics? In our recent pilot study, conducted within the Hiroshima Dental Association in 2003, 17 general dental practitioners, who were trained in the reading of cortical shape, identified 14 (87.5%) of 16 women with osteoporosis and 15 (68.0%) of 22 women with osteopenia (T-score of –1.0 to –2.5) in 61 study subjects who visited their dental clinics, who had had dental panoramic radiographs taken for dental treatment, and who wanted to have BMD assessment by DXA (unpublished data). These dentists misidentified only ten women (16.3%). The sensitivity, specificity, and likelihood ratio for a positive risk result for identifying postmenopausal women with osteoporosis by these dentists were 87.5%, 44.4% and 1.58, respectively. These were similar to those of the OST index in our study. Trained dentists may identify women with osteoporosis with sufficient diagnostic performance compared with the OST index in their own clinics by using their panoramic radiographs.
This study has some limitations. All subjects of this study were not healthy volunteers from the community but were patients who visited our clinic for BMD assessment. Our subjects, therefore, were not representative of the general population of Japanese postmenopausal women. Iki et al. recently reported that, in healthy Japanese women aged 50 to 79 years, the prevalence rate of osteoporosis according to WHO classification was 38.0% at the lumbar spine and 11.6% at the femoral neck [30]. We cannot directly compare our subjects aged 46 to 64 years with the subjects of Iki et al.; however, the prevalence rate of osteoporosis at the lumbar spine (14.6%) was also somewhat higher than that at the femoral neck (10.1%) in our study. The relatively small sample selected by strict inclusion criteria may result in a large 95% CI of likelihood ratio in the high-risk group defined by panoramic measurements. This may limit the interpretation of our findings.
Our previous study in the USA [12], and our present study, suggest that cortical shape may be used both in Japanese and Caucasian women as well. However, it is likely that the cutoff threshold of cortical width may not be directly applied to Caucasian postmenopausal women. Instead of crude cortical width, the cutoff threshold value based on T-score calculated from mean cortical width of young adult Japanese women might be useful for Caucasian women. Further, it would be important for dentists to produce models of expense and benefit if they apply our finding to the entire Japanese population as well as Caucasian population.
In conclusion, cortical measurements detected on dental panoramic radiographs may be useful for identifying younger postmenopausal women with low BMD or osteoporosis. Dentists should refer postmenopausal women with eroded cortex or thin cortical width (<3.0 mm) for bone densitometry. Several studies in populations of other ethnic backgrounds would be necessary to establish screening for osteoporosis in the dental office. Nonetheless, dentists may be able to refer postmenopausal women younger than 65 years for bone densitometry on the basis of incidental findings on dental panoramic radiographs.
References
Bone health and osteoporosis: a surgeon general’s report. (2004)http://www.surgeongeneral.gov/reportspublications.html
Kanis JA, Johnell O (2005) Requirements for DXA for the management of osteoporosis in Europe. Osteoporos Int 16:229–238
Koh LK, Sedrine WB, Torralba TP, Kung A, Fujiwara S, Chan SP, et al (2001) A simple tool to identify Asian women at increased risk of osteoporosis. Osteoporos Int 12:699–705
Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP, Canadian Multicentre Osteoporosis Study (2001) Evaluation of decision rules for referring women for bone densitometry by dual-energy X-ray absorptiometry. JAMA 286:57–63
Cadarette SM, Jaglal SB, Kreiger N, McIsaac WJ, Darlington GA, Tu JV (2000) Development and validation of the Osteoporosis Risk Assessment Instrument to facilitate selection of women for bone densitometry. CMAJ 162:1289–1294
Shimano T, Suzuki Y, Sasaki T (2002) Long-term trend of dental radiographic examination in Japan—analysis on health insurance data (in Japanese). Dent Radiol 42:9–21
Klemetti E, Kolmakov S, Kroger H (1994) Pantomography in assessment of the osteoporosis risk group. Scand J Dent Res 102:68–72
Taguchi A, Suei Y, Ohtsuka M, Otani K, Tanimoto K, Ohtaki M (1996) Usefulness of panoramic radiography in the diagnosis of postmenopausal osteoporosis in women. Width and morphology of inferior cortex of the mandible. Dentomaxillofac Radiol 25:263–267
Nakamoto T, Taguchi A, Ohtsuka M, Suei Y, Fujita M, Tanimoto K, et al (2003) Dental panoramic radiograph as a tool to detect postmenopausal women with low bone mineral density: untrained general dental practitioners’ diagnostic performance. Osteoporos Int 14:659–664
Taguchi A, Sanada M, Krall E, Nakamoto T, Ohtsuka M, Suei Y, et al (2003) Relationship between dental panoramic radiographic findings and biochemical markers of bone turnover. J Bone Miner Res 18:1689–1694
Taguchi A, Suei Y, Sanada M, Ohtsuka M, Nakamoto T, Sumida H, et al (2004) Validation of dental panoramic radiography measures for identifying postmenopausal women with spinal osteoporosis. AJR Am J Roentgenol 183:1755–1760
Bollen AM, Taguchi A, Hujoel PP, Hollender LG (2000) Case–control study on self-reported osteoporotic fractures and mandibular cortical bone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 90:518–524
Persson RE, Hollender LG, Powell LV, MacEntee MI, Wyatt CC, Kiyak HA, et al (2002) Assessment of periodontal conditions and systemic disease in older subjects. I. Focus on osteoporosis. J Clin Periodontol 29:796–802
White SC, Taguchi A, Kao D, Wu S, Service SK, Yoon D, et al (2005) Clinical and panoramic predictors of femur bone mineral density. Osteoporos Int 16:339–346
Devlin H, Horner K (2002) Mandibular radiomorphometric indices in the diagnosis of reduced skeletal bone mineral density. Osteoporos Int 13:373–378
Horner K, Devlin H, Harvey L (2002) Detecting patients with low skeletal bone mass. J Dent 30:171–175
Drozdzowska B, Pluskiewicz W, Tarnawska B (2002) Panoramic-based mandibular indices in relation to mandibular bone mineral density and skeletal status assessed by dual energy X-ray absorptiometry and quantitative ultrasound. Dentomaxillofac Radiol 31:361–367
Halling A, Persson GR, Berglund J, Johansson O, Renvert S (2005) Comparison between the Klemetti index and heel DXA BMD measurements in the diagnosis of reduced skeletal bone mineral density in the elderly. Osteoporos Int 16:999-1003
Geusens P, Hochberg MC, van der Voort DJ, Pols H, van der Klift M, Siris E, et al (2002) Performance of risk indices for identifying low bone density in postmenopausal women. Mayo Clin Proc 77:629–637
Cadarette SM, McIsaac WJ, Hawker GA, Jaakkimainen L, Culbert A, Zarifa G, et al (2004) The validity of decision rules for selecting women with primary osteoporosis for bone mineral density testing. Osteoporos Int 15:361–366
Mauck KF, Cuddihy MT, Atkinson EJ, Melton LJ 3rd (2005) Use of clinical prediction rules in detecting osteoporosis in a population-based sample of postmenopausal women. Arch Intern Med 165:530–536
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
National Osteoporosis Foundation (1998) Physician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation, Washington, DC
Simel DL, Samsa GP, Matchar DB (1991) Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol 44:763–770
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 20:37–46
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Richy F, Gourlay M, Ross PD, Sen SS, Radican L, De Ceulaer F, et al (2004) Validation and comparative evaluation of the osteoporosis self-assessment tool (OST) in a Caucasian population from Belgium. QJM 97:39–46
Lee K, Taguchi A, Ishii K, Suei Y, Fujita M, Nakamoto T, et al (2005) Visual estimation of mandibular cortex on panoramic radiographs in identifying postmenopausal women with low bone mineral densities. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100:226-231
Arifin AZ, Asano A, Taguchi A, Nakamoto T, Ohtsuka M, Tanimoto K (2005) Computer-aided system for measuring the mandibular cortical width on panoramic radiographs in osteoporosis diagnosis. Proc SPIE Med Imaging 2005—Image Processing Conference; Proc SPIE 5747: 813-821
Iki M, Kagamimori S, Kagawa Y, Matsuzaki T, Yoneshima H, Marumo F (2001) Bone mineral density of the spine, hip and distal forearm in representative samples of the Japanese female population: Japanese Population-Based Osteoporosis (JPOS) Study. Osteoporos Int 12:529–537
Acknowledgments
This study was supported by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science (14571786, 16390616).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taguchi, A., Tsuda, M., Ohtsuka, M. et al. Use of dental panoramic radiographs in identifying younger postmenopausal women with osteoporosis. Osteoporos Int 17, 387–394 (2006). https://doi.org/10.1007/s00198-005-2029-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-005-2029-7