Abstract
Introduction and hypothesis
The objective was to assess PD-L1 expression in nonbacterial chronic cystitis (NCC) and bladder cancer (BC).
Methods
The present study included 20 NCC and 20 BC patients. The degree of inflammation of the bladder wall was assessed on slides stained with H&E. Viral pathogens (herpes simplex virus, Epstein–Barr virus, cytomegalovirus, and high-risk HPVs) were detected using real-time polymerase chain reaction analyses of the bladder specimens. Immunohistochemistry was performed to assess the PD-L1 expression in bladder tissue.
Results
Expression of PD-L1 was detected in 40% of NCC patients and 85% of BC patients. Viral pathogens were found in 50% of NCC patients and 60% of BC patients, with EBV being the most common. In NCC patients the immune cell score correlated strongly with the degree of inflammatory infiltration of the bladder wall (r = 0.867, p < 0.001), the presence of lymphoid aggregates in the submucosa (r = 0.804, p < 0.001), koilocytosis (r = 0.620, p = 0.004), and the presence of viral pathogens (r = 0.784, p < 0.001). In BC patients the immune cell score correlated with the degree of inflammatory infiltration of the bladder wall (r = 0.534, p = 0.015) and the presence of viral pathogens (r = 0.626, p = 0.003), but not with the presence of lymphoid aggregates in the submucosa (r = 0.083, p = 0.729), and koilocytosis (r = 0.366, p = 0.112).
Conclusions
Expression of PD-L1 was detected in a cohort of NCC patients, although the PD-L1 positivity rate was lower than that in BC. Our results demonstrate that the degree of PD-L1 expression in bladder tissue is associated with the presence of viral infections and with the degree of inflammatory infiltration of the bladder wall in both NCC and BC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonbacterial chronic cystitis (NCC) is a resumptive term that encompasses nonbacterial infectious and non-infectious chronic cystitis. Nonbacterial infectious cystitis is caused by viruses (e.g., herpes simplex virus [HSV]-1, HSV-2, cytomegalovirus [CMV]) and fungi (Candida spp.) [1, 2]. In cases where no distinct etiological factor is found, a diagnosis of interstitial cystitis/bladder pain syndrome is usually considered and cystoscopy is performed. Although the etiology of interstitial cystitis remains unclear, recent studies have demonstrated that Epstein–Barr virus (EBV) and Varicella zoster virus might be implicated in the pathophysiology of interstitial cystitis [3, 4].
The most common pathological findings include epithelial denudation, inflammatory infiltration of the bladder wall, vasodilation, and submucosal edema [5]. Immune-mediated and autoimmune processes are fundamental to the development of NCC; however, the role of the immune checkpoint PD-1/PD-L1 pathway is underestimated. Programmed cell death-1 (CD-279) is one of the key co-inhibitory receptors expressed on immune cells, including T cells, B cells, NK cells, NKT cells, dendritic cells, and monocytes [6]. The interaction between PD-1 and its ligands, primarily PD-L1 (B7-H1), counters stimulatory signaling from T cell receptors, leading to the downregulation and apoptosis of T cells. Hence, the PD-1/PD-L1 pathway is important in inhibiting immune responses and promoting self-tolerance [6, 7].
Tumor cells and viral pathogens (e.g., HSV, CMV, and EBV) take advantage of the PD-1/PD-L1 pathway by inducing PD-L1 expression in lymphoid and peripheral tissues, which, in turn, helps them to evade host immunity. Increased PD-L1 expression in tumor cells is observed in various malignant diseases, such as breast, colorectal, gastric, and bladder cancers (BC) [8]. Recently, it was found that PD-L1 expression in some nonmalignant diseases (e.g., inflammatory bowel disease, Crohn’s disease, and inflammatory arthritis) correlates with the degree of inflammation [9, 10].
Thus, in this study, we aimed to investigate PD-L1 expression in bladder tissue in NCC and BC and to assess the role of the PD-1/PD-L1 pathway in the pathophysiology of NCC and BC.
Materials and Methods
A total of 40 bladder specimens were obtained during transurethral resections of the bladder: 20 from patients with NCC and 20 from patients with BC. NCC was defined as a recurrent or persistent condition of cystitis-like symptoms for at least 6 months with a negative urine culture. The NCC patients underwent cystoscopy with hydrodistension for 5 min. The saline was drained at the maximum bladder capacity.
The clinical stage of BC was characterized according to the 2017 TNM classification; for grading, the 1973 WHO classification was used. Among the patients with BC, 25% (n = 5) had non-muscle invasive BC and 75% (n = 15) had muscle-invasive BC. For staging, contrast-enhanced computed tomography scans of the chest, abdomen, and pelvis were performed in patients with confirmed muscle-invasive BC.
Histological Evaluation
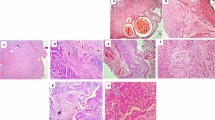
Formalin-fixed and paraffin-embedded bladder tissue slides stained with hematoxylin and eosin were examined. The degree of inflammation of the bladder wall was graded as follows: mild, with scattered immune cells in the specimen; moderate, with inflammatory infiltration of less than 50% of the bladder wall; and severe, with inflammatory infiltration of more than 50% of the bladder wall. Additionally, the presence of lymphoid aggregates in the submucosa and koilocytosis were assessed.
Real-Time Polymerase Chain Reaction
All bladder samples were stored in liquid nitrogen, then slowly thawed and mechanically homogenized with Lysing Matrix A ceramic beads in FastPrep®-24 Classic (MP Biomedicals, USA). DNA was extracted using Maxwell® RSC 48 (Promega, USA). The amplification and detection were conducted on a CFX96® Touch System (Bio-Rad Laboratories, USA), according to the manufacturer's protocol. The RealBest HSV-1 and HSV-2 polymerase chain reaction (PCR) Kits, RealBest EBV PCR Kits, RealBest CMV PCR Kits, and RealBest HPV screen PCR for types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 (Vector Best, Russia) were used. The presence of viral pathogens was determined by measuring the increase in fluorescence of the FAM fluorophore.
Immunohistochemistry
Paraffin-embedded sections of the bladder specimens 5 μm thick were prepared. After deparaffinization and rehydration of the tissue with xylene and graded ethanol series, antigen retrieval was performed using the Dako Target Retrieval Solution (Dako North America, USA). Endogenous peroxidase was blocked with a 3% hydrogen peroxide solution. PD-L1 expression was detected using the EnVision FLEX visualization system on AutostainerLink 48 (Dako) after the application of the monoclonal mouse anti-PD-L1 clone 22C3 (Dako). The immune cell score, which refers to the percentage of the area covered with PD-L1-positive immune cells, was calculated for both NCC and BC specimens. The tumor cell (TC) score was calculated only for BC specimens. PD-L1 expression was defined based on the immune cell score as follows: none (< 1%), mild (≥ 1% and < 5%), moderate (≥ 5% and < 10%), and high (≥ 10%). The pathologist was blinded to the clinical and pathological data.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics (IBM Corp., USA). The Kolmogorov–Smirnov test was used to determine whether the data were normally distributed. Means with 95% confidence intervals were calculated for continuous variables, and percentage frequencies were used to present categorical data. Differences between study groups were tested using Fisher’s exact test. The associations between the two variables were evaluated using Spearman’s rank correlation coefficient, as either (or both) variables were ordinal. Probability values (p-values) less than 0.05 were considered statistically significant.
Results
Tables 1 and 2 provide clinical information about the patients with NCC (average age: 35.2 ± 4.3 years) and BC (average age: 69.5 ± 4.0 years), accordingly. The results of the pathological examination and real-time PCR of the bladder tissue biopsies are shown in Tables 3 and 4. Viral pathogens were detected in 50% of NCC patients and 60% of BC patients. In both NCC and BC, the EBV was most commonly found (30% and 55% respectively). Severe inflammatory infiltration of the bladder wall was observed more often in BC than in NCC (50% vs 15%, p = 0.041). There were no statistically significant differences in the presence of lymphoid aggregates and koilocytosis between NCC and BC.
In NCC, the presence of viral DNA correlated positively with the degree of inflammatory infiltration of the bladder wall (r = 0.624, p = 0.003), the presence of lymphoid aggregates in the submucosa (r = 0.577, p = 0.008), and koilocytosis (r = 0.816, p < 0.001). In BC, the presence of viral DNA also correlated positively with the degree of inflammatory infiltration of the bladder wall (r = 0.791, p < 0.001) and the presence of lymphoid aggregates in the submucosa (r = 0.471, p = 0.036), but not with koilocytosis (r = 0.375, p = 0.103).
PD-L1 Expression in the Bladder Wall
Among NCC patients, PD-L1 expression was positive in 40% of cases (8 out of 20): mild expression was seen in 25% of patients (5 out of 20), moderate expression in none, and high expression in 15% (3 out of 20). Meanwhile, PD-L1 expression was detected significantly less often in NCC patients with cystoscopic findings than in NCC patients without these findings (10% vs 70%, p = 0.020). The immune cell score in NCC correlated strongly with the degree of inflammatory infiltration of the bladder wall (r = 0.867, p < 0.001), the presence of lymphoid aggregates in the submucosa (r = 0.804, p < 0.001), koilocytosis (r = 0.620, p = 0.004), and the presence of viral pathogens (r = 0.784, p < 0.001). No statistically significant correlation between the immune cell score and the age of NCC patients was found (r = −0.379, p = 0.100).
Expression of PD-L1 was positive in 85% of BC patients (17 out of 20): mild expression was detected in 60% of patients (12 out of 20), moderate expression in 20% (4 out of 20), and high expression in 5% (1 out of 20). The immune cell score correlated with the degree of inflammatory infiltration of the bladder wall (r = 0.534, p = 0.015) and the presence of viral pathogens (r = 0.626, p = 0.003), but not with the presence of lymphoid aggregates in the submucosa (r = 0.083, p = 0.729), and koilocytosis (r = 0.366, p = 0.112). The TC score correlated with the presence of lymphoid aggregates in the submucosa (r = 0.627, p = 0.003) and the presence of viral pathogens (r = 0.562, p = 0.010), but not with the degree of inflammatory infiltration of the bladder wall (r = 0.325, p = 0.163), and koilocytosis (r = 0.407, p = 0.075). There was no correlation between the TC score and the age of BC patients (r = −0.285, p = 0.223) and between the immune cell score and the age of BC patients (r = 0.001, p = 0.997).
Discussion
Currently, the upregulation of the PD-1/PD-L1 inhibitory pathway in BC is unquestionable [8, 11]. It was proven that high PD-L1 expression in the bladder tissue is associated with more advanced tumors, higher recurrence rates, and lower survival rates [8, 12]. In contrast, PD-L1 (B7-H1) expression is not observed in normal tissues, particularly in the bladder tissue [13,14,15,16].
Schistosomiasis and viral infections (high-risk human papilloma viruses [HPVs] and EBV) are known to contribute to the development of BC [17, 18]. According to recent findings, cancer cells and some viral pathogens (e.g., EBV, CMV, HIV) can modulate the PD-1/PD-L1 axis by inducing the overexpression of PD-L1 in tissues [6, 19]. Activation of this inhibitory axis helps viral pathogens to subvert the host antiviral immune response. For instance, PD-L1 is expressed in EBV-positive cancers, such as Hodgkin lymphoma, nasopharyngeal cancer, gastric cancer, as well as chronic active EBV infection [19]. HPV oncoproteins E5 and E6/E7 can upregulate the PD-1/PD-L1 pathway [20]. We verified the PD-L1 overexpression in a cohort of BC patients and its strong association with the presence of viral DNA in bladder tissue. EBV-positive bladder specimens and koilocytosis were seen in more than half of BC patients. Associated with HPV infection, koilocytes are squamous cells with nuclear features of low-grade squamous intraepithelial lesions. HPV infection in BC patients was detected in previous studies; however, clear associations between HPV and urothelial cancer have not yet been established [21]. As we performed PCR analyses for only 14 high-risk HPV types, the HPV-positive bladder specimen count may have been underestimated.
Upregulation of the PD-1/PD-L1 pathway is seen not only in malignant diseases. On the one hand, this signaling pathway plays a substantial role in immunoregulation by limiting immune-mediated tissue damage during infection. On the other hand, virus-induced PD-L1 expression is considered an immune evasion strategy [6]. Chronic bacterial and viral infections cause persistent stimulation of antigen-specific T cells, which additionally induces early T cell exhaustion [19, 20]. Benedict et al. demonstrated that CMV-infected dendritic cells in mice express PD-L1, thereby contributing to antigen-specific T cell anergy [22]. PD-1 blockade can potentially reverse this functional immunodeficiency [23].
Programmed death ligand-1 is usually expressed by T and B cells, epithelial cells, endothelial cells, tumor cells, dendritic cells, and macrophages in areas of dense inflammatory infiltration [8]. It has been reported that CD8 T cells, proinflammatory cytokines (e.g., IFN-γ, TNF-α, IL-4), transcription factors, and microRNAs positively affect the PD-L1 expression in tissues. The results of our study are consistent with those of previous studies; PD-L1 expression correlated significantly with the inflammatory infiltration of the bladder wall and the presence of lymphoid aggregates in both NCC and BC patients but did not correlate with the age of those patients [15, 24].
Recently, viral infections have been investigated as potential etiological factors of IC. Jhang et al. showed that EBV was present in 87.5% of bladder specimens from ulcerative IC patients and in 17.4% of specimens from non-ulcerative IC patients [3]. In our study, 30% of the bladder specimens from NCC patients were EBV positive. Novel findings included the detection of PD-L1 expression and its association with the presence of viral pathogens in NCC patients. However, the PD-L1 positivity rate in NCC patients is lower than in BC patients, partially due to the lower prevalence of concomitant viral infections. Additionally, the PD-L1 positivity rate in NCC patients with no cystoscopic findings was found to be higher than that in IC patients. Previously Chen et al. detected PD-L1 expression in a cohort of patients with relatively severe IC and found a positive correlation between the degree of PD-L1 expression and the effectiveness of hydrodistension [5].
We acknowledge that the limited number of study participants (n = 40) led to some results being statistically insignificant. More prospective and retrospective studies at the molecular level are required to confirm the associations between the PD-L1 expression, viral infections, and inflammatory infiltration of the bladder wall found in this study. An in-depth analysis of NCC pathophysiology may lead to the appreciation of the PD-1/PD-L1 pathway as a potential therapeutic target.
Conclusions
Expression of PD-L1 was detected in a cohort of NCC patients, although the PD-L1 positivity rate was lower than that in BC. The degree of PD-L1 expression in bladder tissue is associated with the presence of viral infections and the degree of inflammatory infiltration of the bladder wall in both NCC and BC.
Data availability
The authors confirm that all data generated or analyzed during this study are available within the article.
Abbreviations
- BC:
-
Bladder cancer
- CMV:
-
Cytomegalovirus
- EBV:
-
Epstein–Barr virus
- HPV:
-
Human papillomavirus
- HSV:
-
Herpes simplex virus
- IC/BPS:
-
Interstitial cystitis/bladder pain syndrome
- IUC:
-
Infiltrating urothelial carcinoma
- NCC:
-
Nonbacterial chronic cystitis
- NPUC:
-
Non-invasive papillary urothelial carcinoma
- PCR:
-
Polymerase chain reaction
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death ligand-1
- TC score:
-
Tumor cell score
References
Barsegian VA, Kosova IV. Role of the lower urinary tract viral infections in the development of female micturition disorders. Urologiia. 2022;5:117–22.
Odabasi Z, Mert A. Candida urinary tract infections in adults. World J Urol. 2020;38(11):2699–707. https://doi.org/10.1007/s00345-019-02991-5.
Jhang JF, Hsu YH, Peng CW, Jiang YH, Ho HC, Kuo HC. Epstein-Barr virus as a potential etiology of persistent bladder inflammation in human interstitial cystitis/bladder pain syndrome. J Urol. 2018;200(3):590–6. https://doi.org/10.1016/j.juro.2018.03.133.
Hsu CY, Lin CL, Kao CH. Association between chronic interstitial cystitis and herpes zoster. Int J Environ Res Public Health. 2020;17(7):2228. https://doi.org/10.3390/ijerph17072228.
Chen Y, Yu W, Yang Y, et al. Expression of programmed death ligand-1 on bladder tissues is detected in a clinically and histologically well-defined interstitial cystitis cohort. Neurourol Urodyn. 2018;37(4):1396–404. https://doi.org/10.1002/nau.23459.
Schönrich G, Raftery MJ. The PD-1/PD-L1 axis and virus infections: a delicate balance. Front Cell Infect Microbiol. 2019;13(9):207. https://doi.org/10.3389/fcimb.2019.00207.
Patsoukis N, Wang Q, Strauss L, Boussiotis VA. Revisiting the PD-1 pathway. Sci Adv. 2020;6(38):eabd2712. https://doi.org/10.1126/sciadv.abd2712.
Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727–42.
Szczepaniak K, Paskal W, Kuśmierczyk Z, et al. Evaluation of spatial PD1 and PD-L1 expression in inflammatory bowel disease samples—a pilot study. Pol J Pathol. 2022;73(1):50–9. https://doi.org/10.5114/pjp.2022.117178.
Canavan M, Floudas A, Veale DJ, Fearon U. The PD-1:PD-L1 axis in inflammatory arthritis. BMC Rheumatol. 2021;5(1):1. https://doi.org/10.1186/s41927-020-00171-2.
Kosova IV, Loran OB, Sinyakova LA, et al. Immunohistochemical characteristics of bladder cancer in patients with virus-positive tumors. Onkourologiya. 2018;14(2):142–54. https://doi.org/10.17650/1726-9776-2018-14-2-142-154.
Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7–H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56(8):1173–82. https://doi.org/10.1007/s00262-006-0266-z.
Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. https://doi.org/10.1038/nm730. Erratum: 8(9):1039.
Wang Y, Zhuang Q, Zhou S, Hu Z, Lan R. Costimulatory molecule B7–H1 on the immune escape of bladder cancer and its clinical significance. J Huazhong Univ Sci Technolog Med Sci. 2009;29(1):77–9. https://doi.org/10.1007/s11596-009-0116-2.
Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7–H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109(8):1499–505. https://doi.org/10.1002/cncr.22588.
Ghosh C, Luong G, Sun Y. A snapshot of the PD-1/PD-L1 pathway. J Cancer. 2021;12(9):2735–46. https://doi.org/10.7150/jca.57334.
Sun JX, Xu JZ, Liu CQ, et al. The association between human papillomavirus and bladder cancer: evidence from meta-analysis and two-sample Mendelian randomization. J Med Virol. 2023;95(1):e28208. https://doi.org/10.1002/jmv.28208.
Panagiotakis GI, Papadogianni D, Chatziioannou MN, Lasithiotaki I, Delakas D, Spandidos DA. Association of human herpes, papilloma and polyoma virus families with bladder cancer. Tumour Biol. 2013;34(1):71–9. https://doi.org/10.1007/s13277-012-0512-2.
Murata T. Human herpesvirus and the immune checkpoint PD-1/PD-L1 pathway: disorders and strategies for survival. Microorganisms. 2021;9(4):778. https://doi.org/10.3390/microorganisms9040778.
Jubel JM, Barbati ZR, Burger C, Wirtz DC, Schildberg FA. The role of PD-1 in acute and chronic infection. Front Immunol. 2020;24(11):487. https://doi.org/10.3389/fimmu.2020.00487.
Jørgensen KR, Jensen JB. Human papillomavirus and urinary bladder cancer revisited. APMIS. 2020;128(2):72–9. https://doi.org/10.1111/apm.13016.
Benedict CA, Loewendorf A, Garcia Z, Blazar BR, Janssen EM. Dendritic cell programming by cytomegalovirus stunts naive T cell responses via the PD-L1/PD-1 pathway. J Immunol. 2008;180(7):4836–47. https://doi.org/10.4049/jimmunol.180.7.4836.
Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. https://doi.org/10.1038/ni.1679.
Nechifor-Boilă IA, Loghin A, Nechifor-Boilă A, et al. PD-L1 expression in muscle invasive urothelial carcinomas as assessed via immunohistochemistry: correlations with specific clinical and pathological features, with emphasis on prognosis after radical cystectomy. Life (Basel). 2021;11(5):404. https://doi.org/10.3390/life11050404.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
I.K.: study conception and design, data collection, revision of the manuscript; V.B.: study conception and design, data collection, analysis and interpretation of results, draft manuscript preparation; L.G.: data collection, revision of the manuscript; D.K.: data collection, revision of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Local Ethics Committee of the Russian Medical Academy of Continuous Professional Education (Protocol #15, 16/11/2021) and conducted in accordance with the principles of the Helsinki Declaration. All patients signed an informed consent form before participating in the study.
Conflicts of interest
None.
Additional information
Handling Editor: Gin-Den Chen
Editor in Chief: Maria A. Bortolini
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kosova, I., Barsegian, V., Gundorova, L. et al. PD-L1 Expression in Nonbacterial Chronic Cystitis and Bladder Cancer. Int Urogynecol J 35, 1069–1075 (2024). https://doi.org/10.1007/s00192-024-05782-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-024-05782-8