Abstract
Introduction and hypothesis
The aim of this study is to report cognitive dysfunction with commonly used antimuscarinic overactive bladder medications in patients suffering from overactive bladder disorder with and without baseline neurologic conditions.
Methods
We conducted an Ovid MEDLINE, Embase, and PsycINFO search from January 1998 to December 2018 using PRISMA guidelines. Eighteen studies met the inclusion criteria, including 5 randomized controlled trials and 13 observational studies.
Results
Cognitive decline was reported with oxybutynin use (5 of 8 studies) and tolterodine use (4 of 7 studies) among patients with and without baseline cognitive impairment. Oxybutynin use was linked to functional, mental, and behavioral decline among patients with Alzheimer’s disease (2 studies). No cognitive decline was detected among patients with and without baseline cognitive impairment taking trospium (6 studies), darifenacin (3 studies), imidafenacin (2 studies), and fesoterodine (1 study). Solifenacin was not associated with cognitive decline (2 studies) but was linked to an increased risk of dementia among patients with diabetes (1 study).
Conclusion
In this review, cognitive decline was reported with oxybutynin and tolterodine use and should be used with caution in adults over 65 years of age. Solifenacin, fesoterodine, and imidafenacin showed mixed results related to central nervous system effect. Trospium and darifenacin were not associated with cognitive decline among patients with and without baseline cognitive impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overactive bladder (OAB) is defined as urinary urgency, usually accompanied by frequency and nocturia, with or without urge urinary incontinence, in the absence of urinary tract infection or other obvious pathology [1]. The National Overactive Bladder Evaluation (NOBLE) Program found OAB disorder to be highly prevalent with 16.9% of women affected in the US. OAB increases significantly with age affecting 2% of women 18 to 24 years of age and 19.1% of women 65 to 74 years of age [2]. Although prevalence of this disease among men and women is similar, more women experience incontinence with OAB than men, and as a result, women have worse quality of life [2]. OAB is a disorder that not only negatively impacts quality of life, but also carries a significant economic burden. Approximately $7.4 billion is spent on OAB care for women annually, with $1.1 billion due to pharmacologic treatment [3]. With increasing prevalence of OAB with age and the anticipated nearly doubling of the world’s population over 60 years old from 12% to 22% between 2015 and 2050, OAB can be expected to become an even larger public health concern [4].
Although numerous conservative non-pharmacologic treatment options exist for OAB, including behavioral modifications, bladder training, and pelvic floor muscle training [5], the mainstay of therapy is the pharmacologic management of OAB symptoms in the form of anticholinergic medications and beta-3 adrenergic receptor agonists [5,6,7]. Bladder anticholinergics exert their action by blocking the muscarinic M3 receptors on the bladder smooth muscle that control bladder contractions, whereas beta-3 agonists bind β3 adrenergic receptors in the bladder leading to detrusor muscle relaxation [8]. Anticholinergic use is common with an estimated 51% of the general population on these medications [9, 10]. Data on prevalence of OAB medication use in women are limited. According to a cross-sectional study using the 2009 National Ambulatory Medical Care Survey database, active OAB medication use was estimated to occur in approximately 8 million ambulatory visits at an annual rate of 68 visits per 1000 women [3].
Anticholinergic medications have long been used with caution because of their side effects profile, such as dry mouth, constipation, blurry vision, and altered cognition [11]. The Beers Criteria developed by the American Geriatrics Society strongly recommends the avoidance or minimization of the use of anticholinergic drugs due to the associated increased risk of cognitive decline [11]. Bladder antimuscarinic agents including oxybutynin, tolterodine, darifenacin, fesoterodine, solifenacin, and trospium are on the list of medications with strong anticholinergic properties for potentially inappropriate medication use in older adults [11]. Studies have shown that older adults are at increased risk of adverse drug events, particularly related to cognitive decline [9].
Polypharmacy is more common in older adults who are at greater risk of taking multiple medications with anticholinergic properties. These medications are routinely used for the management of common conditions such as asthma, urinary incontinence, and various psychiatric disorders and in aggregate may contribute to a higher anticholinergic burden. Anticholinergic burden refers to the cumulative effect of taking multiple drugs with anticholinergic activity simultaneously [11]. Several anticholinergic risk scales have been developed to quantify anticholinergic burden [12]. Although there is no consensus on a standardized tool for measuring anticholinergic burden, studies have shown that higher anticholinergic burden is associated with poorer cognitive and functional outcomes [12]. Ancelin et al. found that these adverse effects on multiple areas of cognitive dysfunction—reaction time, attention, memory, and recall—may not arise exclusively from using an individual agent with strong anticholinergic effects, but rather from a synergistic effect of multiple medications with varying degrees of anticholinergic effects [9]. Several factors play a role in CNS side effects of OAB anticholinergic agents including M1 subtype muscarinic receptor affinity in the brain, which principally mediates cognition and memory.
Important CNS side effects like cognitive decline in relation to OAB anticholinergic use have not been well studied resulting in insufficient data for metanalyses. In 2018, the Agency for Health and Quality released a systematic review update on nonsurgical treatments for urinary incontinence in women. This review was largely focused on comparative effectiveness of pharmacologic interventions from 95 studies and included limited data on adverse effects pertaining to cognitive function [13]. A recent study by Coupland et al. found evidence linking anticholinergics with an increased risk for sustained cognitive deficits such as dementia [14]. The odds ratio for dementia among patients with high exposure to bladder anticholinergics was 1.65 [14]. The aim of this review is to comprehensively summarize cognitive dysfunction with commonly used overactive bladder medications in patients with and without baseline neurologic conditions.
Material and methods
This systematic review was conducted following the PRISMA guidelines. We conducted an Ovid MEDLINE, Embase, and PsycINFO search for articles between January 1998 and December 2018. Ethics approval was not required for this systematic review. The databases were searched using keywords including overactive bladder (OAB), urinary incontinence (UI), detrusor instability, urge incontinence, cognition, memory, dementia, and OAB medications/drug classes. An a priori systemic review protocol was developed by the authors of this review. Two authors (VD, AI) reviewed the abstracts for content and relevant outcomes. Inclusion criteria were formed using the population, intervention, comparison, outcomes, and study designs. The population of study included female patients > 18 years of age with OAB. The intervention under study was use of bladder anticholinergic medications including darifenacin, oxybutynin, tolterodine, trospium, imidafenacin, solifenacin, and fesoterodine and a beta-adrenergic agonist, mirabegron. Among the studies identified, comparison groups included pre- and post-treatment findings, placebo, or no treatment. The outcomes included were cognitive decline measured by various cognitive-related assessments and diagnosis of dementia or irreversible cognitive decline. Data on mean age, sample size, bladder antimuscarinic medications, length of follow-up, presence or absence of cognitive impairment, severity of cognitive impairment, presence of Alzheimer’s disease or other neurologic conditions, and comorbidities were extracted. Studies were excluded if full text was not available in English or if they did not address the objective of this systematic review. Length of follow-up was not a criterion for exclusion. Both reviewers read the full-text articles to assess if data presented in the articles contributed to the review. Disagreements were resolved through review and discussion of the objectives of the study.
All studies measured level of baseline cognition and cognition following medication use. There is currently no gold standard for cognitive impairment screening and quantifying the severity of cognition [15]. Therefore, numerous forms of cognitive assessments were utilized in the reviewed studies. Most (9/18) studies utilized the Mini-Mental Status Exam (MMSE) [16,17,18,19,20,21,22,23,24]. Three studies used activities of daily living (ADL) as a measurement [18, 23, 25]. Three studies assessed cognition using Hopkins Verbal Learning Test-Revised (HTLV-R) [21, 26, 27]. Two studies measured cognition with the D2 test of attention [28, 29]. Two studies used Alzheimer’s Disease Assessment Scale (ADAS–Cog) [16, 30]. Other cognitive assessment tools that were used to measure changes in cognition included the Brief Visuospatial Memory Test-revised, California Verbal Learning Test, Cognitive Drug Research (CDR) computerized testing, cognitive performance scale, confusion assessment method, digit span, divided attention subtest from the test battery of Zimmermann and Fimm, Epworth Sleepiness Scale, frontal assessment battery, Geriatric Depression Scale, gestalt closure test from the Kaufmann Assessment Battery, Lawton Brody Instrumental ADL Scales, MDS Cognition Scale, memory scanning sensitivity, Mini-Mental Status X, Mini-Cog evaluation, number combination test, Orientation, Memory & Concentration short form, severe impairment battery, speed of choice reaction time, Stroop test, Thurstone word fluency test, Trails Making Test Part A and B, visuospatial performance subtests from the Hamburg-Wechsler Adult Intelligence Test, word recognition sensitivity, and Yesavage Geriatric Depression Scale.
The quality of each of the included studies was assessed by the two authors (VD, AI). Studies were graded as good (A), fair (B), or poor (C), based on study design, study length, population, and outcome measures. The aforementioned criteria were all taken into account in determining the overall quality of evidence using modified guidelines taken from the Grades for Recommendation, Assessment, Development and Evaluation (GRADE) system [31]. Disagreements about grading were resolved through thorough review of the evidence and discussion of criteria in question. An assessment of the quality of evidence reported by the studies included in this review are given in Table 1. All data are reported in accordance with the PRISMA statement.
Results
The MEDLINE, PubMed, and PsycINFO searches yielded a total of 427 articles: 151 articles from Ovid MEDLINE, 211 from Embase, and 65 from PsycINFO. Eighteen studies met the inclusion criteria, including 5 randomized controlled trials and 13 observational studies. A diagram showing the flow of information through the different phases of review is given in Fig. 1. Studies included seven out of eight OAB medications that were identified prior to conducting this review. These include oxybutynin, tolterodine, solifenacin, trospium, imidafenacin, fesoterodine, and darifenacin. There were no cognitive studies identified for mirabegron, a beta-adrenergic agonist included in this review, during the selected time period. Levels of cognition and function were measured with varying cognitive assessments including most commonly the Mini-Mental Status Exam (MMSE), Hopkins Verbal Learning Test-Revised (HTLV-R), and Activities of Daily Living (ADL).
Cognitive decline was reported with oxybutynin (5 of 8 studies) [13, 25, 32,33,34] and tolterodine (4 of 7 studies) [15, 16, 25, 33] use among patients with and without baseline cognitive impairment. Oxybutynin use was linked to functional, mental, and behavioral decline among OAB patients with Alzheimer’s disease (2 studies) [16, 25]. No cognitive decline was detected among OAB darifenacin (4 studies) [19, 20, 23, 30], imidafenacin (2 studies) [21, 22], and fesoterodine (1 study) [29]. Solifenacin was not associated with cognitive decline (2 studies), [22, 32] but was linked to an increased risk of dementia among patients with diabetes (1 study) [33].
Neurocognitive dysfunction by bladder antimuscarinic agent
Oxybutynin
Of the 18 studies included in this review, 8 investigated the effect of oxybutynin use on cognition among patients receiving treatment for overactive bladder. Of the eight studies, five showed cognitive decline with oxybutynin use [13, 25, 32,33,34]. Seven out of the eight studies included patients with baseline cognitive impairment [16, 17, 23, 25, 30, 32, 34]. Yang et al. conducted a cohort study of diabetic patients without baseline dementia, which showed increased dementia event rates in patients receiving oxybutynin compared to nonusers in a 6-year follow-up period [33]. The adjusted hazard ratios compared to nonusers of oxybutynin was 2.35 (95% CI, 1.96 to 2.81) [33]. There was no difference in scores determined by the confusion assessment method, a quick and simple diagnostic tool for identification of delirium between oxybutynin (5 mg ER daily) and placebo in a randomized control trial in a short-term treatment period of 4 weeks [17].
Tolterodine
Seven studies investigated the effect of tolterodine use on cognition among patients receiving treatment for overactive bladder. Of the seven studies, four showed cognitive decline with tolterodine use [15, 16, 25, 33]. Of these four studies, one study included patients with dementia who were also taking cholinesterase inhibitors (ChIs) [25]. This prospective cohort study by Sink et al. demonstrated that dual use of CI and tolterodine or oxybutynin may result in greater rates of functional decline than uses of ChI alone in higher functioning participants with dementia, although no significant differences in ADL scores were found [25]. The study by Diefenbach et al. detected a significant decrease in rapid eye movement sleep in tolterodine users with at least one deficient CYP2D6 allele, although no changes in cognition were observed [28]. In one prospective observational study, there was no negative impact observed in cognitive function in older adults with or without Alzheimer’s disease with tolterodine use in a 6-month follow-up period [23].
Solifenacin
Of the four studies identified in this review, two showed an association of cognitive decline with solifenacin use [29, 33]. In a study of patients with diabetes exclusively, solifenacin use showed increased dementia event rates compared to nonuse within a 6-year follow-up period (3.9%, 1.2%, p < 0.001) [33]. In this study, solifenacin use had the lowest risk of subsequent diagnosis of dementia for adjusted hazard ratios compared to nonuse compared to oxybutynin and tolterodine [33]. A prospective cohort study investigated the effect of solifenacin on cognition in patients with a mean age of 78 years and did not observe a change in MMSE scores at baseline and after 12 weeks of treatment [22]. Krebs et al. investigated the effect of solifenacin, fesoterodine, or darifenacin on cognition in patients with spinal cord injury and detrusor overactivity and found a decline in immediate recall, but no significant change among other measures of cognition in 29 individuals [29].
Trospium
Of the 18 studies included in this review, 6 investigated trospium use on cognition in overactive bladder treatment. Two studies included participants with baseline cognitive impairment. Of the six studies that investigated trospium use, no cognitive decline was detected among OAB patients with and without baseline cognitive impairment [18, 21, 23, 27, 32]. One prospective cohort study demonstrated that dual use of galantamine, a cholinesterase inhibitor, and trospium for 6 months in the elderly with Alzheimer’s disease did not have negative effects on cognition based on MMSE and ADL scores [18]. Staskin et al. showed that use of extended release trospium chloride 60 mg daily for 10 days was undetectable in cerebrospinal fluid samples [27]. Concurrently, there was no decline in performance on tests of memory and recall detected before and after trospium administration [27].
Imidafenacin
Sakakibara et al. investigated the effect of imidafenacin on cognitive function among 62 patients with neurogenic OAB and a mean MMSE score of 21.8 (0–30 scale, normal > 24) [19]. No changes were observed in cognitive function, which was assessed with MMSE scores, ADAS-cog, and frontal assessment battery over a 3-month follow-up. Patients had a mean age of 70 years and various baseline neurocognitive diseases including Alzheimer’s dementia, Parkinson’s disease, Lewy body dementia, multiple system atrophy, and frontotemporal dementia [19]. A subsequent post-marketing surveillance study by Sakakibara et al. showed no change in cognition after a longer follow-up period of 1 year [20]. Mini-Mental Status Exams administered prior to and after imidafenacin use showed that the drug had no effects on cognitive function of patients with mild cognitive impairment [20].
Fesoterodine
Krebs et al. studied the use of fesoterodine, solifenacin, and darifenacin among cognitively intact patients with neurogenic bladder following spinal cord injuries in a prospective cohort study of 29 individuals (control group 19, antimuscarinic group 10) [29]. Of the ten individuals in the antimuscarinic group, two took fesoterodine 8 mg once daily during the 3-month treatment period [29]. Overall, there was no cognitive deterioration in the spinal cord injury patients with antimuscarinic treatment based on neuropsychologic testing [29].
Darifenacin
Four studies analyzed the cognitive effects of darifenacin among patients being treated for overactive bladder. Among both patients with and without baseline cognitive impairments, darifenacin was not found to negatively impact cognitive function [23, 29, 30, 35]. In a double-blind, cross-over study, Lipton et al. found that both immediate release and controlled released darifenacin had no cognitive impact on patients with mild or no baseline cognitive impairment [35]. Three studies, all of which included darifenacin as one of their antimuscarinic treatment arms, found that the use of darifenacin did not impact short-term cognition among patients with varying degrees of baseline CNS impairment including dementia, Parkinson’s disease, and spinal cord injury [23, 29, 30].
Discussion
This systematic review provides an evaluation of the current literature on the cognitive impact of overactive bladder medications by specifically examining the neurocognitive effect of specific OAB medications on healthy patients and patients with baseline neurologic conditions including, but not limited to, those with dementia and Alzheimer’s disease. This review subdivides each medication, which may help healthcare providers when making decisions on which continence medication to prescribe. In this review, cognitive decline was reported with oxybutynin and tolterodine use. Solifenacin showed mixed results related to central nervous system effect. Trospium and darifenacin were not associated with cognitive decline among patients with and without baseline cognitive impairment. There were too few of studies for imidafenacin and fesoterodine to come to a conclusion regarding CNS effects. During the 10-year search period for this review, there were no studies identified evaluating the cognitive effect of mirabegron, the single beta-adrenergic agonist approved for use at that time. Recently, the results of a phase 4, randomized, placebo-controlled study (PILLAR) have become available. The PILLAR study measures differences in cognitive function between mirabegron and placebo using the Montreal Cognitive Assessment (MoCA) test, a validated clinical screening instrument for mild cognitive impairment [36]. It concluded that the 12-week treatment with mirabegron does not impact cognitive function compared to placebo in patients 65 years or older [36].
For the 18 studies included in this systematic review, we were unable to perform metanalysis because of heterogeneity in study design and outcomes (see Table 1). To date, no standardized cognitive screening tool has been adopted as a universally accepted measure of cognitive dysfunction to be used in research, as reflected by the diversity of cognitive assessment tools observed in the studies included in this review. Significant heterogeneity was observed, which may have resulted from different study populations. One study looked at patients with spinal cord injuries [29], whereas another study looked exclusively at patients with type 2 diabetes [33]. The majority of the studies were observational and deemed low to fair in quality of evidence. Confounding factors, including polypharmacy, might have introduced bias into observational studies looking at the relationship of cognition and OAB medications. Comparative trials of different bladder antimuscarinics were not identified. Follow-up periods for the included studies were short term, ranging from 2 weeks to 6 years. Future clinical research should focus on standardization of cognitive assessment in a clinical setting, comparative studies of cognition decline among OAB pharmacologic therapies, as well as long-term and reversibility of effects on neurocognitive dysfunction, while translational research might focus on other than systemic delivery methods for these pharmacologic agents.
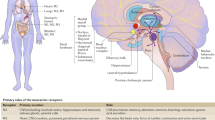
Aside from these limitations, differences among bladder anticholinergics with respect to CNS side effects were observed in this systematic review. These differences may be related to differences in their pharmacokinetic properties in relationship to central cholinergic pathways. There are two main types of acetylcholine receptors, nicotinic and muscarinic, based on their natural alkaloid agonists, nicotine and muscarine, respectively. Muscarinic receptors are abundantly expressed throughout the brain and can be found in various other tissues in the body, such as the salivary glands, bladder, and gastrointestinal system. The M1 receptor is the most abundant muscarinic acetylcholine receptor subtype in brain [8]. It plays an essential role in many cognitive functions such as learning and memory.
Pharmacokinetic factors play an essential role in CNS side effects of OAB anticholinergic agents. Available bladder antimuscarinics vary in their selectivity for muscarinic receptor types.
Both oxybutynin and tolterodine do not have M3 over M1 subtype selectivity. In this review, newer bladder anticholinergics like solifenacin, fesoterodine, and imidafenacin showed mixed results related to central nervous system effect and are known to have higher M3 over M1 subtype selectivity.
Properties of crossing the blood-brain barrier (BBB) also play a role in CNS side effects of bladder antimuscarinics. Oxybutynin and tolterodine are lipophilic molecules easily penetrating the BBB and centrally binding M1 subtype receptors. Trospium, a quaternary amine, is a large, hydrophilic molecule that does not readily cross the BBB [8]. Active drug efflux transporters located on endothelial cells of the BBB are another component of barrier function. These transporters, such as P-glycoprotein (P-gp), actively pump substances from the central circulation back into the systemic circulation. Darifenacin and trospium have been shown to be substrates of P-gp, limiting their cerebral vasculature permeability [8].
Aside from known pharmacokinetic properties of bladder anticholinergics, there is growing evidence that older adults are particularly sensitive to anticholinergic effects because of the age-related decrease in cholinergic neurons in the brain, reduction in hepatic and renal drug metabolism, and an increase in BBB permeability [8]. From animal studies, there is a suggested loss of M1 receptors in the brain [37] and possibly a stronger affinity of the anticholinergic medication binding to these M1 receptors. With use of nonselective anticholinergics like oxybutynin and tolterodine that bind and antagonize the already diminished M1 receptors in the brain, there is a risk of cognitive impairment. Newer bladder anticholinergics are more selective for M3 receptors. The risk of using these newer agents to bind to M1 receptors in the brain is lower, and they are expected to cause less cognitive impairment, which may explain what was seen in the studies that were included in this review.
Polypharmacy and the risk of higher anticholinergic burden disproportionately affect older adults [9]. A systematic review and meta-analysis performed in 2015 showed that exposure to drugs with anticholinergic effects (DACEs) as a class was associated with an increased odds ratio of cognitive impairment (OR 1.45; 95% CI, 1.16, 1.73) [38]. Of the 18 studies included in the 2015 systematic review, only 3 examined individual drugs with anticholinergic properties with oxybutynin and tolterodine as the only OAB anticholinergics included in their search strategy. This systematic review offers additional data on cognitive dysfunction and newer bladder anticholinergics.
Another component that compromises the BBB is the presence of comorbid conditions, such as multiple sclerosis, type 2 diabetes mellitus, Parkinson disease, and Alzheimer’s. In this review, a single study showed solifenacin use was associated with cognitive decline in diabetic patients [28]. In such conditions like type 2 diabetes mellitus, all anticholinergic agents can more easily cross the compromised or more permeable BBB.
Exposure to anticholinergics—a broad class of medications which includes antidepressants, anti-Parkinson drugs, antipsychotic drugs, bronchodilators for respiratory disorders, and bladder antimuscarinics—has come under scrutiny given emerging evidence which shows an association with increased risk of developing dementia [14]. In particular, Coupland et al. suggest that 10% of all dementia cases may be due to anticholinergic medication exposure—a much higher proportion when compared to estimates of modifiable risk factors for dementia such as 5% for mid-life hypertension, 3% for diabetes, and 6.5% for physical inactivity [24]. Tertiary-tier therapies, including posterior tibial sacral nerve stimulation, sacral neuromodulation, and intravesical injection of botulinum toxin A should be considered not only in refractory cases, but also in cases with heightened concern for cognitive dysfunction. Based on these existing studies and the findings from our review, we propose and summarize these major points:
-
Oxybutynin and tolterodine should be used with caution in elderly adults suffering from OAB symptoms, especially in those who have baseline CNS deficits.
-
Consider beta-3 agonist mirabegron for the treatment of OAB symptoms in patients with baseline cognitive decline.
-
Use caution with bladder anticholinergics in diabetic patients.
-
Health care providers should perform individualized risk assessment and routine evaluation of elderly adults prescribed OAB anticholinergic medications.
References
Haylen BT, De Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–36.
Ju R, Garrett J, Wu JM. Anticholinergic medication use for female overactive bladder in the ambulatory setting in the United States. Int Urogynecol J. 2014;25(4):479–84.
Ageing and health [WHO Web site]. February 5, 2018. Available at: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. Accessed December 1, 2020.
Gormley EA, Lightner DJ, Faraday M, Vasavada S. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015;193(5):1572–80.
Lightner DJ, Gomelsky A, Souter L, Vasavada S (2019) Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults: AUA/SUFU Guideline Amendment 2019. J Urol 101097JU0000000000000309.
ACOG Practice Bulletin No. 155: Urinary Incontinence in Women. Obstet Gynecol. 2015;126(5):e66–81.
Cetinel B, Onal B. Rationale for the use of anticholinergic agents in overactive bladder with regard to central nervous system and cardiovascular system side effects. Korean J Urol. 2013;54:806–15.
Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332:455–9.
Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311–20.
American Geriatrics Society. Updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–46.
Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31. https://doi.org/10.1186/s12877-015-0029-9.
Balk E, Adam GP, Kimmel H, et al (2018) Nonsurgical Treatments for Urinary Incontinence in Women: A Systematic Review Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); (Comparative Effectiveness Review, No. 212.)
Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. 2019;179(8):1084–93.
Cullen B, O'Neill B, Evans JJ, Coen RF, Lawlor BA. A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry. 2007;78(8):790–9.
Jewart RD, Green J, Lu CJ, Cellar J, Tune LE. Cognitive, behavioral, and physiological changes in Alzheimer disease patients as a function of incontinence medications. Am J Geriatr Psychiatry. 2005;13(4):324–8.
Lackner TE, Wyman JF, McCarthy TC, Monigold M, Davey C. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc. 2008;56(5):862–70.
Isik AT, Celik T, Bozoglu E, Doruk H. Trospium and cognition in patients with late onset Alzheimer disease. J Nutr Health Aging. 2009;13(8):672–6.
Sakakibara R, Tateno F, Yano M, Takahashi O. Imidafenacin on bladder and cognitive function in neurologic OAB patients. Clin Auton Res. 2013;23(4):189–95.
Sakakibara R, Hamano H, Yagi H. Cognitive safety and overall tolerability of Imidafenacin in clinical use: a long-term, open-label, post-marketing surveillance study. Low Urin Tract Symptoms. 2014;6(3):138–44.
Geller EJ, Dumond JB, Bowling JM, et al. Effect of Trospium chloride on cognitive function in women aged 50 and older: a randomized trial. Female Pelvic Med Reconstr Surg. 2017;23(2):118–23.
Hampel C, Betz D, Burger M, Nowak C, Vogel M. Solifenacin in the elderly: results of an observational study measuring efficacy, tolerability and cognitive effects. Urol Int. 2017;98(3):350–7.
Esin E, Ergen A, Cankurtaran M, et al. Influence of antimuscarinic therapy on cognitive functions and quality of life in geriatric patients treated for overactive bladder. Aging Ment Health. 2015;19(3):217–23.
Yamada S, Kuraoka S, Osano A, Ito Y. Characterization of bladder selectivity of antimuscarinic agents on the basis of in vivo drug-receptor binding. Int Neurourol J. 2012;16(3):107–15.
Sink KM, Thomas J, Xu H, et al. Dual use of bladder anticholinergics and cholinesterase inhibitors: long-term functional and cognitive outcomes. J Am Geriatr Soc. 2008;56(5):847–53.
Geller EJ, Crane AK, Wells EC, et al. Effect of anticholinergic use for the treatment of overactive bladder on cognitive function in postmenopausal women. Clin Drug Investig. 2012;32(10):697–705.
Staskin D, Kay G, Tannenbaum C, Goldman HB, Bhashi K, Ling J, et al. Trospium chloride has no effect on memory testing and is assay undetectable in the central nervous system of older patients with overactive bladder. Int J Clin Pract. 2010;64(9):1294–300.
Diefenbach K, Jaeger K, Wollny A, Penzel T, Fietze I, Roots I. Effect of tolterodine on sleep structure modulated by CYP2D6 genotype. Sleep Med. 2008;9(5):579–82.
Krebs J, Scheel-sailer A, Oertli R, Pannek J. The effects of antimuscarinic treatment on the cognition of spinal cord injured individuals with neurogenic lower urinary tract dysfunction: a prospective controlled before-and-after study. Spinal Cord. 2018;56(1):22–7.
Moga DC, Carnahan RM, Lund BC, et al. Risks and benefits of bladder antimuscarinics among elderly residents of veterans affairs community living centers. J Am Med Dir Assoc. 2013;14(10):749–60.
Kavanagh BP. The GRADE system for rating clinical guidelines. PLoS Med. 2009;6(9):e1000094.
Wagg A, Dale M, Tretter R, Stow B, Compion G. Randomised, multicentre, placebo-controlled, double-blind crossover study investigating the effect of solifenacin and oxybutynin in elderly people with mild cognitive impairment: the SENIOR study. Eur Urol. 2013;64(1):74–81.
Yang YW, Liu HH, Lin TH, Chuang HY, Hsieh T. Association between different anticholinergic drugs and subsequent dementia risk in patients with diabetes mellitus. PLoS One. 2017;12(4):e0175335.
Lampela P, Lavikainen P, Garcia-horsman JA, et al. Anticholinergic drug use, serum anticholinergic activity, and adverse drug events among older people: a population-based study. Drugs Aging. 2013;30(5):321–30.
Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: effects of darifenacin. J Urol. 2005;173(2):493–8.
Griebling TL, Campbell NL, Mangel J, Staskin D, Herschorn S, Elsouda D, et al. Effect of mirabegron on cognitive function in elderly patients with overactive bladder: MoCA results from a phase 4 randomized, placebo-controlled study (PILLAR). BMC Geriatr. 2020;20(1):109. https://doi.org/10.1186/s12877-020-1474-7.
Schwarz RD, Bernabei AA, Spencer CJ, Pugsley TA. Loss of muscarinic M1 receptors with aging in the cerebral cortex of fisher 344 rats. Pharmacol Biochem Behav. 1990;35(3):589–93.
Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and all-cause mortality in older adults: a systematic review and meta-analysis. Br J Clin Pharmacol. 2015;80(2):209–20.
Financial disclaimer/conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Contributions
Vi Duong: Project development, data collection/management, data analysis, manuscript writing/editing.
Aya Iwamoto: Data collection/management, data analysis, manuscript writing/editing.
Jon Pennycuff: Data analysis, manuscript writing/editing.
Bela Kudish: Project development, manuscript editing.
Cheryl Iglesia: Project development, manuscript editing.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 64 kb)
Rights and permissions
About this article
Cite this article
Duong, V., Iwamoto, A., Pennycuff, J. et al. A systematic review of neurocognitive dysfunction with overactive bladder medications. Int Urogynecol J 32, 2693–2702 (2021). https://doi.org/10.1007/s00192-021-04909-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-021-04909-5