Abstract
A damaged sphincteric unit or support system, unstable urethral deformability or damaged sensory innervation are all potential causes of a dysfunctional urethral sphincter. With the current improvement in pharmacological targets and urodynamic understanding, studies have begun quantifying individual structures and their importance in closure pressure and consequently urethral continence. However, when it comes to the function of the longitudinal urethral smooth muscle layer, there is currently no consensus. The intent of this structured review is to critically examine literature regarding the female urethral anatomy and closure mechanism. We hypothesized that the longitudinal smooth muscle is a prerequisite for sufficient urethral closure and not merely involved during micturition. Overall opinions on a dysfunctional closure mechanism are controversial. Nonetheless, basic mechanics may be applied to understand simple urodynamics. With the assumption of longitudinal muscles forming a plug when contracted, this could have a substantial effect on the continence mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the urethral closure mechanism and dysfunction in female stress urinary incontinence (SUI) has been hotly debated by many investigators for decades. Numerous hypotheses have been offered to try to explain the accurate function of the urethral structures and the shared impact on a healthy urinary mechanism. Considering the many contributing factors behind SUI, nothing less should be expected when trying to understand the contribution of each anatomical layer. A damaged sphincteric unit or support system, urethral deformability or damaged sensory innervation are all potential causes of an insufficient closure mechanism [1,2,3]. A recent structured literature review presented a lack of consensus regarding the function of the longitudinal urethral smooth muscle layer (USM). Many scholars concluded significance only during micturition [4,5,6,7,8] (by shortening the urethra and pulling down the bladder neck to facilitate urination). Others were convinced that the peri-luminal location was deliberate in terms of maximizing coaptation [9,10,11]. There are inadequate detailed observations of this structure. This structured review is aimed at reviewing the overall urethral anatomy and innervation and correlating these with biomechanical principles and physiological properties of the longitudinal urethral smooth muscle.
It is based on a structured review of English literature with no preferences of study design. An assortment of three major databases was elected to incorporate the broadest range of articles: PubMed/MEDLINE, Embase and Web of Science. A clear distinction was made when selecting articles, between striated and smooth urethral sphincter as the latter holds longitudinal smooth muscle. Each search engine was used with four search terms. [Anatomy and histology of urethra] AND [nerve innervation of urethra] AND [physiology of urethral smooth muscle] AND [female urethral sphincter] were applied, generating a total of 5,714 articles. A further selection was made based on title and abstract, generating 156 articles. Inclusion criteria were urethral smooth muscle, clinical trials and reviews, human subjects, adult females (+19), and studies in English. A conclusive number of articles was selected. Initially, articles on anatomy, physiology, and nerve innervation of the female urethra were reviewed, and consensus established. Hereafter, a search for observational or experimental data regarding the physiology of the urethral smooth muscle and the urethral closure mechanism was pursued. A timeframe filter of articles from 1950 was placed.
Anatomy of female urethral continence
The urethral closure mechanism
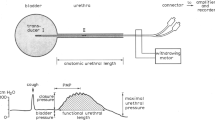
The level of SUI depends upon the ability of the urethra to maintain a robust urethral closure pressure, during fluctuations of intra-abdominal pressure [12, 13]. The intraurethral pressure must therefore be higher than or equal to bladder pressure. This pressure is sustained through a combination of active and passive closure forces generated by the urethral wall and surrounding structures and mechanisms. During rest, permanent closure forces seal off the lumen. These are submucosal vessels, elastin and connective tissue, smooth muscle layer, and neuronal stimuli [4, 6]. During coughing or sneezing, adjunctive forces add additional support by activating voluntary muscles of the pelvic floor and intra-abdominal pressure transmission [14].
Striated urethral sphincter
The external urethral sphincter (EUS), rhabdosphincter or striated urogenital sphincter (as referred to in older literature) is the external skeletal layer of the urethra. It extends from the neck to the perineal membrane and accounts for 20–80% of the total urethral length [2]. The layer forms a sleeve over the entire urethra, with the thickest part in the middle third of the urethra, the high pressure zone [12]. The muscle can be divided into three distinct muscles: m. sphincter urethrae, m. compressor urethrae, and the m. urethrovaginal sphincter [15]. According to El-Badawi and Schenk, the EUS is histochemically triple-innervated. This suggests a coordinated function with the bladder, bladder neck, and urethral smooth muscle simultaneously [16].
Urethral smooth muscle
Many agree that the tubular structure of the urethra is a histological continuation of the trigone [4]. It is composed of two muscle layers: an inner thick longitudinal smooth muscle layer (LSM) and an outer thin circular layer (circular smooth muscle layer [CSM]). The smooth muscle layer is present throughout the upper four fifths of the urethra [4]. The combined muscle layer is thickest in the proximal urethra and thins out distally [15]. This in contrast to the EUS, which is more dense in the mid-urethral region [2, 17, 18]. The thick inner longitudinal layer measures approximately 1 mm, with closely packed muscle fibers [4]. The muscle bundles are extensively separated by populous collagen and elastic fibers. The circular layer surrounds the longitudinal layer and is one tenth the thickness of the longitudinal layer [4]. Separate circular fibers are more prominent in the upper portion of the urethra [2]. Posterolaterally, the two smooth muscle layers attach to the trigonal plate (Fig. 1) [4, 16].
Submucosal vasculature
Luminal to urethral smooth musculature, a thick submucosal vasculature is present. Three venous systems have been identified. The longitudinal veins in the proximal plexus are located below the internal urethral meatus with a tortuous course. Berkow used the term a cavernous vascular submucosa [4, 19]. The distal plexus is oriented in different directions and located above the external urethral meatus. The last venous system has longitudinal course veins and extends from the internal to external urethral meatus. The arterial supply consists of branches from the inferior vesical artery, which penetrates the deep trigone. Four arteries have been identified: two muscular branches and two submucosal ones.
Submucosa
In the pre- and postmenopausal urethra, the lumen is initially slit-like, but becomes increasingly star-shaped beneath the urogenital diaphragm. The submucosa is composed of loosely woven connective tissue, numerous longitudinal oriented muscle bundles, and elastin [4].
Epithelium
The proximal urethra is lined with transitional epithelium (also called urothelium), which distally transforms into nonkeratinizing squamous epithelium [4].
Bladder neck sphincteric system
The urethra and bladder neck should be considered a synergic unit, as their anatomy and function work co-dependently upon one another [15, 20]. The bladder neck is unique as it is a mixture of various directional muscle components. It is considered sphincteric as it continues throughout the urethra [15]. Many consider the bladder neck as the location of the predominant continential properties, as the elastic tissues here are immense [4]. It consists of the distal portion of the detrusor (including the trigone) and continues into the proximal urethra. Circular and longitudinally oriented muscle fibers are both present in the trigonal ring, with both layers continuing as the inner longitudinal and outer circular smooth muscle of the urethra [4].
Nerve innervation of the female bladder neck and urethra
The nerve innervation of the female lower urinary tract consists of combined efforts from the parasympathetic, sympathetic, and somatic pathways. Each pathway works harmoniously with the other, interchangeably activated during various stages of storage and micturition [20, 21]. Pharmacological studies have outlined countless targets, neurotransmitters, and integrated reflexes involved in ensuring proper urethral function and control.
Efferent pathways: pelvic nerve
Efferent parasympathetic fibers originate from the intermediolateral column in S2–S4 of the spinal cord and come together as the pelvic nerve. Upon termination on postganglionic neurons in the pelvic ganglion and detrusor, parasympathetic preganglionic axons release acetylcholine (ACH) [20]. Modulatory mechanisms at the synapse exist in the form of muscarinic, adrenergic, purinergic, and enkephalinergic influence [20, 21]. Gosling et al. noted an extensive cholinergic parasympathetic nerve supply of the urethral smooth muscle, identical to that which supplies the detrusor [20, 22]. Other authors have observed noradrenergic nerve terminals [23, 24]. Simultaneously, parasympathetic fibers reach urethral smooth muscle where nitric oxide synthase is found. Nitric oxide (NO) is synthesized prior to release, causing proximal urethral relaxation simultaneous with bladder contraction (Fig. 2) [20,21,22].
Parasympathetic nerve innervation of the lower urinary tract. Fibers from the spinal cord (S2-S4) travel to synapse at the pelvic ganglion and further terminate in the bladder detrusor and urethral muscles. This leads to simultaneous contraction of the bladder and relaxation of the urethra. Ach acetylcholine, ATP adenosine triphosphate, No nitric oxide. Michelle Alexandra Mistry®
Hypogastric nerve
The sympathetic preganglionic neurons, or the hypogastric nerve, are located in the intermediolateral column of T10–L2 [20]. Before reaching the urethral smooth musculature, synaptic circuits are established the inferior mesenteric ganglion, paravertebral ganglia, and pelvic ganglia. The actual effector transmission is carried out by norepinephrine (NE), and the bladder neck and in particular the urethra (proximal to the mid-urethral region) have a dense population of -adrenoceptors [20, 21, 25, 26]. 1- and 2-adrenoceptors are both present in the human urethral smooth muscle, with the former being predominant [20]. Activation of the -adrenoceptors results in a collective smooth muscle contraction and rise in the intraurethral pressure. In addition, Nakamura et al. demonstrated inhibitory functions of 2-adrenoceptor and 1-adrenoceptor on parasympathetic neurons [27]. -adrenoceptors are also present in the lower urinary tract. Three subtypes are found (1, 2, and 3) with the latter most predominant in the human detrusor [21]. 2-adrenoceptors are found in the bladder neck and an administering of 2-agonists or NE results in relaxation (Fig. 3).
Sympathetic nerve innervation of the lower urinary tract. Fibers from the spinal cord (T10-L2) travel to synapse with postganglionic neurons in the inferior mesenteric, paravertebral, and pelvic ganglia. This leads to bladder detrusor relaxation and urethral smooth muscle contraction. This increases bladder capacity and intraurethral pressure for continence. NE norephinephrine, β2, β3 beta-adrenoceptors, α1A alpha-adrenoceptors. Michelle Alexandra Mistry®
Pudendal nerve
Voluntary or somatic control originates from motor neurons in Onuf’s nucleus of the anterior horn of segments S2–S4 in the spinal cord [20]. Onuf’s nucleus has a dense population of noradrenergic and serotonergic terminals. The pudendal nerve releases Ach, which binds directly to nicotinic receptors (N1) on striated muscle fibers of the EUS and periurethral muscles of the pelvic floor, leading to an voluntary, amplified contraction for continence (Fig. 4) [20, 21].
Somatic nerve innervation of the lower urinary tract. Motor neurons of the anterior horn in the spinal cord are densely populated with noradrenergic and serotonergic terminals. These form the pudendal nerve; which innervates the skeletal (voluntary) muscle of the external urethral sphincter. This can add additional muscle strength during continence. Michelle Alexandra Mistry®
Additional neuromodulators
Non-adrenergic and noncholinergic transmitters
Additional neuromodulators have been identified as altering the autonomic neural response. Non-adrenergic and noncholinergic neurotransmitters (NANC) can be either excitatory or inhibitory [27]. NO is found in urothelium and afferent nerves. It is the major inhibitory NANC of the bladder and urethral systems [22, 28]. Postsynaptic purinergic receptors are also present on detrusor smooth muscle [21, 29] suggesting that extracellular adenosine triphosphate might be an additional neurotransmitter. Vasoactive intestinal peptide was found in the human urogenital system, causing smooth muscle relaxation [20]. Tachykinins such as substance P, neurokinin A, and neurokinin B are rapid effectors shown to cause rapid urethral contraction [20].
Afferent fibers to the urethra
Afferent parasympathetic fibers in the lower urinary tract are located in the suburothelial layer and detrusor muscle, as a nerve plexus. These fibers can either be myelinated A-fibers or C fibers [21]. From the urethra and bladder, the fibers travel back to the spinal cord as either the pelvic or the hypogastric nerves. The suburothelial plexus is sparse in the dome of the bladder, denser towards the bladder neck, and very extensive in the trigone [23]. These fibers detect stretching and overall fulness of the urogenital tissues.
Afferent fibers to the striated urethral sphincter
The afferent fibers of the EUS leave through the pudendal nerve towards the sacral region of the spinal cord. The interaction of the smooth muscle in the bladder and urethra, striated muscles, and peripheral neural control all help to store or eliminate urine. These are regulated or inhibited through several supraspinal centers. The binary function of the lower urinary tract requires intricate regulation of the neuronal input through reflexes [23].
Storage
During storage, the bladder maintains a low and constant pressure by allowing the detrusor fibers to accommodate increasing volume through stretching and simultaneously inhibiting the parasympathetic input to the bladder [21]. As the volume increases, afferent volume receptors (A-fibers) in the bladder wall are activated and turn on an intersegmental spinal reflex pathway [30]. The afferent signals travel back to the spinal cord, and trigger activation of the sympathetic input to the bladder. This leads to inhibition of the bladder activity and a contraction of the bladder neck and proximal urethra. While this is taking place, afferent bladder fibers co-activate pudendal motor neurons on their circuit into the spinal cord. In addition, the activated pudendal motor neurons stimulate the striated EUS, providing additional force for maintenance of urinary continence. This reflex is known as the guarding reflex [20, 21].
Micturition
In an appropriate setting, elimination of urine occurs by voluntary activation of the micturition reflex. Here, an inhibitory input to the somatic center and a stimulatory input to the parasympathetic center is generated. This leads to a relaxation of the urethra and contraction of the bladder [20].
Evidence regarding urethral smooth muscle
A structured literature review was launched with the aim of finding studies that identified differences in the USM arrangements. Variances in USM receptor presentation or verified antagonistic activation was of particular interest. No study assessing the actual impact of LSM was found. Most authors believe that contraction of the LSM opened and shortened the urethra, by pulling down the bladder neck during micturition [4, 15]. Others denied this entirely and reasoned that the unique organization with an inner longitudinal filler and surrounding circular structure is crucial for optimal continence and sphincter mechanism [9]. Pharmacological studies have provided insight into the effect of the receptors and thus the different muscle layers.
Cholinergic influence on the urethral pressure
Ek et al. demonstrated a large number of cholinergic nerves in the urethral smooth muscle, suggesting the influence of antimuscarinic drugs [25]. Fesoterodine is an antimuscarinic drug. The drug had previously been shown to increase the maximal urethral pressure in an animal study [31]. However, in a small study of women with SUI, administration of fesoterodine did not change urethral tone in the resting stage nor did it decrease the numbers of SUI episodes. Similar findings were confirmed by Ek et al., who in addition studied all parts of the urethra distal to the bladder neck junction. ACH was also administered, producing no effect or a slight contraction [25].
Adrenergic influences on the urethral pressure
A tight concentration of -adrenoceptors has been found along the entire length of the female urethra. Ek et al. showed a uniform response to adrenoceptor-stimulating drugs [25]. The resultant contraction was responsible for maintaining intraurethral pressure along the entire length of the urethra. In the same study, phentolamine, a reversible nonselective -adrenergic antagonist was also examined and found to decrease the urethral pressure profile across the entire length [25]. The idea of smooth muscle playing a role in resting urethral pressure was confirmed in a study by Reitz et al. Tamsulosin, a selective alpha-blocker, significantly reduced urethral pressure over the entire urethral length [32]. The EUS can be stimulated through Onuf’s nucleus in the spinal cord and the urethral pressure can thereby be increased. Both serotonin–noradrenaline reuptake inhibitor and noradrenaline reuptake inhibitors (NRIs) have an effect on urethral tone. Reboxetine (RBX), a NRI, seems to be the most efficient drug, with the highest opening urethral pressures [33].
The theory of a sphincter: arrangement of the sphincter components
A sphincter is a mechanical arrangement of active and passive structures enclosing a tubular lumen. Mechanically, an annular shape enables a tube to seal and open in response to changes of tubal components. For instance, in the urethra, the lumen and resulting closure pressure must therefore reflect changes in muscle fiber lengths, the resulting forces thereof and structural arrangements of urethral components. The circumferential length is directly proportional to the size of the lumen. If the sphincter only consisted of circular fiber, a closure of the lumen would require a contraction to zero length [9]. This is because the length around a closed lumen is zero. Practically, such a contraction cannot be carried out by circular fibers alone. Therefore a central filler substance must be present in order to enable circular fibers to compress the lumen width.
Because muscle fibers are incompressible matter, when they contract, they become thicker, forming a plug in the urethra (Fig. 5).
Relaxed urethra. Longitudinal smooth muscle fibers are relaxed, which allows the pressure of urine to flow through and open the lumen. Contracted urethra. When longitudinal smooth muscle fibers are contracted, the muscle fibers are shortened in length. Located close to the lumen, they end up sealing as a plug with additional pressure from the circular smooth muscle fibers. This ensures proper closure of the lumen. Michelle Alexandra Mistry®
It is hypothesized that a central filler volume determines opening and closing. This central filler volume must consist of all peri-luminal structures: LSM, submucous plexus, submucosa, and epithelium [9]. In the example of a Charriére 24 hematuria cathether, the cross-section is equivalent to 50 mm [2]. If the central filler volume is 1 mm [2] circular fibers are required to stretch seven times their length to enable placement of the catheter. This would cause breakage of the circular fibers owing to overstretching. However, considering a central filler volume of 12 mm2, circular fibers are only required to double to increase the lumen to approximately 50 mm2. This suggests that the central filler volume might determine the sphincter’s ability to open and close as the volume affects the length/force relationship of the circular muscle fibers. This is in a similar manner to blood-filled heart chambers affecting the contraction ability of cardiac muscle fibers (Frank–Starling mechanism). The central filler principle is presumably also used during urethral bulking, where bulking material (briefly) increases the strength of the urethral sphincter [11].
In addition, Rud et al. demonstrated that urethral blood pressure was responsible for approximately one third of the maximum urethral pressure [34]. The submucosal plexus is postulated to be important in a similar manner to blood flow enabling a penile erection. Zinner et al. concluded that both volume and plasticity were vital to urethral continence. The inner soft tissue was studied in a model and confirmed the importance of the viscoelastic properties of each urethral component [10]. Submucosal veins are plastic and conformable. Zinner et al. established three essential factors pertaining to continence: wall tension or external compression, filler material, and inner urethral softness. These all correspond to urethral components [10].
An anatomical study of urethral sphincteric function was sought and elaborated through this literature review. The exact biomechanical interaction needs further functional studies in humans to quantify the contribution of each tissue layer such as the epithelium, vessels, elastin, and connective tissue. Nevertheless, contraction of the LSM increases the central filler volume, resulting in luminal obstruction. This suggests that the LSM might be active throughout continence and relaxed when voiding. Thus, the LSM and CSM functions are synergic as they share equal nerve innervation. This has been confirmed upon stimulation or inhibition, where the resting urethral pressure either increased or decreased, further proving combined smooth muscle activation or inhibition.
Conclusions
Through a structured review of current literature on urethral anatomy and function combined with biomechanical considerations, we believe that the longitudinal smooth muscle is in fact crucial for urethral continence. The contracted longitudinal fibers become a thick plug when contracted and strengthen the sphincter mechanism. The plug mechanism would also improve coaptation of the luminal mucosal folds, with an overall improvement of the sealing mechanism. Further experimental in vivo studies would be needed to verify these conclusions.
Abbreviations
- ACH:
-
Acetylcholine
- CSM:
-
Circular smooth muscle layer
- EUS:
-
External urethral sphincter
- LSM:
-
Longitudinal smooth muscle layer
- NANC:
-
Non-adrenergic and noncholinergic neurotransmitters
- NE:
-
Norepinephrine
- NO:
-
Nitric oxide
- NRI:
-
Noradrenaline reuptake inhibitor
- SUI:
-
Stress urinary incontinence
- USM:
-
Urethral smooth muscle layer
References
Jung J, Ahn HK, Huh Y. Clinical and functional anatomy of the urethral sphincter. Int Neurourol J. 2012;16(3):102–6. https://doi.org/10.5213/inj.2012.16.3.102.
Ashton-Miller JA, DeLancey JOL. Functional anatomy of the female pelvic floor. Ann N Y Acad Sci. 2007;1101:266–96. https://doi.org/10.1196/annals.1389.034.
Kamo I, Kaiho Y, Canon TW, et al. Functional analysis of active urethral closure mechanisms under sneeze induced stress condition in a rat model of birth trauma. J Urol. 2006;176(6):2711–5. https://doi.org/10.1016/j.juro.2006.07.139.
Huisman AB. Aspects on the anatomy of the female urethra with special relation to urinary continence. Contrib Gynecol Obstet. 1983;10:1–31 http://europepmc.org/abstract/MED/6685603.
Tanagho EA, Meyers FH, Smith DR. Urethral resistance: its components and implications. I. Smooth muscle component. Investig Urol. 1969;7(2):136–49 http://europepmc.org/abstract/MED/4309609.
Gosling JA. The structure of the female lower urinary tract and pelvic floor. Urol Clin North Am. 1985;12(2):207–14 http://europepmc.org/abstract/MED/3992742.
Delancey JOL, Ashton-Miller JA. Pathophysiology of adult urinary incontinence. Gastroenterology. 2004;126(1 Suppl 1):S23–32. https://doi.org/10.1053/j.gastro.2003.10.080.
DeLancey JOL, Trowbridge ER, Miller JM, et al. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol. 2008;179(6):2286–90. https://doi.org/10.1016/j.juro.2008.01.098.
Schäfer W. Some biomechanical aspects of continence function. Scand J Urol Nephrol Suppl. 2001;(207):44–60 discussion 106—25. http://europepmc.org/abstract/MED/11409614.
Zinner NR, Sterling AM, Ritter RC. Role of inner urethral softness in urinary continence. Urology. 1980;16(1):115–7. https://doi.org/10.1016/0090-4295(80)90352-0.
Klarskov N, Lose G. Urethral injection therapy: what is the mechanism of action? Neurourol Urodyn. 2008;27(8):789–92. https://doi.org/10.1002/nau.20602.
Saaby ML. The urethral closure function in continent and stress urinary incontinent women assessed by urethral pressure reflectometry. Dan Med J. 2014;61(2):1–15.
Aoki Y, Brown HW, Brubaker L, Cornu JN, Daly JO, Cartwright R. Urinary incontinence in women. Nat Rev Dis Prim 2017;3:17097. doi:https://doi.org/10.1038/nrdp.2017.42.
Lose LG. Simultaneous recording of pressure and cross-sectional area in the female urethra: a study of urethral closure function in healthy and stress incontinent women. Neurourol Urodyn. 1992;11(2):55–89. https://doi.org/10.1002/nau.1930110202.
Oelrich TM. The striated urogenital sphincter muscle in the female. Anat Rec. 1983;205(2):223–32. https://doi.org/10.1002/ar.1092050213.
Elbadawi A, Schenk EA. A new theory of the innervation of bladder musculature. 4. Innervation of the vesicourethral junction and external urethral sphincter. J Urol. 1974;111(5):613–5. https://doi.org/10.1016/s0022-5347(17)60028-4.
DeLancey JO. Functional anatomy of the female lower urinary tract and pelvic floor. Ciba Found Symp. 151:57–69; discussion 69–76.
Perucchini D, DeLancey JOL, Ashton-Miller JA, Peschers U, Kataria T. Age effects on urethral striated muscle. I. Changes in number and diameter of striated muscle fibers in the ventral urethra. Am J Obstet Gynecol. 2002;186(3):351–5. https://doi.org/10.1067/mob.2002.121089.
Berkow SG. The corpus spongiosum of the urethra: its possible role in urinary control and stress incontinence in women. Am J Obstet Gynecol. 1953;65(2):346–51 http://europepmc.org/abstract/MED/13040386.
Yoshimura N, Chancellor MB. Neurophysiology of lower urinary tract function and dysfunction. Rev Urol. 2003;5(Suppl 8):S3–S10 https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/16985987/?tool=EBI.
Canda AE, Cinar MG, Turna B, Sahin MO. Pharmacologic targets on the female urethra. Urol Int. 2008;80(4):341–54. https://doi.org/10.1159/000132690.
Mamas MA, Reynard JM, Brading AF. Nitric oxide and the lower urinary tract: current concepts, future prospects. Urology. 2003;61(6):1079–85. https://doi.org/10.1016/s0090-4295(03)00131-6.
Gosling JA, Dixon JS, Lendon RG. The autonomic innervation of the human male and female bladder neck and proximal urethra. J Urol. 1977;118(2):302–5. https://doi.org/10.1016/s0022-5347(17)57981-1.
Tanagho EA, Smith DR. The anatomy and function of the bladder neck. Br J Urol. 1966;38(1):54–71. https://doi.org/10.1111/j.1464-410x.1966.tb09679.x.
Ek A, Alm P, Andersson KE, Persson CG. Adrenergic and cholinergic nerves of the human urethra and urinary bladder. A histochemical study. Acta Physiol Scand. 1977;99(3):345–52. https://doi.org/10.1111/j.1748-1716.1977.tb10387.x.
Miller KL. Stress urinary incontinence in women: review and update on neurological control. J Womens Health (Larchmt). 2005;14(7):595–608. https://doi.org/10.1089/jwh.2005.14.595.
Nakamura T, Yoshimura M, Shinnick-Gallagher P, Gallagher JP, Akasu T. Alpha 2 and alpha 1-adrenoceptors mediate opposing actions on parasympathetic neurons. Brain Res. 1984;323(2):349–53. https://doi.org/10.1016/0006-8993(84)90312-3.
Förstermann U, Closs EI, Pollock JS, et al. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension. 1994;23(6 Pt 2):1121–31. https://doi.org/10.1161/01.hyp.23.6.1121.
Sjögren C, Andersson KE, Mattiasson A. Effects of vasoactive intestinal polypeptide on isolated urethral and urinary bladder smooth muscle from rabbit and man. J Urol. 1985;133(1):136–40. https://doi.org/10.1016/s0022-5347(17)48822-7.
Yoshimura N, de Groat WC. Neural control of the lower urinary tract. Int J Urol. 1997;4(2):111–25. https://doi.org/10.1111/j.1442-2042.1997.tb00156.x.
Klarskov N, Darekar A, Scholfield D, Whelan L, Lose G. Effect of fesoterodine on urethral closure function in women with stress urinary incontinence assessed by urethral pressure reflectometry. Int Urogynecol J. 2014;25(6):755–60. https://doi.org/10.1007/s00192-013-2269-6.
Reitz A, Haferkamp A, Kyburz T, Knapp PA, Wefer B, Schurch B. The effect of tamsulosin on the resting tone and the contractile behaviour of the female urethra: a functional urodynamic study in healthy women. Eur Urol. 2004;46(2):235–40; discussion 240. https://doi.org/10.1016/j.eururo.2004.04.009.
Klarskov N, Cerneus D, Sawyer W, Newgreen D, van Till O, Lose G. The effect of single oral doses of duloxetine, reboxetine, and midodrine on the urethral pressure in healthy female subjects, using urethral pressure reflectometry. Neurourol Urodyn. 2018;37(1):244–9. https://doi.org/10.1002/nau.23282.
Rud T, Andersson KE, Asmussen M, Hunting A, Ulmsten U. Factors maintaining the intraurethral pressure in women. Investig Urol. 1980;17(4):343–7 http://europepmc.org/abstract/MED/7188694.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mistry, M.A., Klarskov, N., DeLancey, J.O. et al. A structured review on the female urethral anatomy and innervation with an emphasis on the role of the urethral longitudinal smooth muscle. Int Urogynecol J 31, 63–71 (2020). https://doi.org/10.1007/s00192-019-04104-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-019-04104-7