Abstract
Introduction and hypothesis
SDF-1 chemokine enhances tissue regeneration through stem cell chemotaxis, neovascularization and neuronal regeneration. We hypothesized that non-viral delivery of human plasmids that express SDF-1 (pSDF-1) may represent a novel regenerative therapy for stress urinary incontinence (SUI).
Methods
Seventy-six female rats underwent vaginal distention (VD). They were then divided into four groups according to treatment: pSDF-1 (n = 42), sham (n = 30), PBS (n = 1) and luciferase-tagged pSDF-1 (n = 3). Immediately after VD, the pSDF-1 group underwent immediate periurethral injection of pSDF-1, and the sham group received a vehicle injection followed by leak point pressure (LPP) measurement at the 4th, 7th and 14th days. Urogenital tissues were collected for histology. H&E and trichrome slides were analyzed for vascularity and collagen/muscle components of the sphincter. For the luciferase-tagged pSDF-1 group, bioluminescence scans (BLIs) were obtained on the 3rd, 7th and 14th days following injections. Statistical analysis was conducted using ANOVA with post hoc LSD tests. The Mann-Whitney U test was employed to make pair-wise comparisons between the treated and sham groups. We used IBM SPSS, version 22, for statistical analyses.
Results
BLI showed high expression of luciferase-tagged pSDF-1 in the pelvic area over time. VD resulted in a decline of LPP at the 4th day in both groups. The pSDF1-treated group demonstrated accelerated recovery that was significantly higher than that of the sham-treated group at the 7th day (22.64 cmH2O versus 13.99 cmH2O, p < 0.001). Functional improvement persisted until the 14th day (30.51 cmH2O versus 24.11 cmH2O, p = 0.067). Vascularity density in the pSDF-1-treated group was higher than in the sham group at the 7th and 14th days (p < 0.05). The muscle density/sphincter area increased significantly from the 4th to 14th day only in the pSDF-1 group.
Conclusions
Periurethral injection of pSDF-1 after simulated childbirth accelerated the recovery of continence and regeneration of the urethral sphincter in a rat SUI model. This intervention can potentially be translated to the treatment of post-partum urinary incontinence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress urinary incontinence (SUI) is defined as unintentional loss of urine on exertion, sneezing or coughing. It is estimated that 40% of childbearing women develop SUI [1]. The prevalence of incontinence in US women is expected to increase from 18 million in 2010 to 28 million in 2050 [2]. SUI may begin soon after delivery or years later because of tissue and nerve damage during delivery [1]. There are multiple options for treatment of SUI varying from noninvasive to invasive procedures; however, few effective options are available prior to surgery. Synthetic mid-urethral slings are the most popular and effective modality of therapy. However, with the emergence of mesh-related concerns, a strong interest in regenerative medicine as an alternative treatment for SUI is surfacing [3]. This is exemplified in a recent recommendation of the European Commission’s Scientific Committee on Emerging Health Risks report (SCENIHR) on the use of surgical meshes, encouraging regenerative technologies for SUI treatment [4].
Urethral injectable materials may offer an acceptable option for young women because of the low risk of complications [5]. Existing injectable therapies are either pharmacologic or cell based. Pharmacologic injectables are bulking agents such as calcium hydroxylapatite and Macroplastique [6]. Pharmacologic injectables have been in clinical use for several years; however, their efficacy and utility have been limited [6]. While cell-based injectable therapy is promising, several issues remain that need to be addressed before the therapy becomes mainstream clinical practice. Some of the issues relate to the cell type, therapeutic approach and timing, cost effectiveness and FDA regulations [7]. Cell-based therapy with the use of autologous muscle-derived skeletal myoblasts is offered in phase I/II clinical trials as a regenerative therapy. The results showed safety and potential efficacy at 12 months’ follow-up [8]. Two other alternative regenerative injectable options include the use of either conditioned media products of stem cells (secretome) [9] or a single chemokine injection such as stromal-derived factor-1 (SDF-1).
SDF-1, also known as CXC motif chemokine 12 (CXCL12), is a chemokine expressed in many tissues and cells. SDF-1 is mainly released from mesenchymal stem cells (MSCs) and damaged tissues in the regenerative microenvironment [10]. SDF-1 is involved in immunomodulation, angiogenesis and chemo-attraction of stem cells into injury sites. SDF-1 is a paracrine component of MSCs that also improves the efficiency of MSCs’ other paracrine components (positive feedback) that promote healing and tissue repair [10, 11]. Researchers have harnessed the chemo-attractant characteristic of SDF-1 to develop protein/plasmid-based cell therapeutics for clinical applications. Non-viral human SDF-1 plasmid (pSDF-1) has shown safety, an increase in vascularity and improved patient status in ischemic heart and vascular diseases [12, 13].
We hypothesized that non-viral delivery of human DNA plasmids that express SDF-1 at the periurethral tissue immediately after childbirth injury may improve continence function through amelioration of injury impact on the host tissue continence mechanism, improving vascularity and potentially decreasing scar formation. pSDF-1 may represent a novel pharmaceutical regenerative injectable therapy for SUI without the limitations of cell-based therapies.
The vaginal distension (VD) model is an established reproducible model of childbirth injury [14]. VD results in direct and stretch-induced trauma to the urethra, vagina and pelvic floor [15]. The VD model is associated with ischemic insult and nerve injury. This injury generates a model with measurable continence dysfunction, which has been used as a preclinical model to explore the potential of regenerative therapies for SUI [16].
This study aimed to assess the effect of pSDF-1 peri-urethral injection on continence outcomes following VD as measured by assessment of leak point pressure (LPP) and to associate the changes with histology over time. In vivo proof of LPP restoration and histologic proof of regeneration will support the use of pSDF-1 as a potential injectable therapy for SUI applications.
Materials and methods
Seventy-six Sprague-Dawley rats, 2 months old, weighing 225–250 g, were randomly divided into 2 experimental groups, 4 rats for evaluation of expression of backbone plasmid expressing luciferase in the pelvis over time and 72 rats for functional LPP evaluation after pSDF-1 (JVS-100, Juventas Therapeutics, Cleveland, OH) treatment and histologic correlation. Animal care and experimental protocols were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University (IACUC#2013-0081) in compliance with the Animal Welfare Act and the guide for the care and use of laboratory animals.
All 76 rats underwent VD under intraperitoneal (IP) ketamine (75 mg/kg)/xylazine (10 mg/kg)-induced anesthesia. VD was performed as described before [14, 16]. The vagina was accommodated to a larger capacity by serial lubricated bougie dilators (24–32 Fr). A sterile modified 10-Fr Foley catheter with the tip cut off was inflated gradually with 3 ml water inside the vagina and secured for 4 h to mimic childbirth injury.
To verify the uptake of the plasmid upon peri-urethral injection, we injected 150 ul luciferase plasmid with the same backbone as pSDF-1 into three rats following VD. Another one rat was injected with an equal volume of phosphate-buffered saline (PBS) as a control. For imaging, rats were anesthetized with inhalation isoflurane (flow rate 3%) after VD and pSDF-1 or PBS peri-urethral injection. Luciferase-expression was determined using an IVIS-200 bioluminescence (BLI) scanner (PerkinElmer, Waltham, MA, USA). Prior to imaging (15 min), animals received 0.8 ml injection of d-luciferin (50 mg/ml dissolved in 1 × PBS; filter sterilized). BLI data were obtained at the 3rd, 7th and 14th days following VD.
Seventy-two rats were used to examine the efficacy of pSDF-1 in restoring LPP. pSDF-1 and dextrose 5% were injected immediately following VD; 150 ul of pSDF-1 (2 mg/ml) was delivered through the vaginal wall to the connective tissue surrounding the urethra into 42 rats (Fig. 2a). The doses of pSDF-1 were roughly estimated based on organ size and the doses used in a porcine myocardial ischemia model [17]. Thirty rats were sham-injected with vehicle (dextrose 5%) alone. Rats survived until three time points: 4, 7 and 14 days following VD. At each time point, 14 rats from the pSDF1-treated group and 10 rats from the vehicle-treated group were randomly selected for LPP testing under urethane anesthesia (1.2 g/kg; IP).
LPP was performed as described before [16] through a suprapubic bladder catheter. A 26-gauge tube was placed into the dome of the bladder. The distal end of the tubing was then connected to a syringe pump to deliver 0.9% saline solution at a rate of 5 ml/h during LPP testing. Leaks were induced by applying gentle pressure on the mid-full bladder. We observed the urethral meatus for leakage. At least ten leak pressure measurements were recorded per animal during the induced leaks. Immediately after final measurement, urogenital tissues (bladder, vaginal and urethra) were harvested, and the animals were subsequently killed.
Specimens were prepared into serial sections obtained from the mid-urethra for H&E and Masson’s trichrome. Masson’s trichrome stain demonstrates the urethral striated muscles in dark red and collagen in blue. Quantitative histology of the urethra was performed using the Photoshop and ImageJ software programs. In each cross-section, urethras were morphometrically evaluated. Trichrome slides were quantified for collagen and muscle ratios at the area of the inner longitudinal muscle of the sphincter (Fig. 4a). H&E-stained mid-urethral images were quantified for vascular density in pixels. We divided the sphincter complex depending on the relation to the vagina into the vaginal and anterior halves. We reported the vascularity values of the vaginal half.
Blinded LPP measurement and histologic analysis were performed based on the animal code. The level of significance between time points was determined using analysis of variance (ANOVA) with post hoc Fisher’s LSD test. Mann-Whitney U test was employed to make post hoc pair-wise comparison between the pSDF-1-treated versus sham-treated groups. p < 0.05 was considered statistically significant. We used IBM SPSS v.22 software for statistical analyses.
Results
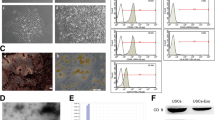
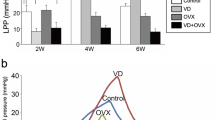
Bioluminescence scans showed high signal expression for the treatment group (pSDF-1-luciferase tagged) versus sham treatment group (PBS) in the pelvic area. The expressions were persistent for two rats up to the 14th day and for one rat up to the 7th day (Fig. 1). Six rats in the pSDF1-treated group (two rats/time point) and one in the sham-treated group did not undergo LPP measurement because of either an incompetent suprapubic tube or dying. The LPP reached a nadir at the 4th day (as expected) in both groups after VD (Fig. 2b). The pSDF1-treated group demonstrated an accelerated recovery where the LPP median incremented to a level significantly different from that of the sham-treated group at the 7th day, 22.64 cmH2O (IQR = 26.87–18.72) versus 13.99 cmH2O (IQR = 14.72–13.02) respectively, p < 0.001. The improvement in LPP continued in both groups at the 14th day, with the statistical difference approaching significance, 30.51 cmH2O (IQR = 42.26–27.38) versus 24.11 cmH2O (IQR = 27.89–22.82), respectively, p = 0.067. The mean LPP in control rats without surgery or injection was 28.56 mmHg in our previous study [16].
Upper images show luciferase expression determined using Xenogen IVIS® bioluminescence (PerkinElmer, Waltham, MA, USA). Rats 1, 2 and 3 were locally treated with luciferase backbone simulating pSDF-1 after VD. The control rat received PBS injection after VD. Lower curves show rat 1 presented persistent high signal till the last time point. Rat 2 presented high expression till the 7th day and then the signal faded by the 14th day. Rat 3 presented high signal till the last time point with an upward-sloping curve
a Rat vagina exposed by retractors. Dashed lines represent the urethra. The injection site was superficially through the vagina on either side of the urethra (see x on circle). b Box-and-whisker plot shows leak point pressure changes in the pSDF-1-treated group versus dextrose 5%-treated group. Vehicle-treated group median LPP and interquartile range (IQR) were 15.66 cmH2O (IQR = 17.16–13.92), 13.99 cmH2O (IQR = 14.73–13.02) and 24.11 cmH2O (IQR = 27.89–22.82) for 4-, 7- and 14-day time points, respectively. The pSDF-1-treated group median LPP and IQR were 16.83 cmH2O (IQR = 19.88–16.03), 22.64 cmH2O (IQR = 26.87–18.72) and 30.51 cmH2O (IQR = 42.26–27.38) for the 4-, 7- and 14-day time points, respectively. The p value of the pSDF-1-treated versus vehicle-treated rats per time point were 0.167, < 0.001 and 0.074 for the 4-, 7- and 14-day time points, respectively. Connecting bars refer to significant differences between groups (p < 0.05). Leak point pressure measurements were conducted for N#14 animals per time point for the pSDF-1-treated groups and for N#10 animals per time point for the sham-treated groups
Qualitative histologic evaluation of N#5 animals per group revealed regular-shaped cells and nuclei with no abnormal cellular differentiation in response to pSDF-1. Quantitative image analysis for vascular density in the urethra revealed higher vascular density in the pSDF1-treated animals at the 7th and 14th days post-treatment compared with the sham-treated groups. The p value was 0.032 and 0.004, respectively (Fig. 3a). Interestingly, we noticed higher vascularity more pronounced at the injection site (right/left of the urethra) in the pSDF1-treated group slides (Fig. 3b). Mean of the muscle density/sphincter area increased significantly from the 4th day to 14th day for the pSDF1-treated group (62.46% ± 5.4% vs. 72.48% ± 2.7%, respectively, p = 0.03), but not for the sham-treated groups. Muscle density tended to be higher than in the sham-treated animals at the 7th and 14th days. On the other hand, collagen density values demonstrated a downward-trending pattern in the pSDF1-treated animals, but this was not statistically different from the sham-treated group (Fig. 4b).
a Mean area occupied by blood vessels in pixels over time at the vaginal half of the urethra. Error bars represent standard deviation. pSDF-1-treated animals had higher vascularity values than sham. pSDF-1-treated animals’ mean vascularity peaked at day 7 (15,529 ± 2634, 22,641 ± 5364 and 17,199 ± 3355 pixels for day-4, -7 and -14 groups, respectively), while the sham-treated (dextrose 5%) group exhibited a downward-sloping curve over time (13,512 ± 7431, 9692 ± 4684 and 5658 ± 2788 pixels for the 4-, 7- and 14-day groups, respectively). One-way analysis of variance between treatment groups revealed significant differences at the day-7 and -14 time points for SDF-1 versus sham (p = 0.625, 0.032 and 0.004 for the day 4-, 7- and -14 groups, respectively). *Significant difference, p < 0.05. b H&E-stained mid-urethral slides representing the urethra, vagina and vascularity around the urethra. a A slide from the pSDF-1-treated group harvested 7 days after injection. Injection was done to the right of the urethra as marked. b A slide from the pSDF-1-treated group harvested 14 days after injection. The injection was done to the left of the urethra as marked. c A slide from the dextrose 5% vehicle-treated group harvested 14 days after injection. *Injection site
a Collagen quantification methodology; 10× histologic picture of a Masson’s trichrome mid-urethral section. The lower magnification picture shows the layers of the urethral sphincter complex. Collagen appears blue in Masson’s trichrome. b We separated the urethra into two halves, vaginal and urethral. We did super selection of the collagen areas at the inner muscle layer and then stained it with bright blue for easy subtraction and analysis. We used Adobe Photoshop CC selection tools. c The subtracted collagen. We analyzed the subtracted images using Image J software. b Red bars show means of the muscle ratio per skeletal muscle area of the mid-urethral sphincter. Blue bars show means of the collagen component per skeletal muscle area of the mid-urethral sphincter. Error bars represent standard deviation. For pSDF-1-treated animals, muscle component (mean ± standard deviation percentage) of the inner part of the sphincter complex was: 62.46 ± 5.39%, 64.44 ± 10.65% and 72.48 ± 2.74% for the day-4, -7 and -14 groups, respectively. Vehicle (dextrose 5%)-treated animals; muscle component (mean ± standard deviation percentage) of the inner part of the sphincter complex was: 62.42 ± 5.14%, 62.75 ± 7.32% and 68.11 ± 9.63% for the day-4, -7 and -14 groups, respectively. Connecting bars refer to significant differences between groups (p = 0.03)
Discussion
Periurethral-injection of pSDF-1 after simulated childbirth promoted the recovery of continence, and this was associated with increased vascular density in the urethral sphincter area, which coincided with the timing of continence recovery. There was a pattern of increased muscle density in the pSDF-1-treated group with a decrease in the collagen content of the urethra over the time points studied, which was significant in the former but not statistically significant in the latter.
To the best of our knowledge, we are the first to explore the potential of pSDF-1, a non-viral gene therapy that expresses SDF-1, as an injectable treatment for incontinence. We propose that injection of pSDF-1 immediately after injury releases SDF-1 chemokine, which may ameliorate the injury impact on the host tissue continence mechanism or promote healing by improving vascularity and potentially decreasing scar formation, thus leading to increased functional outcome seen at 7 days post-treatment.
In our study, BLI demonstrated that the plasmid backbone was taken up by the peri-urethral tissues (injury and injection site) as evidenced by an increased luciferase expression signal over time (Fig. 1). This observation is concurrent with the proven upregulation of SDF-1 at the injury site and consistent with injection of this plasmid in cardiac tissues demonstrating expression [17]. Delivery of non-viral human plasmid has shown comparable expression to viral-promoted transfection [18]. In another experiment, we tracked the SDF-1 expression in urogenital tissue after pSDF-1 injection up to 2 weeks. SDF-1 presented a constant high expression versus controls (data not shown).
VD resulted in a decrease in LPP that reached a nadir at day 4, as reported before. pSDF-1 injection at the time of VD injury resulted in an accelerated recovery of continence function, evident by the significant increase in LPP in the treated group at day 7. pSDF-1-treated animals tended to have a higher LPP than the sham group at all time points, comparable to a previous observation of the LPP curve after MSC treatment following injury in a rat model of VD [16]. LPP reduction at day 4 is expected because of the acute insult of injury.
The improvement in functional recovery was seen to be associated with higher vascularity and a trend to higher muscle density in the sphincter in the pSDF1-treated group compared with the sham-treated group over time. Our results of vascular density at the urethral half facing the vagina (site of injection and injury) may support the vascular regenerative effect of pSDF-1.
Evaluation of the muscle and collagen density revealed higher muscle density and high vascularity per sphincter complex in the pSDF-1-treated group. These findings are concurrent with the chronic effect results reported 6 months after injury and treatment in a monkey model [19]. This group explored the chronic effect of CXCL12 chemokine injection into a limited number of pudendal nerve injuries in a monkey model versus skeletal muscle precursor cell (skMPC) injection. Chemokine-treated animals, but not skMPC injections, led to higher maximum urethral pressure and a significant increase of muscle density in the sphincter area [19]. CXCL12 chemokine was tested at the time of induction of chronic injury in rodents with a high safety profile and superiority over skMPCs [20].
Our future overview of this treatment modality might be considered a tertiary prevention option for high-risk postpartum incontinence patients. High-risk pregnancies [21], late pregnancy incontinence [22] and novel biomarkers [23] may predict the progression of SUI. Early treatment may help healing before irreversible tissue damage and fibrosis [24].
SDF-1 is a component of cytokine-cytokine receptor interaction, the TGF-B signaling pathway and VEGF signaling pathway. Cytokine-cytokine receptor interaction signals through the Jak-STAT pathway, G protein-alpha and G protein-beta. Jak-STAT and MAPK signaling pathways control cellular DNA for cytokine production, cellular growth and differentiation, and cell survival and apoptosis. G protein-beta signaling proceeds through Rac, PAK1 and cdc42/WASP to regulate the actin cytoskeleton production and leukocyte trans-endothelial migration. The downstream effect is to control chemotaxis and cell shape changes [25]. SDF-1 enables stem cell migration into the injury site [11]. Stem cells are theoretically responsible for tissue regeneration. SDF-1 induced angiogenesis and accelerated revascularization of ischemic heart, which is a muscular organ [13].
Childbirth injury leads to neuronal crushing [26]. Muscle denervation leads to muscle atrophy [27]. SDF-1/CXCR4 had a role in chemo-attraction of sympathetic neural crest cells. SDF-1 as well as stem cells was tested for induction of recovery of injured cochlear and optic neurons [28].
Limitations of the study included the animal model’s spontaneous recovery. The rat VD model limited the experiment timeline to 2 weeks. While post-partum SUI is prevalent, occurring in 30% [29], it is characterized by spontaneous recovery in the majority of women. Having said that, women who do not recover continence within 3 months after delivery are at the highest risk for development of SUI later in life. Thus, such therapy would be ideal for the high-risk group of post-partum women. Our study provides promising proof-of-concept data in an acute SUI model; however, it would not apply to established SUIs that manifest remotely from the time of the index injury. Collaborative preclinical research with chronic models of SUI and treatment with pSDF-1 at a time frame remote from the injury would be of great value and will be the focus of future studies. Moreover, future comprehensive research should explore the mechanism, appropriate dose, location of treatment and long-term safety of pSDF-1, although it has demonstrated a strong safety profile to date in phase 1 and 2 clinical trials [13, 30].
Conclusions
pSDF-1 injection immediately after simulated childbirth injury accelerated the recovery of continence in a rat animal model. Our data demonstrate pSDF-1 induced increased the amount of sphincter muscle and significantly higher vascular regeneration. This is consistent with previous reports associating SDF-1 upregulation with stem cell homing and tissue regeneration. pSDF-1 seems to be a potential novel regenerative off-shelf therapy for predicted SUI after delivery. However, further research is needed to explain the mechanism of early improvement, safety and long-term efficacy before translation to clinical trials.
Abbreviations
- CXCL12:
-
CXC motif chemokine 12
- IP:
-
intraperitoneal
- IACUC:
-
institutional animal care and use committee
- IQR:
-
interquartile range
- SLPP:
-
leak point pressure
- MSCs:
-
mesenchymal stem cells
- PBS:
-
phosphate-buffered saline
- pSDF-1:
-
plasmid of SDF-1
- SDF-1:
-
stromal-derived factor-1
- skMPC:
-
skeletal muscle precursor cell
- SUI:
-
stress urinary incontinence
- VD:
-
vaginal distention
References
Cheater FM, Castleden CM. Epidemiology and classification of urinary incontinence. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(2):183–205.
Ilie CP, Chancellor MB. Female urology-future and present. Rev Urol. 2010;12(2-3):e154–6.
Chapin K, Khalifa A, Mbimba T, McClellan P, Anderson J, Novitsky Y, et al. In vivo biocompatibility and time-dependent changes in mechanical properties of woven collagen meshes: a comparison to xenograft and synthetic mid-urethral sling materials. J Biomed Mater Res B Appl Biomater. 2018. https://doi.org/10.1002/jbm.b.34138.
Chapple CR, Cruz F, Deffieux X, Milani AL, Arlandis S, Artibani W, et al. Consensus statement of the European Urology Association and the European Urogynaecological Association on the use of implanted materials for treating pelvic organ prolapse and stress urinary incontinence. Eur Urol. 2017;72(3):424–31. https://doi.org/10.1016/j.eururo.2017.03.048.
Robinson D, Castro-Diaz D, Giarenis I, Toozs-Hobson P, Anding R, Burton C, et al. What is the best surgical intervention for stress urinary incontinence in the very young and very old? An international consultation on incontinence research society update. Int Urogynecol J. 2015;26(11):1599–604. https://doi.org/10.1007/s00192-015-2783-9.
Kirchin V, Page T, Keegan PE, Atiemo KO, Cody JD, McClinton S, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:Cd003881. https://doi.org/10.1002/14651858.CD003881.pub4.
Hart ML, Izeta A, Herrera-Imbroda B, Amend B, Brinchmann JE. Cell therapy for stress urinary incontinence. Tissue Eng B Rev. 2015;21(4):365–76. https://doi.org/10.1089/ten.TEB.2014.0627.
Peters KM, Dmochowski RR, Carr LK, Robert M, Kaufman MR, Sirls LT, et al. Autologous muscle derived cells for treatment of stress urinary incontinence in women. J Urol. 2014;192(2):469–76. https://doi.org/10.1016/j.juro.2014.02.047.
Tran C, Damaser MS. Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Adv Drug Deliv Rev. 2015;82-83:1–11. https://doi.org/10.1016/j.addr.2014.10.007.
Luo Q, Zhang B, Kuang D, Song G. Role of stromal-derived factor-1 in mesenchymal stem cell paracrine-mediated tissue repair. Curr Stem Cell Res Ther. 2016;11(7):585–92.
Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther. 2011;11(2):189–97. https://doi.org/10.1517/14712598.2011.546338.
Sundararaman S, Miller TJ, Pastore JM, Kiedrowski M, Aras R, Penn MS. Plasmid-based transient human stromal cell-derived factor-1 gene transfer improves cardiac function in chronic heart failure. Gene Ther. 2011;18(9):867–73. https://doi.org/10.1038/gt.2011.18.
Penn MS, Mendelsohn FO, Schaer GL, Sherman W, Farr M, Pastore J, et al. An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circ Res. 2013;112(5):816–25. https://doi.org/10.1161/circresaha.111.300440.
Boncher N, Vricella G, Kavran M, Xiao N, Hijaz A. Setting a new standard: updating the vaginal distention translational model for stress urinary incontinence. Neurourol Urodyn. 2012;31(1):190–4. https://doi.org/10.1002/nau.21168.
Wood HM, Kuang M, Woo L, Hijaz A, Butler RS, Penn M, et al. Cytokine expression after vaginal distention of different durations in virgin Sprague-Dawley rats. J Urol. 2008;180(2):753–9. https://doi.org/10.1016/j.juro.2008.03.182.
Sadeghi Z, Isariyawongse J, Kavran M, Izgi K, Marini G, Molter J, et al. Mesenchymal stem cell therapy in a rat model of birth-trauma injury: functional improvements and biodistribution. Int Urogynecol J. 2015. https://doi.org/10.1007/s00192-015-2831-5.
Penn MS, Pastore J, Miller T, Aras R. SDF-1 in myocardial repair. Gene Ther. 2012;19:583. https://doi.org/10.1038/gt.2012.32.
Abruzzese RV, Godin D, Burcin M, Mehta V, French M, Li Y, et al. Ligand-dependent regulation of plasmid-based transgene expression in vivo. Hum Gene Ther. 1999;10(9):1499–507. https://doi.org/10.1089/10430349950017833.
Williams JK, Dean A, Badra S, Lankford S, Poppante K, Badlani G, et al. Cell versus chemokine therapy in a nonhuman primate model of chronic intrinsic urinary sphincter deficiency. J Urol. 2016;196(6):1809–15. https://doi.org/10.1016/j.juro.2016.05.106.
Koudy Williams J, Dean A, Lankford S, Andersson KE. Efficacy and initial safety profile of CXCL12 treatment in a rodent model of urinary sphincter deficiency. Stem Cells Transl Med. 2017;6(8):1740–6. https://doi.org/10.1002/sctm.16-0497.
Altman D, Ekstrom A, Gustafsson C, Lopez A, Falconer C, Zetterstrom J. Risk of urinary incontinence after childbirth: a 10-year prospective cohort study. Obstet Gynecol. 2006;108(4):873–8. https://doi.org/10.1097/01.AOG.0000233172.96153.ad.
Johannessen HH, Stafne SN, Falk RS, Stordahl A, Wibe A, Morkved S. Prevalence and predictors of double incontinence 1 year after first delivery. Int Urogynecol J. 2018. https://doi.org/10.1007/s00192-018-3577-7.
Woo LL, Hijaz A, Kuang M, Penn MS, Damaser MS, Rackley RR. Over expression of stem cell homing cytokines in urogenital organs following vaginal distention. J Urol. 2007;177(4):1568–72. https://doi.org/10.1016/j.juro.2006.11.047.
Kaplani K, Koutsi S, Armenis V, Skondra FG, Karantzelis N, Tsaniras SC, et al. Wound healing related agents: ongoing research and perspectives. Adv Drug Deliv Rev. 2018. https://doi.org/10.1016/j.addr.2018.02.007.
Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45(D1):D353–d361. https://doi.org/10.1093/nar/gkw1092.
Palacios JL, Juarez M, Moran C, Xelhuantzi N, Damaser MS, Cruz Y. Neuroanatomic and behavioral correlates of urinary dysfunction induced by vaginal distension in rats. Am J Physiol Ren Physiol. 2016;310(10):F1065–73. https://doi.org/10.1152/ajprenal.00417.2015.
Glass DJ. Molecular mechanisms modulating muscle mass. Trends Mol Med. 2003;9(8):344–50.
Zhang PZ, Cao XS, Jiang XW, Wang J, Liang PF, Wang SJ, et al. Acoustical stimulus changes the expression of stromal cell-derived factor-1 in the spiral ganglion neurons of the rat cochlea. Neurosci Lett. 2014;561:140–5. https://doi.org/10.1016/j.neulet.2013.12.061.
Viktrup L, Rortveit G, Lose G. Risk of stress urinary incontinence twelve years after the first pregnancy and delivery. Obstet Gynecol. 2006;108(2):248–54. https://doi.org/10.1097/01.AOG.0000226860.01127.0e.
Chung ES, Miller L, Patel AN, Anderson RD, Mendelsohn FO, Traverse J, et al. Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized phase II trial. Eur Heart J. 2015;36(33):2228–38. https://doi.org/10.1093/eurheartj/ehv254.
Funding
Juventus Therapeutics, Inc. provided the pSDF-1 for the experiments.
Preliminary data of part of this work were presented as a moderated poster at the American Urological Association meeting, San Diego, CA, 2016. (Reference: Khalifa A, Mahran A, Kavran M, Woda J, Penn M, Hijaz A (2016) MP65-01 stromal cell derived factor-1 accelerates recovery of continence in rat model of vaginal distension injury. J Urol 195 (4):e864. doi:https://doi.org/10.1016/j.juro.2016.02.1212).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Ahmad O. Khalifa: no conflict of interest; Michael Kavran: no conflict of interest; Amr Mahran: no conflict of interest; Ilaha Isali: no conflict of interest; Juliana Woda: former commercial developer of SDF-1 plasmid technology at Juventas Therapeutics; Chris A. Flask: no conflict of interest; Marc S. Penn: no conflict of interest; Adonis Hijaz: speaker for Astellas Pharma.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khalifa, A.O., Kavran, M., Mahran, A. et al. Stromal derived factor-1 plasmid as a novel injection for treatment of stress urinary incontinence in a rat model. Int Urogynecol J 31, 107–115 (2020). https://doi.org/10.1007/s00192-019-03867-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-019-03867-3