Abstract
Introduction and hypothesis
Chronic inflammatory conditions seem to be a shared characteristic in patients with interstitial cystitis (IC) and overactive bladder (OAB). Thus, we measured 40 inflammatory urine markers in IC patients with or without Hunner’s lesions (HIC and NHIC respectively) and OAB patients.
Methods
Urine was collected from consecutive HIC patients, NHIC patients, and age and gender-matched OAB patients with no history of IC, recurrent urinary tract infection or bladder cancer. The diagnosis of IC was based on the Asian IC guideline criteria. A representative 40 inflammatory growth factors, cytokines, and chemokines in urine were measured using a MILLIPLEX immunoassay kit. Statistical differences in these markers among the groups were determined by nonparametric ANOVA followed by multiple comparison test. The diagnostic efficiency of these markers was measured using receiver operating characteristic analysis.
Results
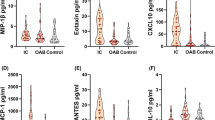
Vascular endothelial growth factor (VEGF), interleukin-1α (IL-1α), IL-6, and chemokines including CCL2, CCL5, CXCL1, CXCL8, and CXCL10 were significantly increased in HIC (n = 30) and NHIC (n = 30) patients compared with OAB (n = 28) patients. The significant increases in CXCL8 and CXCL10 were also found in HIC patients compared with NHIC patients. However, there were no significant differences in the other urine markers among the groups. Area under the curves for VEGF, CXCL10, CXCL8, IL-1α, CCL5, CCL2, IL-6, and CXCL1 to detect IC in these patients were 0.87, 0.86, 0.81, 0.80, 0.80, 0.71, 0.66, and 0.50 respectively.
Conclusions
The increases in angiogenesis-associated proteins such as VEGF and CXCL10 may be pathophysiologically important for the development of IC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the recently reported Asian guideline, interstitial cystitis (IC) is defined as a chronic bladder inflammatory disease diagnosed by three factors [1]:
-
1.
A characteristic complex of lower urinary tract symptoms (LUTS) such as discomfort, pressure or pain in the bladder usually associated with urinary frequency and nocturia
-
2.
Bladder pathology including Hunner’s type IC (HIC) with Hunner’s lesions and non-Hunner’s type IC (NHIC) with mucosal bleeding after hydrodistention in the absence of Hunner’s lesions
-
3.
Exclusions of confusable diseases
A Hunner’s lesion is not considered to be an ulcer, but rather a distinctive inflammatory lesion presenting as a reddened mucosal area with fragile microvessels radiating toward a central scar [2]. Angiogenesis of the bladder seen in IC patients has also been recognized as an important characteristic in other chronic inflammatory diseases, such as diabetic retinopathy, atherosclerosis, and inflammatory bowel disease [3, 4]. Recent studies have suggested that smooth muscle cell function of airway, bladder, and vascular tissues is not limited to contraction, but rather extended to be a potential source of cytokines and chemokines that can act to alter contractility or participate in and coordinate the inflammatory response [5, 6]. Therefore, smooth muscle cells may be crucial for the development of chronic inflammatory diseases such as bronchitis, inflammatory bowel disease, and IC.
Overactive bladder (OAB) is characterized by LUTS of urinary frequency and urgency with or without urinary incontinence [7, 8]. Recent studies have noted the signs of inflammation in biopsied bladder tissues and urine specimens from OAB patients in the absence of urinary tract infection [9, 10]. Overlapping symptoms of IC and OAB often complicate the diagnosis and suitable treatments. In addition, the chronic inflammatory condition seems to be a shared characteristic in patients with IC and OAB. Thus, to investigate the contributing factors of chronic bladder inflammation in IC patients, we measured 40 representative inflammatory urine markers, including growth factors, cytokines, and chemokines from consecutive patients diagnosed as HIC or NHIC after hydrodistention, in addition to age- and gender-matched OAB patients.
Materials and methods
Study design
The protocol for this retrospective study was approved by Jikei University Institutional Review Board. Mid-stream urine was collected from consecutive HIC patients and NHIC patients at least 3 months after the last hydrodistention, and age- and gender-matched OAB patients. According to the Asian guideline for IC [1], IC patients were divided into HIC, NHIC with mucosal bleeding after hydrodistention, or no bladder pathology during hydrodistention (hypersensitive bladder). In the present study, all IC patients underwent hydrodistention and were diagnosed as HIC or NHIC before urine collection. In addition, all OAB patients were diagnosed using OAB symptom score questionnaires [11] and exclusion criteria included no history of IC, recurrent urinary tract infection or bladder cancer before urine collection. Mid-stream urine was collected and immediately centrifuged at 1,500 rpm for 5 min and the supernatant material was frozen at −80°C before analysis. All participants completed the O’Leary–Sant score, including symptom indexes (OSSI) and problem indexes (OSPI), and visual analog scale (VAS) pain scores.
Multiplex analysis
We assayed 40 representative inflammatory cytokines, chemokines, and growth factors using a commercially available immunoassay kit (MILLIPLEX MAP Human Cytokine/Chemokine multiplex kit; Millipore, Billerica, MA, USA) according to the manufacturer’s instructions, as previously described [10]. Three groups of proteins were evaluated in the present study:
-
1.
Growth factors including epidermal growth factor (EGF), fibroblast growth factor-2 (FGF-2), transforming growth factor-α (TGF-α), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), interferon α (IFNα), IFNγ, platelet-derived growth factor-AA (PDGF-AA), PDGF-BB, soluble CD40 ligand (sCD40L), fms-like tyrosine kinase-3 ligand (FLT-3L), tumor necrosis factor α (TNFα), TNFβ, and vascular endothelial growth factor (VEGF).
-
2.
Cytokines including interleukin-1α (IL-1α), IL-1β, IL-1 receptor antagonist (IL-1Ra), IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12p40, IL-13, IL-15, and IL-17.
-
3.
Chemokines including monocyte chemotactic protein-1 (MCP-1/CCL2), macrophage inflammatory protein-1α (MIP-1α/CCL3), MIP-1β/CCL4, regulated upon activation, normal T cell expressed and secreted (RANTES/CCL5), MCP-3/CCL7, EOTAXIN/CCL11, macrophage-derived chemokine (MDC/CCL22), growth-related oncogene (GRO/CXCL1), IL-8/CXCL8, interferon-inducible protein-10, (IP-10/CXCL10).
Statistical analysis
All data were represented as mean values ± standard deviation (SD) of the mean. Statistical differences in median levels of growth factors, cytokines, and chemokines, in addition to age, OSSI, OSPI, and VAS scores among the three groups were determined by Kruskal–Wallis nonparametric ANOVA followed by Dunn’s multiple comparison test. Sex differences among the groups were examined using Chi-squared test. The diagnostic efficiency of these urine markers was measured using receiver-operating characteristic analysis. P < 0.05 was regarded as statistically significant.
Results
Patient characteristics are shown in Table 1. There were no significant age and sex differences among the three groups. OSSI, OSPI, and VAS scores were significantly increased in HIC (n = 30) and NHIC (n = 30) patients compared with OAB (n = 28) patients, although there were no significant differences in OSSI, OSPI, and VAS scores between HIC and NHIC patients.
All 40 inflammatory urine marker levels are shown in Table 2. VEGF (Fig. 1a), IL-1α (Fig. 1b), IL-6 (Fig. 1c), CCL2 (Fig. 1d), CCL5 (Fig. 1e), CXCL1 (Fig. 1f), CXCL8 (Fig. 1g), and CXCL10 (Fig. 1h) were significantly increased in both HIC and NHIC patients compared with OAB patients, whereas the significant increases in CXCL8 (Fig. 1g) and CXCL10 (Fig. 1h) were also detected in HIC patients compared with NHIC patients. However, there were no significant differences in the other inflammatory urine markers among the groups.
Scatter plots of urine a VEGF-A (A), b IL-1α, c IL-6, d CCL2, e CCL5, f CXCL1, g CXCL8 and h CXCL10 levels. VEGF-A, IL-1α, IL-6, and chemokines, including CCL2, CCL5, CXCL1, CXCL8, and CXCL10 were significantly increased in Hunner’s type interstitial cystitis (HIC) and non-Hunner’s type interstitial cystitis (NHIC) patients compared with OAB patients. The significant increases in CXCL8 and CXCL10 were also found in HIC patients compared with NHIC patients. *p < 0.05, **p < 0.01 vs OAB, †p < 0.05, ††p < 0.01 vs NHIC
According to the receiver-operating characteristic analysis of these significantly increased urine markers in IC patients compared with OAB patients, areas under the curve (AUCs) for VEGF, CXCL10, CXCL8, IL-1α, CCL5, CCL2, IL-6, and CXCL1 were 0.87, 0.86, 0.81, 0.80, 0.80, 0.71, 0.66, and 0.50 respectively. In addition, the cutoff values using Youden’s index of VEGF at 23.8 pg/ml, CXCL10 at 9.6 pg/ml, CXCL8 at 11.2 pg/ml, IL-1α at 1.7 pg/ml, and CCL5 at 9.8 pg/ml showed sensitivity of 76.7%, 83.1%, 67.9%, 68.3%, and 70.0%, and specificity of 82.1%, 78.6%, 81.5%, 77.8%, and 77.8% respectively (Table 3).
Discussion
Overlapping symptoms of IC and OAB often complicate diagnosis and suitable treatments [1, 7, 8]. In addition, the chronic inflammatory condition seems to be a common feature in patients with IC and OAB [9, 10, 12], suggesting that it might be useful to identify the differences in molecular signatures of bladder inflammation associated with IC and OAB for their differential diagnosis. It has been reported that the urine proteome consists of over 1,500 proteins [13]; thus, differences in the inflammatory responses of IC and OAB could be discriminated at the urine molecular levels of growth factors, cytokines, and chemokines. Tyagi et al. recently examined urine makers, including VEGF, nerve growth factor (NGF), IL-1Ra, IL-6, CCL2, CCL5, CXCL1, and CXCL10 among groups of HIC, NHIC, and asymptomatic controls (n = 10 each) [14]. The significant increases in NGF, IL-6, CXCL1, and CXCL10 were detected in HIC patients compared with NHIC patients and controls, although there were no significant differences in VEGF, IL-1Ra, CCL2, and CCL5 among the three groups. These results are partially consistent with the present study, in which VEGF, IL-1α, IL-6, CCL2, CCL5, CXCL1, CXCL8, and CXCL10 were significantly increased in HIC and NHIC patients compared with OAB patients, in addition to the significant increases in CXCL8 and CXCL10 that were also found in HIC patients compared with NHIC patients.
It has been reported that angiogenic components of diabetic retinopathy, atherosclerosis, and inflammatory bowel disease contribute to and exacerbate their disease conditions [3, 4]. Recently, increased expression of VEGF has been detected in IC patients and correlated with pain severity and neovascularization [15]. The possible mechanism of the increase in urine VEGF levels could be hypoxia in bladder tissues during the filling phase that up-regulates the expression of VEGF, which then induces bladder fibrosis and reduces bladder capacity after chronic inflammation [16]. This assumption is further supported by recent studies showing that inhibition of angiogenesis attenuates inflammation [4]. Taken together with our results of VEGF upregulation in IC patients, the anti-angiogenic therapy could be a novel and effective approach to chronic inflammatory diseases [4]. VEGF plays a key stimulatory role in angiogenesis [17] whereas CXCL10 is a potent inhibitor of angiogenesis [18]. Therefore, it is reasonable to assume that the increase in CXCL10 in IC patients may be explained as an offsetting homeostatic response to the increase in VEGF.
CXCL10 can induce not only anti-angiogenesis effects by inhibiting the proliferation of endothelial cells, but also pro-inflammatory response by activating T lymphocytes [19]. CCL2 or CCL5 causes chemotactic migration of monocytes, eosinophils, basophils, lymphocytes or mast cells, but not does not act on neutrophils [20], whereas CXCL1 and CXCL8 are mainly chemotactic for neutrophils [21]. In addition, both IL-1, including IL-1α and IL-1β, and IL-6, have many pro-inflammatory effects [22]. Taken together, these results suggest that IL-1α, IL-6, CCL2, CCL5, CXCL1 and CXCL8 might be the markers of inflammatory changes that are not specific to IC, whereas VEGF and CXCL10, which are involved in the modulation of angiogenesis, may be more specific markers of IC. The hypothesis is further supported by the present study, showing that AUCs for VEGF and CXCL10 were larger than those for CXCL8, IL-1α, CCL5, CCL2, IL-6, and CXCL1 for detecting IC in patients with chronic inflammatory conditions such as IC or OAB.
It has recently been reported that lymphocytic inflammation (≥200 cells/mm2) was observed in 93% of HIC bladder specimens (n = 27), but only in 8% of NHIC specimens (n = 39) [23]. In the present study, the significant increases in CXCL8 and CXCL10 were seen in HIC patients (n = 30) compared with NHIC patients (n = 30), although there were no significant differences in the other inflammatory urine markers, including VEGF, IL-1α, IL-6, CCL2, CCL5, and CXCL1 between HIC and NHIC patients. It has also been reported that urine markers including IL-6 and CXCL8 were not significantly different between HIC (n = 8) and NHIC (n = 17) patients [24]. The discrepancy in these results may be due to the small number of IC patients recruited in the current and previous studies. Thus, further studies with a large number of patients are needed to clarify the pathophysiological differences in HIC and NHIC.
Recent studies have shown that chronic inflammation is involved in OAB. It has been reported that significant increases in EGF, sCD40L, IL-5, IL-10, IL-12p40/p70, CCL2, CCL4, and CXCL1were observed in OAB patients (n = 17) compared with asymptomatic controls (n = 8) [10], although a more recent study by the same authors has reported that there were no significant differences in PDGF, IL-1β, CCL2, CXCL1, CXCL8, and CXCL10 between OAB patients (n = 59) and asymptomatic controls (n = 26) [21]. Our results have shown that VEGF, IL-1α, IL-6, CCL2, CCL5, CXCL1, CXCL8, and CXCL10 were significantly increased in IC patients compared with OAB patients, suggesting that chronic inflammatory changes in IC patients might be more severe than those in OAB patients.
The limitation of the current study is the small number of patients, although the 40 representative inflammatory cytokines, chemokines, and growth factors were measured to detect urine markers for IC compared with OAB. Urine was collected from consecutive IC or OAB patients diagnosed according to each guideline [1, 7]; therefore, all participants had already been treated at the time of urine collection. Thus, there is the possibility that a previous history of bladder hydrodistention in IC patients might affect the concentrations of urine markers tested in this study, even though we waited at least 3 months after the last episode of hydrodistention to minimize its effects. In addition, urine levels were expressed as pg/ml without normalizing these to the creatinine concentration, because disease-associated proteins are passively secreted into urine from the bladder [21]. Taken together, a further study with a larger number of patients may be necessary to confirm the findings of this study before utilizing our urine marker data to guide management of IC patients.
Conclusions
Forty inflammatory cytokines, chemokines, and growth factors in patients with HIC or NHIC, or OAB were compared to detect urine markers for IC. IC patients seem to have more severe chronic bladder inflammation, as demonstrated by the significant increases in IL-1α, IL-6, CCL2, CCL5, CXCL1, CXCL8, and CXCL10 compared with OAB patients. In addition, the increases in angiogenesis-associated proteins such as VEGF and CXCL10 may be pathophysiologically important for the development of IC.
Abbreviations
- HIC:
-
Hunner’s type interstitial cystitis
- IC:
-
Interstitial cystitis
- IL:
-
Interleukin
- NHIC:
-
Non-Hunner’s type interstitial cystitis
- OAB:
-
Overactive bladder
- OSSI:
-
O’Leary–Sant score symptom indexes
- OSPI:
-
O’Leary–Sant score problem indexes
- VAS:
-
Visual analog scale
- VEGF:
-
Vascular endothelial growth factor
References
Homma Y, Ueda T, Tomoe H, Lin AT, Kuo HC, et al. Clinical guidelines for interstitial cystitis and hypersensitive bladder updated in 2015. Int J Urol. 2016;23(7):542–9.
Van de Merwe JP, Nordling J, Bouchelouche P, Bouchelouche K, Cervigni M, et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol. 2008;53(1):60–7.
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57.
Chidlow JH, Shukla D, Grisham MB, Kevil CG. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):5–18.
Ammit AJ, Lazaar AL, Irani C, O’Neill GM, Gordon ND, et al. Tumor necrosis factor-alpha-induced secretion of RANTES and interleukin-6 from human airway smooth muscle cells: modulation by glucocorticoids and beta-agonists. Am J Respir Cell Mol Biol. 2002;26(4):465–74.
Bouchelouche K, Alvarez S, Horn T, Nordling J, Bouchelouche P. Human detrusor smooth muscle cells release interleukin-6, interleukin-8, and RANTES in response to proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha. Urology. 2006;67(1):214–9.
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, et al. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the international continence society. Neurourol Urodyn. 2002;21(2):167–78.
Bo K, Frawley HC, Haylen BT, Abramov Y, Almeida FG, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Int Urogynecol J. 2017;28(2):191–213.
Apostolidis A, Jacques TS, Freeman A, Kalsi V, Popat R, et al. Histological changes in the urothelium and suburothelium of human overactive bladder following intradetrusor injections of botulinum neurotoxin type a for the treatment of neurogenic or idiopathic detrusor overactivity. Eur Urol. 2008;53(6):1245–53.
Tyagi P, Barclay D, Zamora R, Yoshimura N, Peters K, et al. Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol. 2010;42(3):629–35.
Torimoto K, Matsushita C, Yamada A, Goto D, Matsumoto Y, et al. Clinical efficacy and safety of mirabegron and imidafenacin in women with overactive bladder: a randomized crossover study (the MICRO study). Neurourol Urodyn. 2017;36(4):1097–103.
Gamper M, Viereck V, Eberhard J, Binder J, Moll C, et al. Local immune response in bladder pain syndrome/interstitial cystitis ESSIC type 3C. Int Urogynecol J. 2013;24(12):2049–57.
Crosley LK, Duthie SJ, Polley AC, Bouwman FG, Heim C, et al. Variation in protein levels obtained from human blood cells and biofluids for platelet, peripheral blood mononuclear cell, plasma, urine and saliva proteomics. Genes Nutr. 2009;4(2):95–102.
Tyagi P, Killinger K, Tyagi V, Nirmal J, Chancellor M, et al. Urinary chemokines as noninvasive predictors of ulcerative interstitial cystitis. J Urol. 2012;187(6):2243–8.
Kiuchi H, Tsujimura A, Takao T, Yamamoto K, Nakayama J, et al. Increased vascular endothelial growth factor expression in patients with bladder pain syndrome/interstitial cystitis: its association with pain severity and glomerulations. BJU Int. 2009;104(6):826–31; discussion 31
Lee JD, Lee MH. Increased expression of hypoxia-inducible factor-1alpha and vascular endothelial growth factor associated with glomerulation formation in patients with interstitial cystitis. Urology. 2011;78(4):971. e11–5
Lazarus A, Keshet E. Vascular endothelial growth factor and vascular homeostasis. Proc Am Thorac Soc. 2011;8(6):508–11.
Strieter RM, Kunkel SL, Arenberg DA, Burdick MD, Polverini PJ. Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem Biophys Res Commun. 1995;210(1):51–7.
Luster AD, Cardiff RD, MacLean JA, Crowe K, Granstein RD. Delayed wound healing and disorganized neovascularization in transgenic mice expressing the IP-10 chemokine. Proc Assoc Am Physicians. 1998;110(3):183–96.
Bouchelouche K, Alvarez S, Andersen L, Nordling J, Horn T, et al. Monocyte chemoattractant protein-1 production by human detrusor smooth muscle cells. J Urol. 2004;171(1):462–6.
Tyagi P, Tyagi V, Qu X, Chuang YC, Kuo HC, et al. Elevated CXC chemokines in urine noninvasively discriminate OAB from UTI. Am J Physiol Ren Physiol. 2016;311(3):F548–54.
Erickson DR, Xie SX, Bhavanandan VP, Wheeler MA, Hurst RE, et al. A comparison of multiple urine markers for interstitial cystitis. J Urol. 2002;167(6):2461–9.
Maeda D, Akiyama Y, Morikawa T, Kunita A, Ota Y, et al. Hunner-type (classic) interstitial cystitis: a distinct inflammatory disorder characterized by pancystitis, with frequent expansion of clonal B-cells and epithelial denudation. PLoS One. 2015;10(11):e0143316.
Erickson DR, Tomaszewski JE, Kunselman AR, Stetter CM, Peters KM, et al. Urine markers do not predict biopsy findings or presence of bladder ulcers in interstitial cystitis/painful bladder syndrome. J Urol. 2008;179(5):1850–6.
Acknowledgements
This study was supported by JSPS KAKENHI grant number JP15K10633.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Furuta, A., Yamamoto, T., Suzuki, Y. et al. Comparison of inflammatory urine markers in patients with interstitial cystitis and overactive bladder. Int Urogynecol J 29, 961–966 (2018). https://doi.org/10.1007/s00192-017-3547-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-017-3547-5