Abstract
Introduction and hypothesis
Pelvic floor disorders affect many women in high-income countries. Since little is known about such disorders in Africa, this study aimed at assessing the prevalence and risk factors in an Ethiopian community. We also assessed the validity of a prolapse questionnaire.
Methods
A community-based cross-sectional study was conducted among 395 women, recruited by a systematic random sampling technique. Women were interviewed about symptoms of urinary incontinence, faecal incontinence and pelvic organ prolapse by female nurses. Additionally, pelvic examinations were performed in 294 (74.2 %) participants to assess anatomical prolapse using the simplified Pelvic Organ Prolapse Quantification staging system. Descriptive statistics and logistic regression analyses were employed.
Results
The median age of participants was 35.0 years. Thirty-one women reported urinary incontinence (7.8 %), 25 (6.3 %) symptomatic pelvic organ prolapse and 2 (0.5 %) faecal incontinence. Anatomical pelvic organ prolapse stage II–IV was detected in 162 (55.1 %) of women who underwent pelvic examination. The questionnaire for prolapse assessment had poor validity (38.3 % sensitivity and 95.4 % specificity) even in cases of clinically relevant prolapse (stage III or IV). After adjustment, carrying heavy objects for 5 or more hours a day, history of prolonged labour and highland rural residence were associated with anatomical pelvic organ prolapse.
Conclusions
Self-reported incontinence seems low in northwest Ethiopia. The prevalence of symptomatic prolapse was low despite a high prevalence of prolapse signs. Notably, heavy carrying and prolonged labour increased the risk of anatomical prolapse stage II–IV. The methods of assessing pelvic floor disorders in a low-income context need further development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary and faecal incontinence and pelvic organ prolapse, classified as ‘pelvic floor disorders’ [1], are commonly found in the female population. Urinary incontinence, defined as the complaint of involuntary leakage of urine [1], is estimated to occur in 15–34 % of adult European women [2]. Faecal incontinence, i.e. involuntary leakage of solid or liquid stools [1], has been reported to affect 2–12 % of community-dwelling adults in studies from the USA [3, 4]. Pelvic organ prolapse occurs when the pelvic floor no longer supports the proper positioning of the pelvic organs, i.e. the vagina, bladder, rectum or uterus. Symptoms of pelvic organ prolapse include a feeling of vaginal bulging and/or pelvic pressure [1], and the prevalence in women is around 6–7 % in US studies [5–7].
Studies from sub-Saharan countries report an astonishing variation in the prevalence of urinary incontinence ranging from 2.8 to 70.8 % [8, 9]. Likewise, the prevalence of genital prolapse varies substantially (3.4–56.4 %) in developing countries [7, 9–11].
Childbirth is a well-known risk factor for pelvic floor disorders in high-income settings [12–14]. However, African-American women are less likely to report urinary incontinence [15, 16] and symptomatic prolapse [5] compared with Caucasian women. Since women in low-income countries have more pregnancies, less access to obstetric care and more physical strain in daily life, it is still possible that pelvic floor disorders may be more common and may affect daily life more severely than suggested by reports from high-income settings. However, very few studies have been conducted on the subject [9].
In Ethiopia, where access to obstetric care is very limited (institutional delivery being only 10 %) and the fertility rate is high (5.5 children per woman) [17], little is known about the prevalence and risk factors for pelvic floor disorders. Among rural Ethiopian women, the prevalence of total urinary incontinence due to obstetric fistula is 0.2 % [18]. Beyond this evidence no population-based studies have been performed on pelvic floor disorders, but reports from hospitals suggest a high burden of pelvic organ prolapse among women at gynaecological outpatient clinics and wards [19, 20]. The large variation of the prevalence in the different studies reflects methodological challenges and calls for more research in low-income contexts [9]. Since population-based epidemiological studies of pelvic floor disorders are rare in low-income countries, and as a first step towards a full-scale study to address these issues, we carried out a population-based pilot study.
The primary aim of this pilot study was to estimate the prevalence of pelvic floor disorders (urinary incontinence, faecal incontinence, symptomatic pelvic organ prolapse and anatomical prolapse) in an Ethiopian context. The secondary aim was to assess the validity of a prolapse questionnaire in relation to anatomical prolapse as defined by pelvic examination.
Materials and methods
The Dabat Incontinence and Prolapse (DABINCOP) Study is a collaborative research project between the University of Gondar, Ethiopia, and the University of Bergen, Norway, conducted in Dabat district, northwest Ethiopia. Dabat is located 75 km north of Gondar town, in the Amhara region. Gondar town hosts the only referral hospital in the area serving about 5 million people. Dabat Research Centre is a Demographic and Health Survey Site in Ethiopia, run by the University of Gondar. The Centre has collected demographic data from a population of about 50,000 inhabitants in the ten kebeles (seven rural and three semi-urban) of the district twice a year since 1996 [21]. In Ethiopia, there are around 30,000 kebeles, which is the smallest administrative unit in the country. There is huge variation in terms of geographic area a kebele covers as well as degree of urbanity. The population size of a kebele generally ranges from 4,000 to 5,000 people.
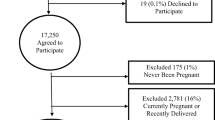
A proportional sample was taken from one randomly selected kebele in each of three different climatic and sociocultural settings (one semi-urban, one highland rural and one lowland rural kebele). In total, there were 13,424 inhabitants in the three kebeles, and for the study 405 women were approached. The total number of households in each kebele was obtained from Dabat Research Centre, and the sample size adjusted proportionally for the three kebeles. Then, households were selected by a systematic random sampling method, i.e. selecting households at a fixed interval throughout the list. Female nurses collected data by face-to-face interview at the participant’s home. They were supervised by Health Officers. One-day training on methods of interview, with emphasis on the introduction, on communication to assess outcomes correctly and on respecting the cultural norms of the women, was given to both data collectors and supervisors and it was supported by practical demonstrations. The questionnaire was pretested in 15 women and appropriate changes were made.
The woman who was head of the selected household aged at least 15 years was invited for participation in the interview study. If there was no eligible woman in the selected household or she was not available at the time of data collection, replacement was done by the next household number. Data collectors explained the objectives of the study, including the reasons for a later pelvic examination, and requested her consent for participation by reading the consent sheet aloud in Amharic (the local language).
The interview questionnaire was composed of five sections (socio-demographic factors, obstetric and gynaecological history, urinary incontinence, faecal incontinence and prolapse symptoms). Women who were responsible for the farming in the household were termed “farmer”. Married women who participated in farming but were not primarily responsible for it were termed “housewife”. The age of the participant was taken to the nearest completed years, and women who were in doubt about their exact age were assisted to determine it by associating with historical events. Maternal obstetric and gynaecological history such as parity, mode and place of delivery etc. were noted. The questionnaire was translated from English to Amharic without back-translation.
Urinary incontinence was assessed by a questionnaire adapted to the current context from the Norwegian EPINCONT questionnaire [22]. If a woman confirmed that she had any involuntary loss of urine (specified as within the last 1 year), further questions were asked. ‘Stress incontinence’ was defined as loss of urine when coughing, sneezing, laughing or lifting heavy items, ‘urgency incontinence’ as involuntary and sudden loss of urine with a strong, sudden need to urinate and ‘mixed incontinence’ if both symptoms were present. Severity of urinary incontinence was graded according to the severity index (mild, moderate or severe), which is the frequency of leakage multiplied by amount of urine per leak [23]. Similarly, faecal incontinence was assessed by asking the woman whether she had experienced involuntary leakage of stool (faecal matter) during the last 1 year.
Symptomatic pelvic organ prolapse was assessed by two questions, previously used in the American RRISK study [12]: Do you have a (1) feeling of bulging/pressure or something seems to be coming down through the vagina? or (2) visible mass protruding via the vagina? If a woman had experienced one or both of these problems in the last 1 year, she was considered as having symptoms of pelvic organ prolapse and further questions followed to assess the duration, associated symptoms, level of bother and perceived impact due to the prolapse. Indications of prolapse based on the questionnaire are hereafter referred to as symptomatic prolapse.
After completing the interview, the participant’s height and weight were measured and recorded by interviewers. Due to weight scale problems, some participants had their weight measured at the health post when attending the pelvic examination. Data collectors reported the appointment time and date for the pelvic examination for each woman at the nearby health post/centre.
All participants were asked to volunteer for a pelvic examination at the nearest health facility. The examination was done by two MDs working in Gondar University Referral Hospital. They had 2 weeks of training and practical demonstrations provided by a gynaecologist, who is part of the research team (MA). The simplified Pelvic Organ Prolapse Quantification (S-POPQ) staging system was applied [24]. Training was assisted by a DVD describing the S-POPQ procedure provided by the team who validated this staging system [24]. There was no formal testing of intra- or inter-evaluator reliability. Pelvic examinations assisted by specifically trained nurses were performed at the health institution at each kebele. Pelvic examination was supervised in the field by the research team gynaecologist. Two virgins were excluded from this part of the study.
Pelvic examination was done after the woman emptied her bladder. After receiving an explanation of the procedure, the participant was requested to lie on an examination couch in the lithotomy position. A disarticulated Graves speculum was inserted into the vagina. The posterior vaginal wall was retracted to observe the descent of the anterior vaginal wall and the degree of protrusion in relation to hymenal ring with strain or cough. Secondly, the anterior vaginal wall was retracted to observe a descent of the posterior vaginal wall during straining. In accordance with the method, no measuring device was used. The examiners estimated the degree of descent by observing the points on the anterior and posterior vaginal segments that were used to represent the respective walls. The point descent in relation to the hymenal ring while performing Valsalva or cough was recorded as the stage (Table 1) in the three areas examined (anterior, posterior and apical/cervix) and the final stage was the maximum one from the three measurements. Reports of stage from the pelvic examination (S-POPQ) are hereafter referred to as anatomical prolapse. Women with anatomical prolapse stage II–IV were defined as cases of anatomical prolapse. In addition, we classified cases with stage III and IV as having clinically relevant prolapse.
Interview results were linked to physical examination reports after data collection had been completed using code numbers. Data were cleaned and entered into a computer using Epi Info version 2002 (Centers for Disease Control and Prevention, Atlanta, GA, USA) and exported to SPSS version 16 (SPSS Inc., Chicago, IL, USA) for analysis.
Statistical analyses were done by bivariate and multivariate methods. Chi-square tests were used when comparing groups. For urinary or faecal incontinence as well as symptomatic prolapse, it was not possible to do further statistical analyses for associated factors due to the low number of cases. Validity measures such as sensitivity, specificity and predictive values were calculated for the prolapse questionnaire with the S-POPQ stage as a reference.
For risk factor analyses of anatomical prolapse, binary and multivariable logistic regression analyses were performed. For this purpose, we used stage II and above as cases and those with stage 0 and I were defined as non-cases. All factors with a p value <0.2 in the bivariate logistic regression were entered into the multivariate model. Odds ratios (OR) with 95 % confidence intervals (CI) were calculated. Statistical significance was accepted at the 5 % level (p < 0.05).
For the validation of the prolapse questionnaire, we categorised anatomical prolapse as stage III and stage IV, because the sensitivity and the specificity for these stages should be high in order to deem the questionnaire valid.
Ethical considerations
Ethical approval of the study was obtained from both the Regional Ethics Review Board in Western Norway and the University of Gondar Institutional Review Board. All participants were requested and gave their verbal consent. A copy of the consent form was given to each participant including information about the study and the confidentiality of information. For the women who came for physical examination, transportation costs and time lost were compensated. Women who were in need of further assessment and hospital treatment were referred to Gondar University Referral Hospital, and transportation costs for the travel were covered.
Results
A total of 405 women were approached and 395 participated, for a response rate of 97.5 %. The median age of participants was 35.0 years (range 16–80) with the majority (81.0 %) in the reproductive age group (15–49 years). Nearly all were of Amhara ethnicity and Orthodox Christians (Table 2).
The mean age at first marriage and delivery was 14.9 and 18.5 years, respectively. The majority (83.3 %) were multiparous, with the mean number of deliveries being 4.8 children. Body mass index (BMI) was calculated for 284 (71.9 %) women of whom nearly a quarter (27.5 %) were underweight and 4.9 % overweight (Table 3).
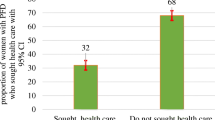
About one in eight women (11.9 %) reported symptoms of any pelvic floor disorder. Urinary incontinence was reported by 31 (7.8 %). Almost half (51.6 %) of incontinent women had moderate incontinence, while 12.9 % had severe urinary incontinence (Table 4). Two women (0.5 %) had faecal incontinence. Symptomatic prolapse was reported by 25 (6.3 %) of the participants. Of these, 14 (56.0 %) reported being bothered by the symptoms though only 1 (4.0 %) had consulted a health professional in connection with the challenge.
Pelvic examination was carried out in 294 (74.2 %) women. The participation rate for pelvic examination differed among the different kebeles (88.2 % of rural women and 58.7 % of women from the semi-urban kebele, p < 0.001) and with number of deliveries (mean number of deliveries 3.7 for non-examined group and 4.7 among the examined women, p < 0.001). There was no significant difference in other characteristics. One third (33.1 %) of the examined women had anatomical prolapse stage I, 48.1 % had stage II while 7.2 % had stage III–IV (clinically relevant prolapse).
In the unadjusted analysis, anatomical prolapse as outcome was significantly associated with maternal age of 35–49 years, living in highland rural kebele, repeated deliveries, carrying heavy objects for ≥5 hours during daily routine work and history of prolonged labour, i.e. ≥2 days (Table 5). After adjustment, only carrying heavy objects (OR 2.13; 95 % CI 1.12–4.07), being from a highland rural kebele (OR 2.30; 95 % CI 1.14–4.62) and having a history of prolonged labour (OR 1.78; 95 % CI 1.10–2.88) were significantly associated with anatomical prolapse stage II–IV (Table 5).
We assessed the validity of the questionnaire for prolapse (symptomatic prolapse) by comparing it with the pelvic examination result (anatomical prolapse) (Table 6). The prolapse questionnaire had low sensitivity even for clinically relevant stages (stage III–IV) of prolapse (38.3 %; 95 % CI 20.8–59.1); however, the specificity was good (95.4 %; 95 % CI 92.4–97.3).
Discussion
This community-based study of pelvic floor disorders in women aged 15 years and above in Ethiopia showed a prevalence of 11.9 % for any of the three common pelvic floor disorders (7.8 % for urinary incontinence, 0.2 % for faecal incontinence and 6.3 % for symptomatic prolapse). There was a significant association between anatomical pelvic organ prolapse and carrying heavy objects for ≥5 h/day, history of prolonged labour and being a resident from a highland rural area. There was a low frequency of reported prolapse symptoms compared with the finding of anatomical prolapse at pelvic examination.
The prevalence of urinary incontinence (7.8 %) is also lower than other reports from Africa [11, 25], though it falls within the range (3.4–56.4 %) of previously reported rates for both developing and developed countries [8, 9, 26, 27]. The relatively low prevalence in our study may be partly explained by the difference in age distribution, as the current population was quite young, with a mean age of about 37 years, compared to a study from Brazil where the mean age was 45 years for continent and 51 years for incontinent women [27]. Additionally, urinary incontinence seems to be less prevalent in women of African origin as compared to Caucasian women [15]. The prevalence in the current study is however higher than a community-based report from Nigeria which reported 2.8 % prevalence [8]. This difference may be due to the fact that we included only women who were head of their household and thus older than those in the Nigerian study (mean age of 33.2 years).
However, we do suspect under-reporting of symptoms with regard to both urinary and faecal incontinence, based on our findings of a substantial discrepancy between reported prolapse symptoms and observed stage III and IV anatomical prolapse. Several explanations may be relevant. Firstly, the questionnaire may not have been perceived as sensible for women with mild symptoms in this context. Secondly, the interview format may have been regarded as somewhat intrusive, and women may have been reluctant to tell about their symptoms even though they were informed that the answers would be treated confidentially. Thirdly, disclosure of symptoms of this nature is uncommon in Ethiopia [28]. In addition to the feeling of embarrassment about the condition, Ethiopian women have very low access to medical assessment and treatment and therefore may have little motivation to discuss these issues. There may however also be additional explanatory factors for the findings, e.g. low intake of fluid and correspondingly less urine production in a hot climate may reduce the problem of urinary incontinence. Additionally, physically fit women with low BMI may better control various degrees of pelvic prolapse than the female population of industrialised countries having a higher BMI [5].
There were only two women (0.5 %) with faecal incontinence in this study which could be due to our study of faecal incontinence for stool (faecal matter) only, not assessing anal incontinence which would include incontinence for flatus or mucus, as well as the already mentioned methodological or disclosure problems. This is lower than previously reported from developing countries (2.5 %) [9].
Symptomatic prolapse was reported by 6.3 % of participants, which is lower than a report from Nigeria (10 %) and the mean prevalence (19.7 %) of pelvic organ prolapse from a review report in developing countries [9, 11]. However, the prevalence from our study is in line with a previous study from the USA reporting a prevalence of 6 % based on the same questionnaire as we used [12].
The prevalence of anatomical pelvic organ prolapse stage II–IV (55.3 %) was higher than in Ghana (12.1 %), but in line with a study from Gambia (46 %) [10, 29]. Wusu-Ansah et al. used the S-POPQ method in the Ghanaian study, and the prevalence was for stage I–IV combined [29]. The prevalence of anatomical prolapse stage II–IV was only 9.1 %, and for clinically relevant prolapse (stage III–IV) 5.2 %. The prevalence of clinically relevant prolapse in Ghana is close to what we found (7.2 %). In the Gambian study, prolapse was based on pelvic examination; however, a formal staging system was not employed and the prevalence reported is based on all degrees of prolapse [10].
The sensitivity of the prolapse questionnaire to identify even overtly visible pelvic organ prolapse cases was very low. In the present study, none of the women with symptomatic prolapse were below stage II. Similarly, in the study from Gambia by Scherf et al., only 13 % of women with moderate or severe prolapse reported symptoms on direct questioning [10]. Other studies also suggest that symptom-based screening methods may lack sensitivity in populations with a low prevalence of pelvic organ prolapse, such as that of the general population [30, 31]. This fact can also be a possible explanation for the low prevalence of other pelvic floor disorders in this study. A discrepancy between symptoms and signs with regard to prolapse is a common finding in studies from high-income contexts [6, 31]. In particular, stage II represents a condition with generally few symptoms, although this varies. At the other end of the spectrum, women with symptoms of prolapse do not necessarily have the condition, making the commonly used term “symptomatic prolapse” a misnomer in such cases.
In this study some well-established factors for prolapse such as increasing age and parity [7, 10, 12] were not significantly associated with anatomical prolapse. This may have at least two reasons. Firstly, we analysed anatomical prolapse, while some others used interview results [7, 11]. Secondly, the small sample size may fail to show associations due to low statistical power. Finally, low variability within each factor (young age and high parity) in this pilot study may have contributed to this result. However, an important risk factor for anatomical pelvic prolapse in this study was a history of long labour. Also, carrying heavy objects many hours a day was associated with anatomical prolapse. These seem to be risk factors specific for women in low-income countries and may be of particular relevance in such settings.
The main strengths of the present study are that it is community based, carried out on an ethnically homogeneous population, with a high response rate and supported by pelvic examinations. One of the limitations of this study is that we only included women who were heads of households. Additionally, the small sample size made generalisations and powerful statistical analyses difficult; specifically we could not investigate the association between urinary, faecal incontinence and symptomatic pelvic organ prolapse. A general limitation is the lack of a culturally validated questionnaire for this particular setting. This shortcoming is partly met by documenting the degree of discrepancy between a symptom-oriented questionnaire and the physical assessment of a pelvic examination.
In conclusion, the prevalence of pelvic floor disorders was found to be low in the study area. Heavy carrying and prolonged labour increased the risk of anatomical prolapse. It is difficult to decide whether the prevalence is actually low or if the finding is due to a problem of non-disclosure. The prevalence of symptomatic prolapse was low despite a high prevalence of anatomical prolapse identified during pelvic examination. As the sensitivity of the prolapse questionnaire was low, pelvic examination should be done in further community-based research on prolapse. Besides, steps should be taken to increase and ensure sensitivity of the questionnaire in identifying pelvic floor disorders.
References
Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J et al (2010) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J 21:5–26
Hunskaar S, Lose G, Sykes D, Voss S (2004) The prevalence of urinary incontinence in women in four European countries. BJU Int 93:324–330
Nelson R, Norton N, Cautley E, Furner S (1995) Community-based prevalence of anal incontinence. JAMA 274:559–561
Bharucha AE, Zinsmeister AR, Locke GR, Seide BM, McKeon K, Schleck CD et al (2005) Prevalence and burden of fecal incontinence: a population-based study in women. Gastroenterology 129:42–49
Whitcomb EL, Rortveit G, Brown JS, Creasman JM, Thom DH, Van Den Eeden SK et al (2009) Racial differences in pelvic organ prolapse. Obstet Gynecol 114:1271–1277
Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J et al (2008) Prevalence of symptomatic pelvic floor disorders in US women. JAMA 300:1311–1316
Lukacz ES, Lawrence JM, Contreras R, Nager CW, Luber KM (2006) Parity, mode of delivery, and pelvic floor disorders. Obstet Gynecol 107:1253–1260
Ojengbede OA, Morhason-Bello IO, Adedokun BO, Okonkwo NS, Kolade CO (2011) Prevalence and the associated trigger factors of urinary incontinence among 5000 black women in sub-Saharan Africa: findings from a community survey. BJU Int 107:1793–1800
Walker GJ, Gunasekera P (2011) Pelvic organ prolapse and incontinence in developing countries: review of prevalence and risk factors. Int Urogynecol J 22:127–135
Scherf C, Morison L, Fiander A, Ekpo G, Walraven G (2002) Epidemiology of pelvic organ prolapse in rural Gambia, West Africa. BJOG 109:431–436
Iyoke CA, Ezugwu FO, Onah HE (2010) Prevalence and correlates of maternal morbidity in Enugu, South-East Nigeria. Afr J Reprod Health 14:121–130
Rortveit G, Brown JS, Thom DH, Van Den Eeden SK, Creasman JM, Subak LL (2007) Symptomatic pelvic organ prolapse: prevalence and risk factors in a population-based, racially diverse cohort. Obstet Gynecol 109:1396–1403
Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S, Norwegian EPINCONT Study (2003) Urinary incontinence after vaginal delivery or cesarean section. N Engl J Med 348:900–907
Rortveit G, Hannestad YS, Daltveit AK, Hunskaar S (2001) Age- and type-dependent effects of parity on urinary incontinence: the Norwegian EPINCONT study. Obstet Gynecol 98:1004–1010
Fenner DE, Trowbridge ER, Patel DA, Fultz NH, Miller JM, Howard D et al (2008) Establishing the prevalence of incontinence study: racial differences in women’s patterns of urinary incontinence. J Urol 179:1455–1460
Thom DH, van den Eeden SK, Ragins AI, Wassel-Fyr C, Vittinghof E, Subak LL et al (2006) Differences in prevalence of urinary incontinence by race/ethnicity. J Urol 175:259–264
Ethiopia Demographic and Health Survey Report. Central Statistical Agency and ICF International 2011
Muleta M, Fantahun M, Tafesse B, Hamlin EC, Kennedy RC (2007) Obstetric fistula in rural Ethiopia. East Afr Med J 84:525–533
Lukman Y (1995) Utero-vaginal prolapse: a rural disability of the young. East Afr Med J 72:2–9
Kiros K, Mariam DH, Zemenfes D (2000) Genitourinary prolapse and joint hypermobility in Ethiopian women. East Afr Med J 77:640–643
Fantahun M, Kumbi S, Degu G, Kebede Y, Admasu M, Haile W et al (2001) Dabat Rural Health Project, North West Ethiopia: report of the baseline survey. Ethiop J Health Dev 15
Hannestad YS, Rortveit G, Sandvik H, Hunskaar S et al (2000) A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of incontinence in the county of Nord-Trøndelag. J Clin Epidemiol 53:1150–1157
Sandvik H, Seim A, Vanvik A, Hunskaar S (2000) A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn 19:137–145
Manonai J, Mouritsen L, Palma P, Contreras-Ortiz O, Korte J, Swift S (2011) The inter-system association between the simplified pelvic organ prolapse quantification system (S-POP) and the standard pelvic organ prolapse quantification system (POPQ) in describing pelvic organ prolapse. Int Urogynecol J 22:347–352
Madombwe JP, Knight S (2010) High prevalence of urinary incontinence and poor knowledge of pelvic floor exercises among women in Ladysmith. S Afr J Obstet Gynaecol 16
Minassian VA, Drutz HP, Al-Badr A (2003) Urinary incontinence as a worldwide problem. Int J Gynaecol Obstet 82:327–338
Amaro JL, Macharelli CA, Yamamoto H, Kawano PR, Padovani CV, Agostinho AD (2009) Prevalence and risk factors for urinary and fecal incontinence in Brazilian women. Int Braz J Urol 35:592–597
Gjerde JL, Rortveit G, Muleta M, Blystad A (2012) Silently waiting to heal: experiences among women living with urinary incontinence in northwest Ethiopia. Int Urogynecol J. doi:10.1007/s00192-012-1951-4
Wusu-Ansah OK, Opare-Addo HS (2008) Pelvic organ prolapse in rural Ghana. Int J Gynaecol Obstet 103:121–124
Barber MD, Neubauer NL, Klein-Olarte V (2006) Can we screen for pelvic organ prolapse without a physical examination in epidemiologic studies? Am J Obstet Gynecol 195:942–948
Tegerstedt G, Miedel A, Maehle-Schmidt M, Nyren O, Hammarström M (2005) A short-form questionnaire identified genital organ prolapse. J Clin Epidemiol 58:41–46
Acknowledgments
We appreciate the Swift group for providing us a DVD for demonstration of the S-POPQ pelvic examination. We also extend our heartfelt thanks to study participants and data collectors. This study was funded by the Western Norway Regional Health Authority and the Nordic Urogynecological Association.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
The study was conducted in Gondar, Ethiopia.
Rights and permissions
About this article
Cite this article
Megabiaw, B., Adefris, M., Rortveit, G. et al. Pelvic floor disorders among women in Dabat district, northwest Ethiopia: a pilot study. Int Urogynecol J 24, 1135–1143 (2013). https://doi.org/10.1007/s00192-012-1981-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-1981-y