Abstract
Introduction and hypothesis
Recommending prophylactic anti-incontinence procedures to continent women undergoing surgery for pelvic organ prolapse (POP) is controversial. We hypothesized that testing for occult incontinence before surgery using four different tests and three defined test combinations would identify individual women at risk for postoperative stress urinary incontinence (POSUI). The diagnostic accuracy of these tests and test combinations were evaluated.
Methods
We tested 137 women before and after surgery. Fisher’s exact test was used when evaluating associations between test results and outcomes. The validity of each test and test combinations was calculated.
Results
We found a statistically significant association between occult incontinence and POSUI in two tests and all test combinations. However, all tests and test combinations displayed poor performance when predicting at individual levels.
Conclusions
This study confirms a positive association between occult incontinence and POSUI. Occult incontinence does not, however, adequately identify individual women in need of prophylactic anti-incontinence surgery when undergoing POP repair.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Postoperative stress urinary incontinence (POSUI) following pelvic organ prolapse (POP) repair in previously continent women is a clinical challenge. De novo stress urinary incontinence (SUI) has been reported to range from 11 % to 44 % after surgical correction of urogenital prolapse [1–3]. Preoperative occult incontinence is generally accepted as a marker for increased risk of POSUI. Occult incontinence is clinically diagnosed when a continent woman with POP displays stress urinary leakage during provocation testing mimicking POP repair. Prophylactic anti-incontinence procedures in patients with occult incontinence have shown a reduced incidence of POSUI [4–6]. However, as long as any anti-incontinence procedure comes with a cost and has potential risks, managing occult incontinence remains controversial. The association between occult incontinence (positive provocation tests) and the risk of developing postoperative incontinence is not yet fully investigated. The lack of standardized diagnostic criteria for occult incontinence makes it difficult to determine the exact prevalence and thereby its true predictive value on POSUI. Different preoperative studies have demonstrated a wide variety of prevalence, from 6 % to 80 %, using various prolapse reduction methods [3–5, 7–13]. The validity of the tests defined by their sensitivity, specificity, positive (PPV) and negative (NPV) predictive values, likelihood ratios, and diagnostic odds ratios (ORs) are, for the most part, unreported. The few studies that have investigated the validity of such tests for occult incontinence have found them to have poor predictive value [3, 5].
This study was aimed at measuring the statistical association of a positive preoperative provocation test (occult incontinence) and the risk for developing POSUI after prolapse surgery in a study design with adequate sample size. We investigated performances of four different provocation tests regularly used in clinical practice for diagnosing occult incontinence and their diagnostic accuracy in predicting POSUI. We also evaluated diagnostic accuracy when combining tests.
Materials and methods
This study was a prospective observational study at the Department of Gynaecology, Oslo University Hospital, Norway. The Regional Committee for Medical and Health Research Ethics in southeastern Norway approved the study in 2007, and written consent was obtained from all study participants. All consecutive patients scheduled for POP repair during a 3-year period (June 2007 to June 2010) without concomitant urinary incontinence (UI) were asked to participate when they attended the outpatient clinic. Four different barrier tests were planned for all patients, which enabled evaluation of test combinations. Inclusion criteria were an indication for surgical POP repair with no subjective or objective UI. The exclusion criteria were any form of preexisting UI, detrusor overactivity during urodynamic evaluation, inability to give informed consent, or lack of language skills in Norwegian or English. Patients with urgency without UI were allowed into the study.
Preoperative evaluation
The prolapse was assessed in the semilithotomic position and staged according to the Pelvic Organ Prolapse Quantification (POP-Q) system (Table 1). A urine dipstick analysis was performed to rule out any urinary tract infection before testing. Women with all types of urogenital prolapse were recruited. The bladder was emptied with a urinary catheter and the test volume of saline installed. The patient was asked to perform a Valsalva maneuver and a cough stress test in the semilithotomic position, first without reducing the prolapse and secondly with the prolapse manually repositioned (test 1). The patient was excluded if there was visual urinary leakage without the prolapse being reduced. A pessary that optimally reduced the prolapse was fitted and the test repeated (test 2). The pessary test was done with both 100 ml (test 2) and 300 ml (test 3) bladder volumes in the semilithotomic position on two different visits. A positive test was defined as visual urine leakage during the Valsalva maneuver or the cough stress test with the prolapse repositioned (tests 1–3). In all tests, care was taken to avoid urethral compression. The pessary was left in situ for a minimum of 1 week between visits to see whether continuous use would elicit UI (test 4), regardless of any leakage on the objective tests. Any subjective feeling of leakage during this long-term pessary use was defined as a positive test. At the second visit, a full urodynamic workup was performed, including a filling cystometry to rule out any involuntary detrusor contractions.
When analyzing data, we defined three test combinations: A, B, C. A positive test combination was defined as having at least one test show positive. Test combination A comprised test 3 and 4, test combination B test 2, 3, and 4, and test combination C all four tests (Table 2).
Surgery
Prolapse repair was performed according to prolapse type, regardless of results from preoperative incontinence testing. No prophylactic incontinence procedure was performed.
Postoperative evaluation
A postoperative evaluation was scheduled after a minimum of 3 months. Three patients developed severe UI and were therefore evaluated before completing the 3-months follow-up period but were not excluded from study results. At the postoperative evaluation, all patients filled out a validated incontinence questionnaire, and POSUI was defined as new onset of subjective symptoms, regardless of severity, with a stress index score > 0 [14]. This was used as the outcome measure in the analysis. Regardless of symptoms, all patients were tested objectively for incontinence with a repetition of test 3 (without pessary).
Statistical analysis
Sample size analysis prior to study initiation was performed. The rate of a positive preoperative test was set at 50 % based on published literature up to 2007, reporting a 44–80 % prevalence of occult incontinence in patients with POP [4, 7–13]. We decided that a clinically significant difference in outcome (POSUI) between those with positive and negative tests must be at least 20 %. We wanted our study to have 80 % test power when the expected outcome (POSUI) in the test positive and test negative groups were 32 % and 12 %, respectively, the latter based on the Norwegian study by Borstad and Rud, who reported an incidence of POSUI after traditional Manchester operation of 22 % [1]. From this we calculated that 136 patients were needed.
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS-PC), version 15.0. Differences in outcome based on positive or negative preoperative tests (dichotomous/categorical data) were tested by two-sided Fisher’s exact test. A probability level of < 0.05 was considered statistically significant. Diagnostic accuracy of tests and test combinations was estimated using sensitivity, specificity, PPV, NPV, and likelihood ratios for positive and negative test results, as well as diagnostic ORs. To test for potential selection bias, we performed a poststudy demographic comparison between study participants and all patients undergoing prolapse surgery at our department during the inclusion period. The parameters compared were age, stage of prolapse, and dominating POP compartment.
Results
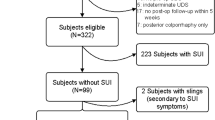
There were 204 patients included in the study (Fig. 1). Forty-seven patients were excluded at the second visit due to subjective or objective incontinence that they had not initially reported. Many of these patients had only slight incontinence that gave little bother and had therefore not reported this to their doctor at the initial inclusion. Of the 140 patients that completed the study, only three were lost to follow-up. We did not plan for any intention to treat analysis and therefore used the final 137 patients for analysis. The preoperative demographic data and types of POP surgery are provided in Table 1.
Table 2 lists positive rates for all preoperative tests and test combinations and shows that not all patients managed to complete all four tests. The incidence of a positive preoperative test (occult SUI) varied from 4 % to 19 % for any single test and from 27 % to 38 % for test combinations. Mean postoperative follow-up was 5 (range 1–15) months. The incidence of POSUI was 17 % when defined as subjective incontinence (outcome evaluated from the validated questionnaire) and 10 % when defined as both subjective and objective incontinence (repeat of test 3 without pessary). When defined as subjective stress incontinence and stratified into the dominating compartments of anterior, mid, or posterior, POSUI incidences were 20 %, 18 %, and 7 % respectively. There was a clear difference in outcome (POSUI) between patients with positive and negative preoperative tests (Table 2). This difference was statistically significant for all test combinations and for single tests 3 and 4 (p < 0.05). Test combination A had the highest OR (9.0, 95 % CI 2.3–35.8, p = 0.002). To evaluate test performance for predicting outcome on an individual level, we tested the diagnostic accuracy of both positive and negative test results. Thus, we calculated sensitivity, specificity, PPV, NPV, likelihood ratios, and diagnostic ORs for all tests and test combinations (Table 3). Sensitivity for the different single tests varied from 9 % to 50 %, with specificity values ranging from 88 % to 97 %. For test combinations, sensitivities ranged from 67 % to 73 %, with specificities varying from 71 % to 82 %. PPV ranged from 22 % to 50 % for single tests, with NPV varying from 83 % to 89 %. For test combinations, PPV ranged from 39 % to 44 % and NPV from 91 % to 92 %. Likelihood ratios ranged from 1.4 to 4.9 for positive tests and from 2.6 to 3.7 for positive test combinations. For negative tests, likelihood ratios varied from 0.57 to 0.97 for single tests and from 0.38 to 0.41 for test combinations. The highest diagnostic OR of 9.0 was seen for test combination A.
Only six of the 137 patients developed postoperative SUI severe enough to need treatment. After further clinical evaluation, four of these received pelvic floor muscle training and two underwent a tension-free vaginal tape (TVT) procedure. Our study population and the total population of patients undergoing POP surgery at our department during the inclusion period were similar for the parameters stage of prolapse and dominating POP compartment (data not shown) but differed slightly in mean age (59 vs 63 years).
Discussion
We found a statistically significant increased risk for POSUI in patients having a positive preoperative provocation test (occult incontinence) for tests 3 and 4 and all test combinations. The increased risk for POSUI with a positive test 1 or 2 did not reach statistically significant values, probably due to small sample size. Our results are in agreement with those observed in the nonintervention arm of the Colpopexy and Urinary Reduction Efforts (CARE) trial, showing a 20 % higher risk of POSUI in those with a positive preoperative test (occult incontinence) compared with those with negative tests (58 % vs 38 %) [5]. To properly counsel individual women whether or not to undergo a prophylactic anti-incontinence procedure at time of POP repair, there is a need to evaluate the validity of preoperative tests. The validity describes test performance for predicting outcome on an individual level by means of sensitivity, specificity, PPV, NPV, likelihood ratios for a positive and negative test result, and diagnostic ORs. In this study, we followed the guidelines recommended by the Standard for Reporting of Diagnostic Accuracy (STARD) initiative from 2003 [15], with the exception of testing each index test against a reference test. Testing against a reference test was not possible, as there is no established consensus on a reference test for occult incontinence. All preoperative tests and test combinations used for occult incontinence in this study showed disappointing performances. Not unexpectedly, we found the highest sensitivity for the test combinations, but none of the individual tests or test combinations had PPV >0.5 or positive likelihood ratios >5. Such poor performance, and the fact that ORs demonstrated significant associations between occult incontinence and POSUI, illustrates that ORs may not be an appropriate tool for describing the ability of a risk marker such as occult incontinence to identify persons likely to end up with POSUI. ORs and relative risks (RRs) are epidemiological instruments frequently used to assess associations but should be used with caution, or perhaps not at all, when using risk markers to predict outcome for individuals [16]. In this study, no test was performed with bladder volumes >300 ml. Other authors demonstrated that there is no additional information gained in retesting beyond 300 ml up to maximal cystometric capacity [5].
The mechanisms by which de novo postoperative SUI can develop are not fully understood. Some women with urogenital prolapse are suggested to maintain continence because of either urethral kinking or direct compression of the urethra by the descending prolapse [9, 11]. Performing prophylactic anti-incontinence procedures at the time of POP repair is a controversial topic. It is of ethical concern when the prophylactic procedure may induce greater morbidity than the potential problem it is meant to correct. Long-term postoperative complications following anti-incontinence surgery, such as TVT, include bladder outlet obstruction, de novo urge incontinence, and vaginal mucosa graft erosions [17, 18]. The risk of intervention following urinary outlet obstruction in patients with occult incontinence undergoing concomitant TVT and POP repair was shown in one study to be as high as 9.7 % [19].
Our study revealed that even patients with negative provocation tests (no occult incontinence) were at risk of POSUI. We found a POSUI incidence of 9 % when the patients tested negative on all four tests (test combination C, Table 2). Our findings correspond well with results from other recent studies [2, 3, 19]. The findings differ significantly, however, from older studies with smaller sample sizes that reported only a slight or no risk for patients without occult incontinence to develop POSUI [6, 10, 20, 21].
Only six (4.4 %) patients in our observational study developed symptoms of postoperative SUI great enough to need treatment, and only two of the 137 patients needed an anti-incontinence procedure (TVT) after reconstructive POP surgery (1.5 %). The remaining four had less severe symptoms and received pelvic floor muscle training. The number of patients needing intervention for SUI was even lower than the recent study from Ballert et al., who reported that only 8.3 % of their patients undergoing POP repair were in need of postoperative intervention for SUI, if no preoperative subjective or objective stress incontinence was demonstrated [19]. Furthermore, several large studies have reported that POSUI may still occur after concomitant prophylactic anti-incontinence procedures at the time of POP surgery [2, 4]. Our study participant group was similar to the total group of patients undergoing prolapse surgery at our department during the inclusion period when POP-Q stage and dominating prolapsed compartment were compared. Our selected group did, however, differ slightly in age, being on average 3 years younger than the total group. We interpreted this as sign of more frequently manifest preoperative incontinence in older than younger POP patients, which precluded them from recruitment to our observational study.
To our knowledge, this is the only study, besides the nonintervention arm of the CARE trial [5], which has, with adequate sample size, demonstrated a statistically significant association between occult incontinence and increased risk of POSUI. Our study is also one of few nonintervention studies to investigate the true incidence of POSUI after reconstructive prolapse repair, enabling us to report on the clinical need for later anti-incontinence intervention. The majority of our patients were tested for POSUI <6 months after surgery, and one could argue that there might have been more patients in need of POSUI treatment had we awaited the 1-year postoperative results. However, a 2-year follow-up of the patients in the CARE trial showed no increased incidence of POSUI compared with postoperative assessment at 3 months [22].
Patients with minor SUI were excluded from participation in the study, even when their incontinence was not considered clinically relevant. This was to enable us to test the patients with true occult incontinence. We recognize, however, that excluding this many patients could reduce the clinical relevance of the study, as many POP patients have a minor degree of SUI. It could also have contributed to the low percentage of clinically significant POSUI after prolapse repair. Our study included heterogeneous patients regarding type of prolapse and type of prolapse surgery performed. This heterogeneity was deliberate, as one of the study goals was to identify a preoperative occult incontinence test or combination of tests simple enough to be implemented in an ordinary clinical setting, which implies a heterogeneous POP population. It is well known that occult UI may be demonstrated by prolapse reduction in any compartment, even in isolated defects of the posterior vaginal wall [23]. A test with high performance in predicting POSUI for any individual woman with POP, regardless of dominating compartment, would therefore be preferable. Our sample size did not allow meaningful subanalyses for the various dominating POP compartments in relation to POSUI prediction. We also recognize that type of surgery could have an impact on the incidence of POSUI, as different techniques may alter the vaginal axis differently when aiming for restoration of normal anatomy. However, the majority (67 %) of our patients underwent a traditional Manchester operation (Table 1), consisting of an anterior and posterior colporrhaphy and cervical amputation, and the performance of the various preoperative tests (individual or combinations) was similar for this largest group as for the entire study group (data not shown). Neither the surgeon nor the doctor performing the postoperative follow-up was blinded to the results of the preoperative testing. In our opinion, a lack of blinding did not influence results, as type of surgery was decided upon before testing and primary outcome was defined as subjective information supplied by the patient on a validated questionnaire. The patient herself was not actively informed about her preoperative testing status.
This study demonstrates a positive association between occult incontinence and risk of developing POSUI. However, it also illustrates that a statistical risk demonstrated by a measure of association (such as OR) poorly describes the tests’ ability as a risk marker for individual women and thereby the tests’ clinical usefulness. Even when combining provocation tests, we found poor predictive values. Based on this study, the risk of being in need of treatment for POSUI after prolapse surgery was as low as 4.4 % if there were no pre-existing subjective or objective incontinence. We therefore believe that incontinence surgery should be reserved for the few patients who develop bothersome de novo SUI after reconstructive pelvic surgery. We suggest that incontinence surgery should not be offered as a prophylactic procedure to patients demonstrating occult incontinence.
References
Borstad E, Rud T (1989) The risk of developing urinary stress-incontinence after vaginal repair in continent women. A clinical and urodynamic follow-up study. Acta Obstet Gynecol Scand 68:545–549
Brubaker L, Cundiff GW, Fine P et al (2006) Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med 354:1557–1566
Ellstrom Engh AM, Ekeryd A, Magnusson A, Olsson I, Otterlind L, Tobiasson G (2011) Can de novo stress incontinence after anterior wall repair be predicted? Acta Obstet Gynecol Scand 90:488–493
Groutz A, Gold R, Pauzner D, Lessing JB, Gordon D (2004) Tension-free vaginal tape (TVT) for the treatment of occult stress urinary incontinence in women undergoing prolapse repair: a prospective study of 100 consecutive cases. Neurourol Urodyn 23:632–635
Visco AG, Brubaker L, Nygaard I et al (2008) The role of preoperative urodynamic testing in stress-continent women undergoing sacrocolpopexy: the Colpopexy and Urinary Reduction Efforts (CARE) randomized surgical trial. Int Urogynecol J Pelvic Floor Dysfunct 19:607–614
Liang CC, Chang YL, Chang SD, Lo TS, Soong YK (2004) Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol 104:795–800
Ghoniem GM, Walters F, Lewis V (1994) The value of the vaginal pack test in large cystoceles. J Urol 152:931–934
Gordon D, Groutz A, Wolman I, Lessing JB, David MP (1999) Development of postoperative urinary stress incontinence in clinically continent patients undergoing prophylactic Kelly plication during genitourinary prolapse repair. Neurourol Urodyn 18:193–197
Gregorio G, Hillemanns HG (1990) Urethral closure function in women with prolapse. Int Urogynecol J 1:143–145
Klutke JJ, Ramos S (2000) Urodynamic outcome after surgery for severe prolapse and potential stress incontinence. Am J Obstet Gynecol 182:1378–1381
Richardson DA, Bent AE, Ostergard DR (1983) The effect of uterovaginal prolapse on urethrovesical pressure dynamics. Am J Obstet Gynecol 146:901–905
Rosenzweig BA, Pushkin S, Blumenfeld D, Bhatia NN (1992) Prevalence of abnormal urodynamic test results in continent women with severe genitourinary prolapse. Obstet Gynecol 79:539–542
Bergman A, Koonings PP, Ballard CA (1988) Predicting postoperative urinary incontinence development in women undergoing operation for genitourinary prolapse. Am J Obstet Gynecol 158:1171–1175
Kulseng-Hanssen S, Borstad E (2003) The development of a questionnaire to measure the severity of symptoms and the quality of life before and after surgery for stress incontinence. BJOG 110:983–988
Bossuyt PM, Reitsma JB, Bruns DE et al (2003) Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Clin Chem Lab Med 41:68–73
Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P (2004) Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol 159:882–890
Daneshgari F, Kong W, Swartz M (2008) Complications of mid urethral slings: important outcomes for future clinical trials. J Urol 180:1890–1897
deTayrac R, Gervaise A, Chauveaud-Lambling A, Fernandez H (2004) Combined genital prolapse repair reinforced with a polypropylene mesh and tension-free vaginal tape in women with genital prolapse and stress urinary incontinence: a retrospective case–control study with short-term follow-up. Acta Obstet Gynecol Scand 83:950–954
Ballert KN, Biggs GY, Isenalumhe A Jr, Rosenblum N, Nitti VW (2009) Managing the urethra at transvaginal pelvic organ prolapse repair: a urodynamic approach. J Urol 181:679–684
Chaikin DC, Groutz A, Blaivas JG (2000) Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol 163:531–534
Kleeman S, Vassallo B, Segal J, Hungler M, Karram M (2006) The ability of history and a negative cough stress test to detect occult stress incontinence in patients undergoing surgical repair of advanced pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 17:27–29
Brubaker L, Nygaard I, Richter HE et al (2008) Two-year outcomes after sacrocolpopexy with and without burch to prevent stress urinary incontinence. Obstet Gynecol 112:49–55
Myers DL, Lasala CA, Hogan JW, Rosenblatt PL (1998) The effect of posterior wall support defects on urodynamic indices in stress urinary incontinence. Obstet Gynecol 91:710–714
Acknowledgement
Study assistance from Øystein Bjørnerud, urotechnician at Department of Urology, Oslo University Hospital, contributing to the preoperative testing and data collection, is greatly appreciated.
Financial Disclosure/Conflicts of Interest
R. Svenningsen: None; Ellen Borstad has received speaker fees from Pfizer, Astellas, and Lilly and served on advisory boards for Astellas and Lilly; Anny Spydslaug has received speaker fees and served as paid consultant for Pfizer; L. Sandvik: None; AC Staff: None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Svenningsen, R., Borstad, E., Spydslaug, A.E. et al. Occult incontinence as predictor for postoperative stress urinary incontinence following pelvic organ prolapse surgery. Int Urogynecol J 23, 843–849 (2012). https://doi.org/10.1007/s00192-012-1764-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-1764-5