Abstract
Introduction and hypothesis
The aim of the study was to determine which magnetic resonance imaging (MRI) reference line for staging pelvic organ prolapse, the pubococcygeal line (PCL) vs. the midpubic line (MPL), has the highest agreement with clinical staging.
Methods
A retrospective study of women with pelvic floor complaints who underwent dynamic pelvic MRI from January 2004 to April 2007 was conducted. Two radiologists staged descent on MRI for each pelvic compartment (anterior, apical, posterior) by consensus, using PCL and MPL reference lines. Agreement between MRI and clinical staging was estimated using weighted kappas.
Results
Twenty women were included. Agreement between clinical and PCL staging was fair in the anterior (κ = 0.29) and poor in the apical (κ = 0.03) and posterior (κ = 0.08) compartments. Agreement between clinical and MPL staging was fair in the anterior (κ = 0.37), apical (κ = 0.31), and posterior (κ = 0.25) compartments.
Conclusions
The MPL has higher agreement with clinical staging than the PCL. However, neither reference line has good agreement with clinical staging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse is a relatively common, but complex condition for which several different imaging techniques, including fluoroscopy, ultrasound, and magnetic resonance imaging (MRI), can be used to complement or clarify physical examination findings. MRI has several advantages over more conventional fluoroscopic and sonographic imaging methods for assessing pelvic organ prolapse including its ability to simultaneously evaluate all of the pelvic organs in multiple planes and relatively noninvasively with high soft tissue and temporal resolution and without the use of ionizing radiation. In addition, MRI can visualize the muscular and ligamentous pelvic floor support structures and detect incidental findings that might alter patient management including uterine fibroids and adnexal masses. In a recent study of 50 patients with fecal incontinence, MRI also reportedly changed the surgical approach in 22 (67%) of 33 patients who underwent surgery [1].
While the use of MRI for evaluating pelvic organ prolapse has been well described [2–9], a standardized system for assessing and documenting the findings of pelvic organ prolapse on MRI does not currently exist. A variety of reference points and lines for staging pelvic organ prolapse with imaging have been proposed. The two most commonly described are the pubococcygeal line (PCL; from the inferior aspect of the symphysis pubis to the last joint of the coccyx) and midpubic line (MPL; midsagittal long axis of the symphysis pubis). Initial studies of MRI for pelvic organ prolapse used the PCL reference line as it is based on fixed bony points of reference. The PCL is also believed to provide an estimate of the pelvic floor as it lies along the plane of the pubococcygeal and puborectalis muscles [10]. More recently, Singh et al. described the use of the MPL for MRI staging of pelvic organ prolapse. They confirmed on cadaveric dissection that the midpubic line extends through the level of the vaginal hymen, the landmark used for clinical staging [11].
In contrast, a standardized system for assessing and documenting pelvic floor prolapse on physical exam, called the pelvic organ prolapse quantification (POP Q) system exists and was proposed by the International Continence Society in 1995. Since then, this clinical staging system has been widely adopted [12]. In this system, the descent of each compartment (anterior: bladder and urethra; apical: uterus and cervix and/or small bowel; posterior: rectum and anal canal) is measured with the patient at a 45° supine position during Valsalva, using the vaginal hymen as a reference point.
To date, there are limited data comparing both MRI PCL and MPL staging of pelvic organ prolapse with clinical staging in symptomatic women. This can create disparity between clinical and radiological evaluations of the pelvic compartments, decreasing accuracy of communication and comparison of clinical and radiologic findings. For imaging to be an applicable clinical tool, a standardized and reproducible staging system that correlates with and translates to clinical findings is needed to report imaging findings. Accurate assessment of all three compartments is important to help guide management, particularly in women considering surgical treatment. Therefore, the primary objective of this retrospective study is to determine which MRI reference line (PCL vs. MPL) has the highest agreement with POP Q clinical staging.
Materials and methods
This study was approved by the Institutional Review Board of Women and Infants’ Hospital and Rhode Island Hospital (Providence, RI, USA). A retrospective search of the radiology report database for all women over the age of 18 referred for dynamic MRI evaluation of pelvic organ prolapse at our institution from January 2004 to June 2007 was performed. Clinical records were reviewed, and women were included in the study if POP Q staging was available.
The MRI examinations for all women were performed using either a 1.5-T (Symphony; Siemens, Malvern, PA, USA) or 1.0-T (Harmony; Siemens) magnet with a pelvic phased array coil. Immediately prior to the MRI exam, the woman’s rectum was filled with 60 to 120 ml of sonography gel. All women emptied their bladder immediately prior to the MRI study, and water absorbent pads were placed around the women. Imaging was performed head first in the supine position with the woman’s knees flexed over a support cushion. No intravenous contrast was administered.
Following the acquisition of localizing images, sagittal T2-weighted half-Fourier-acquired single-shot turbo spin echo (HASTE) sequences through the pelvis were obtained from the femoral head to femoral head with the woman at rest, during Kegel maneuver (maximal voluntary contraction of the anal sphincter and pelvic floor muscles), and during maximal Valsalva. The imaging parameters for the 1.0-T magnet were as follows: T2-weighted HASTE in the sagittal and coronal plane: TR 750 ms; TE 90 ms; field of view 38 cm; section thickness 4 mm; intersection gap 1 mm; and matrix 104 × 512. The imaging parameters for the 1.5-T magnet were as follows: T2-weighted HASTE in the sagittal and coronal planes: TR 1,150; TE 100; field of view 35 cm; section thickness 7 mm; intersection gap, none; and matrix 224 × 256. Axial and coronal T2-weighted turbo spin echo images were also acquired through the pelvis with the following parameters: TR 5,640 ms; TE 120 ms; field of view 30 cm; slice thickness 4 mm; skip, none; and matrix 300 × 384.
To ensure adequate patient Kegel and Valsalva during the examination, the technologists reviewed the maneuvers with the women immediately prior to the exams and also communicated with them throughout the exams. Average exam time, including patient preparation, was 30 min.
Two radiologists with experience reading pelvic MRI, blinded to all clinical data, reviewed and staged all of the MRI examinations in random order by consensus read, first using the PCL and then the MPL reference line. All MRI studies were reviewed on workstations (Synapse; Fujifilm, Stamford, CT, USA and Cerner PACS, Kansas City, MO, USA) with zoom capability and electronic measurement calipers. PCL and MPL consensus reads for staging were temporally spaced by 4 weeks to minimize potential recall bias.
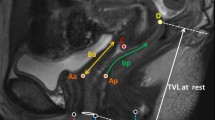
PCL measurements for each exam were performed by drawing a midsagittal line from the inferior margin of the symphysis pubis to the last joint of the coccyx. Data acquisition was divided into three compartments: anterior, apical, and posterior. Perpendicular lines were then drawn from anatomic reference points in each compartment to the PCL line (Fig. 1a). In the anterior compartment, the reference point was the most posterior and inferior aspect of the bladder base. In the apical compartment, the reference point was the anterior cervical lip or the posterior superior vaginal apex if the woman was status post hysterectomy. In cases of asymmetric cervical lips, the most anterior and inferior aspect of the cervix was chosen as the point of reference. In the posterior compartment, the anterior aspect of the anorectal junction served as the point of reference [2, 4, 7, 13].
a, b A 52-year-old female with constipation. Sagittal T2-weighted HASTE MR image at rest demonstrating the a PCL and b MPL measurements. a The PCL (white line) extends from the inferior symphysis pubis to the last coccygeal joint. Compartment measurements made from the bladder base (white arrow) in the anterior compartment, superior vaginal cuff (black arrow), or anterior cervical lip in the apical compartment, and the anterior inferior anorectal junction (curved black arrow) in the posterior compartment. b The MPL (white line) is parallel to the long axis of the midsymphysis pubis. Compartment measurements made from the bladder base (white arrow) in the anterior compartment, superior vaginal cuff (long white arrow), or anterior cervical lip in the apical compartment, and the anterior inferior anorectal junction (black arrow) in the posterior compartment
MPL measurements were performed by drawing a midsagittal line caudally through the long axis of the symphysis pubis. From the same points of reference in the anterior, apical, and posterior compartments as used for PCL staging, perpendicular lines were drawn to the MPL line (Fig. 1b) [3, 5, 7, 11]. The total vaginal length was also measured for MPL staging. Pelvic compartment measurements with respect to the PCL and MPL lines were reported in units of centimeter on the viewing workstations. Measurements for each reference line were obtained during rest, Kegel, and Valsalva positions in all women. The largest measurement during Valsalva, defined as the measurement furthest below the reference line or closest to the reference line if located above the PCL or MPL, was used for further analysis.
In order to compare the MRI measurements with the standardized clinical POP Q staging system, clinical stages were applied to the PCL and MPL measurements as outlined in Table 1. Prior studies have used a similar PCL staging system considering organ prolapse <3 cm below the PCL small, 3 to 6 cm below moderate, and >6 cm below the PCL large prolapse [4, 14]. Prior MRI studies using the MPL reference line have applied the clinical POP Q staging system directly to MPL measurements since both are based on the level of the vaginal hymen [5, 6, 11]. The MPL along the midaxis of the pubic bone has been shown to approximate the level of the vaginal hymen on cadaveric dissection [11]. We did not attempt to propose a new staging system for MRI as the clinical staging system is well established and this same staging system can be applied to MRI measurements. We choose the clinical POP Q exam as our study reference given that it has been validated and is a widely accepted standard.
The MRI studies were also analyzed for pelvic floor structural abnormalities. These included rectoceles, which were defined as a >2-cm rectal wall bulge ventral to a line drawn parallel to the long axis of the anterior anal canal, and enteroceles, sigmoidoceles, and peritoneoceles, defined as extension of small bowel, sigmoid colon, or peritoneal fat, respectively, between the posterior vaginal wall and anterior rectal wall for a length >2 cm [4, 9].
Medical charts for all women were reviewed by a fellowship trained urogynecologist blinded to the MRI results. Demographic and clinical data, including symptoms of pelvic floor dysfunction and pelvic organ prolapse staging using the POP Q, were recorded. All recorded POP Qs were performed under the supervision of one of the two fellowship-trained urogynecologists. Each patient was examined by one urogynecologist.
Agreement between MRI staging and clinical staging was estimated using weighted κ coefficients (poor agreement, κ = <0.2; fair agreement, κ = 0.21–0.40; moderate agreement, κ = 0.41–0.60; good agreement, κ = 0.61–0.80; and very good agreement, κ = 0.81–1.00) [15]. All statistical analyses were performed using STATA 9.0 (Stata Corporation, College Station, TX, USA).
Results
Twenty women were included in this study. Mean age was 54.9 years (range 41–84 years) and median parity was 2 (range 0–3). Clinical patient characteristics included prior pelvic surgery in 15/20 (75%), urinary incontinence symptoms in 13/20 (65%), pelvic organ prolapse symptoms in 17/20 (85%), pelvic pain symptoms in 5/20 (25%), constipation symptoms in 5/20 (25%), and fecal incontinence symptoms in 3/20 (15%).
Table 2 outlines the MRI stages for prolapse in the anterior, apical, and posterior compartments using the PCL and MPL lines as compared to the clinical POP Q stage for each patient. Overall, MRI staging with the PCL reference line compared to clinical staging resulted in similar staging of 22, understaging of 31, and overstaging of seven measurements in the anterior, apical, and posterior compartments combined. MRI staging with the MPL reference line compared to clinical staging resulted in similar staging of 34, understaging of nine, and overstaging of 17 measurements in the anterior, apical, and posterior compartments combined (Figs. 2, 3, and 4). Additional MRI findings included anterior rectoceles in 12 women, sigmoidoceles in three women, and peritoneoceles in two women.
A 62-year-old female with pelvic pain and incontinence. Sagittal T2-weighted HASTE (1,150/100) MR image at stress demonstrates uterine descent with the anterior cervical lip (arrow) extending 4.5 cm below the PCL (stage 2) and within 1 cm of the MPL (stage 2). Clinical POP Q for the apical compartment was stage 1
A 45 year old female with fecal incontinence. Sagittal T2-weighted HASTE (1,150/100) MR image at stress depicts rectal descent. The anterior inferior aspect of the anorectal junction (arrow) is 6 cm below the PCL (stage 2) and within 1 cm of the MPL (stage 2). Clinical POP Q for the posterior compartment was stage 2
Statistical analysis of clinical stage by compartment as compared to the two MRI reference lines revealed that both the PCL (κ = 0.29, standard error (SE) 0.15) and MPL (κ = 0.37, SE 0.12) had fair agreement with clinical staging in the anterior compartment. For the apical and posterior compartments, MPL agreement with clinical staging was fair in both compartments (κ = 0.31, SE 0.12 for apical and κ = 0.25, SE 0.14 for posterior compartment) while PCL agreement was poor in both compartments (κ = 0.03, SE 0.06 for apical and κ = 0.08, SE 0.6 for posterior compartment).
Discussion
This is the first published study documenting the comparison of MPL and PCL reference lines to POP Q staging of women with symptomatic pelvic organ prolapse. In our study population, the MPL demonstrates better agreement with clinical staging than the PCL. However, neither line has greater than fair to poor agreement with clinical staging, and therefore, neither the PCL nor the MPL can be considered a direct correlate for clinical POP Q staging.
Several studies have shown that MRI is a useful method for diagnosing and staging pelvic organ prolapse, having similar detection rates as fluoroscopic techniques and often revealing more extensive organ prolapse than physical exam alone [1, 4, 6, 8]. MRI has the additional advantages of allowing for noninvasive, multiplanar assessment of all three pelvic compartments simultaneously, assessing pelvic organ prolapse dynamically, and evaluating the integrity of the pelvic floor musculature. At our institution, dynamic MRI of the pelvis is often chosen as a diagnostic tool when physical exam findings and patient symptoms do not correlate, or in women with clinically suspected multicompartment or posterior compartment abnormalities. Despite the well-documented usefulness and advantages of MRI for evaluating pelvic organ prolapse, increased clinical use of MRI for pelvic prolapse has been limited. This limited use is likely in part due to the absence of a standardized protocol and reporting system for MRI staging of pelvic organ prolapse, making it difficult to correlate clinical and MRI staging and complicating the translation of MRI findings to a clinical picture.
Additional prior studies have compared various MRI reference points and lines to clinical staging with variable results [3, 5, 7, 9, 11, 13]. In a study of 20 women with pelvic organ prolapse and ten controls, Singh et al. found that MRI staging using the MPL correlated with clinical findings in 75% of cases and overstaged the degree of pelvic organ prolapse in the remaining 25% of cases [11]. Lienemann et al. compared MRI staging with the PCL, MPL, and axial line (horizontal line along the inferior aspect of the symphysis pubis) to clinical POP Q staging in 41 asymptomatic women and found that none of the reference lines were consistently comparable to clinical staging in all three compartments [7]. Cortes et al. compared MRI staging of the apical compartment in 51 women after hysterectomy and found that the MPL did not correlate with clinical POP Q staging in the apical compartment [5]. In one of the more recent studies by Etlik et al. in 2005, clinical staging using the Baden classification system was compared to MRI staging using the MPL in 46 women with prolapse symptoms and 30 asymptomatic women. They found the physical exam and MRI findings to be concordant (p < 0.001 for the anterior and apical compartments and p < 0.05 for the posterior compartment) [3].
Our results of the MPL reference line showing higher agreement with clinical staging than the PCL, and, when not in agreement, more often overstaging than understaging organ prolapse in the anterior and posterior compartments, support the findings of Singh et al. that the MPL correlates with or overstages pelvic organ prolapse compared to clinical staging [11]. Our study results are also consistent with the findings of Lienemann et al. that the MPL has higher clinical agreement than the PCL in the posterior compartment [7]. However, neither the MPL nor the PCL showed good agreement with clinical staging in our study which may be due to multiple factors, including differences in patient positioning between clinical (45° supine/lithotomy) and MRI (supine) examinations, differences in patient Valsalva effort, and differences in the precision with which measurements can be made clinically (physician using a device scored at 1 cm increments) and with MRI (electronic tool measuring to 1/100 of a centimeter). Agreement in the apical compartment may have also been limited by the use of the posterior superior vaginal cuff as the point of reference on MRI in patients status post hysterectomy, which does not directly compare to the clinical reference point, point C, for staging the apical compartment.
Even though the PCL and MPL agreement with clinical staging was the same in the anterior compartment, the results of our study suggest that while still only fair to poor, the MPL has a higher agreement with clinical staging than the PCL. In addition, both the MPL and the clinical POP Q examination are based on the level of the vaginal hymen, which may allow for more consistent comparison of clinical and MRI findings even when women are staged differently.
Limitations of our study include its retrospective design and relatively small sample size. The study population was limited to women who had clinical records available for review that included a detailed description of the clinical POP Q examination. Our study also likely included more complicated pelvic prolapse patients as simple anterior and apical prolapse patients are not typically referred for MRI at our institution. In addition, MRI examinations performed over the course of 4 years on two different magnets were included in this study resulting in slight differences in MRI technique between women. However, the examinations for all women included the requisite rest, Kegel, and Valsalva sagittal T2-weighted HASTE sequences. Finally, MR measurements were made by consensus, so no kappa statistic could be calculated to demonstrate the effect of reader measurement variability on staging. Similarly, each patient was only examined by one of two urogynecologists, which did not allow for inter-rater reliability assessment. A larger prospective study could be performed to address many of these issues and to confirm our findings that while still only fair to poor, the MPL reference line has higher agreement with clinical staging than the PCL reference line.
In conclusion, we found that neither MR-based reference line had good agreement with clinical staging. However, the MPL had higher agreement than the PCL reference line with POP Q clinical staging in women with pelvic floor symptomatology, especially for the apical and posterior compartment. In addition, the MPL reference line more often overstaged women with respect to clinical staging than did the PCL reference line. This suggests that the MPL may classify more women with greater degrees of pelvic organ prolapse than physical exam alone, helping to further guide patient management. Appropriate treatment of pelvic organ prolapse relies on accurate diagnosis and evaluation. Coming to an agreement on a standardized MRI measuring and reporting system for pelvic organ prolapse would likely help to improve correlation with clinical findings, communication, and ultimately patient management.
References
Hetzer FH, Andreisek G, Tsagari C, Sahrbacher U, Weishaupt D (2006) MR defecography in patients with fecal incontinence: imaging findings and their effect on surgical management. Radiology 240:449–457
Kelvin FM, Hale DS, Maglinte DD, Patten BJ, Benson JT (1999) Female pelvic organ prolapse: diagnostic contribution of dynamic cystoproctography and comparison with physical examination. AJR 173:31–37
Etlik Ö, Arslan H, Odabaşi O, Odabaşi H, Harman M, Celebi H, Sakarya ME (2005) The role of the MR-fluoroscopy in the diagnosis and staging of the pelvic organ prolapse. Eur J Radiol 53:136–141
Kelvin FM, Maglinte DDT, Hale DS, Benson JT (2000) Female pelvic organ prolapse: a comparison of triphasic dynamic MR imaging and triphasic fluoroscopic cystocolpoproctography. AJR 174:81–88
Cortes E, Reid WMN, Singh K, Berger L (2004) Clinical examination and dynamic magnetic resonance imaging in vaginal vault prolapse. Obstet Gynecol 103:41–46
Kaufman HS, Buller JL, Thompson JR, Pannu HK, DeMeester SL, Genadry RR et al (2001) Dynamic pelvic magnetic resonance imaging and cystocolpoproctography alter surgical management of pelvic floor disorders. Dis Colon Rectum 44:1575–1583
Lienemann A, Sprenger D, Janßen U, Grosch E, Pellengahr C, Anthuber C (2004) Assessment of pelvic organ descent by use of functional cine-MRI: which reference line should be used? Neurourol Urodyn 23:33–37
Gufler H, Laubenberger J, DeGregorio G, Dohnicht S, Langer M (1999) Pelvic floor descent: MR imaging using a half-Fourier RARE sequence. J Magn Reson Imaging 9:378–383
Comiter CV, Vasavada SP, Barbaric ZL, Gousse AE, Raz S (1999) Grading pelvic prolapse and pelvic floor relaxation using dynamic magnetic resonance imaging. Urology 54:454–457
Yang A, Mostwin JL, Rosenshein NB, Zerhouni EA (1991) Pelvic floor descent in women: dynamic evaluation with fast MR imaging and cinematic display. Radiology 179:25–33
Singh K, Reid WMN, Berger LA (2001) Assessment and grading of pelvic organ prolapse by use of dynamic magnetic resonance imaging. Am J Obstet Gynecol 185:71–77
Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JOL, Klarskov P, Shull BL, Smith ARB (1996) The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 175:10–17
Hodroff MA, Stolpen AH, Denson MA, Bolinger L, Kreder KJ (2002) Dynamic magnetic resonance imaging of the female pelvis: the relationship with the pelvic organ prolapse quantification staging system. J Urol 167:1353–1355
Bertschinger KM, Hetzer FH, Roos JE, Treiber K, Marincek B, Kilfiker PR (2002) Dynamic MR imaging of the pelvic floor performed with patient sitting in an open-magnet unit versus patient supine in a closed-magnet unit. Radiology 223:501–508
Altman DG (1991) Practical statistics for medical research. Chapman and Hall, London
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woodfield, C.A., Hampton, B.S., Sung, V. et al. Magnetic resonance imaging of pelvic organ prolapse: comparing pubococcygeal and midpubic lines with clinical staging. Int Urogynecol J 20, 695–701 (2009). https://doi.org/10.1007/s00192-009-0865-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-009-0865-2