Abstract
The rapid deterioration of the environmental situation comes mainly from environmental pollution by industrial and municipal wastes in the form of industrial and landfilled industrial and municipal wastes. The only way out of this threatening situation is the urgent utilization of all these wastes as valuable raw materials alternative to traditional natural materials. This article describes in detail the result of the complete replacement of natural materials with four industrial wastes—from iron ore processing, cellulose and pulp production, concrete production and demolition, and lime production—without changing the technological process nor creating new residues. The samples’ axial resistance values reached up to 4.94 MPa after 3 curing days, 5.75 MPa after 7 days, and 11.47 MPa on the 720th day of outdoor curing. The XRD, SEM, EDS, and LAMMA methods demonstrated the sol–gel solutions’ chemical interaction process and their gradual densification to a stone-like state. The developed materials have high mechanical and environmental properties and can be used to produce construction materials, such as bases of road and airfield runways, levee cores, industrial and municipal dumps, building foundations, bricks, and blocks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The steadily deteriorating environmental situation on our planet is becoming increasingly threatening for humanity’s survival in the near future. One of the main reasons for this impending disaster is the atmosphere’s clogging with gasses and dust from industrial and municipal waste. Therefore, the only escape from this problematic situation is the scientifically based emergency use of all types of wastes at the industrial level.
The aforementioned problem served as the basis for the development of construction materials, such as cementless concrete from four different types of industrial wastes, namely, iron ore processing waste (IOW) in composites with paper and cellulose pulp waste (CPW), concrete production and demolition waste (CW, and lime production waste (LPW), without the application of any traditional natural components, such as sand, gravel, and rubble, whose quarries irreversibly destroy nature.
Iron ore reserves in Brazil represent 11.9% of world reserves [1]. Thus, iron ore extraction is a vital economic activity in Brazil, corresponding to 87.7% of primary goods exported and 4% of BGP (Brazilian Gross Product). In Brazil, approximately 379 thousand tons of IOW is generated daily, which is dumped in 672 tailings dams [2]. IOW’s annual discharge in China is 300 million tons; however, IOW reuse is inferior to 10% [3]. In Chile, 1.6 million tons of IOW is produced every day [4]. More than 1.2 billion tons of IOW has already been stored in the European Union and billions of tons globally [5], increasing between 5 and 7 billion tons per year in emerging countries [6].
In the rupture of Mariana’s tailing dam, in 2015, considered one of the biggest environmental disasters in Brazil and the world, more than 35,000,000 m3 of mud was released into the environment and reached the Atlantic Ocean, affecting ecosystems over 662 km downstream of the Doce River [7]. A similar tragedy occurred again in the city of Brumadinho in 2019, leaving 259 deaths and 11 people missing from this huge environmental disaster.
The use of mining waste as a raw material resource can be a good proposal for the supply of an alternative material [8] that also adds significant economic value to the generating companies. Do and Mun [9] used a type of IOW to produce cement clinker by the conventional sintering process, varying the IOW amounts from 0 to 20% by weight.
The pulp and paper mills also engender a wide variety of organic and inorganic residues, becoming a primary environmental concern because about 300 kg of sludge is generated for every ton of paper produced [10]. Global paper and cardboard production reached approximately 419.7 million tons in 2017 [11], which means that approximately 126 million tons of highly alkaline wastes are discharged into industrial dumps each year, increasing and creating serious environmental problems for our planet. Therefore, there are a considerable number of studies on methods of disposal for this waste.
In this study, three main types of waste from the last stage of the kraft caustification process of paper and pulp production were used, called dregs, grits, and lime mud. Iyaras [12] determined the influence of the paper mill’s and carbonation’s sludges on the clay-based bricks’ physical, mechanical, and thermal properties. Lime mud can be used as low-cost substrates with a primary focus on wastes from agro, food, brewery, sugar industries, lignocellulosic biorefineries, and textile and pulp mills [13]. Gemelli et al. [14] studied the influence of the application of cellulose and paper residues: dregs, grits, ash, and fibers, in construction mortars. The kraft pulp dregs’ high alkalinity was used to neutralize acid mine drainage from the Witwatersrand goldfields in Johannesburg, South Africa [15].
Silva et al. [16] applied dregs and lime mud residues in the construction of road foundation and sub-foundation Lime mud from Swedish pulp and paper mills was employed by Hu et al. [17] in the stainless steel manufacturing process, partially replacing primary lime.
Machado et al. [18] studied the excellent synergy between cellulose fiber and natural rubber latex. Besides, cellulose fibers are predominantly stripped from the matrix during the fracture of the composites. Mokhena and John [19] published a review describing recent advances in the production and application of cellulose nanomaterials.
Concrete waste (CW) from the demolition process goes through three stages—screening to remove any impurities, such as steel, wood, plaster, masonry, glass, and plastic; grinding; and sieving to separate the aggregate particle sizes. The total amount of waste generated in the European Union in 2010 was over 2.5 billion tons, of which almost 35% (about 860 million tons) came from construction and demolition, with the recycling of up to 46% in 2018 [20]. The global annual consumption of natural aggregates was around 15 billion tons [21]. The concern about the availability of natural aggregates and the effect of their extraction on the environment are quite pronounced and worrying. Some authors [22] reported CW production’s level until 40% of the worldwide industrial and municipal waste generation.
Bassani et al. [23] looked into different by-products as alternative binders for stabilizing bases and sub-bases using aggregates of CW, with positive results for constructing pavement layers. The 2019 statistic specified the total world lime production at 430 million tons [24]. Under Brazilian requirements [25], the total amount of hydrates, CO2, and other elements (such as Al2O3, SiO2, Fe2O3) in commercial lime must not exceed 12%. Otherwise, this material is called lime production waste (LPW). No information was found on the amount of LPW. However, it is known that LPW is widely used simultaneously with carbonates in the production of Portland cement, mortars, in road construction, in the iron and steel industry for slag formation, aluminum, and other non-ferrous metals, such as copper, nickel, and zinc, for the suppression of bacteria in the sludge of municipal sanitation stations. It was used in composites with hazardous sewage sludge [26] and bauxite red mud [27] for construction materials production.
Many other studies on the use of these four industrial wastes have been found in the world scientific and technical literature. The fundamental difference from these for the current study is the 100% rejection of natural materials application as components of the developed materials to increase their environmental and economic efficiency.
1.1 Objectives of the study
The objectives of the study are the following: development of new sustainable composites based only on wastes from industries such as pulp and cellulose production, iron ore processing, concrete production and demolition, and lime production for civil construction with properties that meet the demands of Brazilian standards; characterization of the properties and structures of the industrial wastes used as raw materials; and investigation of the physical–chemical processes of the developed composite structure formation and their properties’ changes during hydration and cure.
2 Materials and methods
2.1 Raw materials and test samples (TSs) preparation
Concrete (CW) and lime production (LPW) wastes were collected from companies in the metropolitan region of Curitiba, Brazil. The iron ore waste (IOW) was obtained at the Fundão dam after its burst (2015) in Mariana, MG, Brazil. For the full use of all three wastes from the paper and cellulose production, a mixture was prepared at the local Berneck plant, after the last caustification stage, in proportion to their annual production, namely, 34.33% drags, 37.12% grits, and 28.55% lime sludge, with the common name “cellulose production waste” (CPW).
The dry mix of components was manually homogenized; hydrated at 15–17%; compacted at a force of 10 MPa for 30 s in a Bovenau manual hydraulic press, in a cylindrical format with a height and diameter of 20 mm; and stored outdoors for multiple test periods; 55 test samples were made for each of the 19 compositions. The total test sample quantity was 1140 pieces. To reduce the tables and the article’s volume, 8 of the most informative compositions were selected.
2.2 Methods
A complex of modern methods was applied to study the raw materials, the developed compositions, and the physicochemical processes of their structure’s formation. The particle size distribution was determined by laser diffraction analysis in combination with the sieve method. The chemical composition of the raw materials was established by X-ray fluorescence analysis (XRF). The mineral composition was analyzed by X-ray diffraction (XRD), using the minerals’ automated database PDF-2. The new formations’ chemical composition was investigated by the energy-dispersive X-ray spectroscopy (EDS) method and the isotope composition by laser micro-mass analysis (LAMMA). The samples’ morphological structure was studied by scanning electron microscopy (SEM), and solubility and leaching of metals were determined by atomic absorption analysis (AAA).
The axial resistance under compression, coefficients of linear dilatation and shrinkage, water absorption, and water resistance of the developed ceramic materials were determined in different hydration periods.
2.3 Calculations
The water resistance coefficient (CWR) was determined based on the ratio [28]:
where, RSAT is the axial compressive strength of the saturated TSs after 24-h total water immersion, and RD is the axial compressive strength of the oven-dried specimen at 100 °C for 24 h.
Water absorption (WA, %) values were calculated under NBR 9778 [29], using the equation:
where, MD is the sample mass after 24-h oven drying at a temperature of 110 °C (g), and Ms is the sample mass after 24-h water immersion at room temperature (g).
2.4 Characterization of the raw materials
2.4.1 Particle size distribution of the raw materials
The particle size distribution of the studied wastes was classified as fine grains by the sieve method, through the material’s flow in a mesh of 4.76 mm to 250 µm, per NBR 248 [30]. The particles with sizes from 180 to 1 µm were analyzed in a Cilas 1064 laser granulometer. The head part of the IOW (77.89%) was 1.68–2.38 mm in size (Table 1), with a specific mass of 3.280 kg/m3, and a pH value of 10.21.
Concrete waste (CW) mainly consisted (87.8%) of particle sizes between 4.76–2.00 mm with a specific mass of 2.280 kg/m3 and pH = 10.34. Silva et al. [16] obtained very different results, with up to 90% of CW particles inferior to 1.19 mm in diameter.
The LPW’s particle size distribution curve showed two peaks with high particle size content: the first one between 1.68–2.38 mm with 43.4% of particles, and the second one with 37.6% of particles at 1.19 mm. LPW specific mass was 1.250 kg/m3 and pH = 11.00 [31].
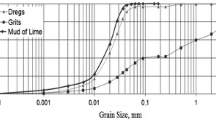
The maximum volume (65.98%) of CPW particles (Table 2) had a diameter 0.010–0.028 mm with pH = 11.62, which is well in line with the pH range = 10–12.8 presented by Manskinen et al. [32]; Piotto [33] reported an even higher pH for CPW, from 13 to 14.
2.4.2 Chemical composition of the raw materials
The chemical composition of IOW (Table 3) consisted mainly of Fe2O3 and SiO2 (sum 90.0%), CW – of CaO and SiO2 (sum 71.6%), LPW – of CaO and MgO (sum 84.0%), and CPW – of CaO (52.3%) with an extremely high calcination loss (C.L. = 41.3%) due to the high carbonate content.
For the same reason, C.L. was 16.2% for LPW and 10.6% for CW. Other authors have also observed high carbonates CaCO3 content in cellulose production sludge [15, 32].
2.4.3 Mineral composition of the raw materials (by XRD method)
Mineral composition of iron ore waste (IOW, Fig. 1A) comprised three iron oxides—hematite Fe2O3, magnetite Fe3O4, and goethite FeO(OH); the mineral of clay kaolinite Al2Si2O5(OH)4; and, the most common mineral in the Earth’s crust, quartz SiO2. All mineral peaks had an extremely low intensity, except for one peak of quartz at the angle of 2Θ° = 26.8° and one common peak of hematite and magnetite, which coincided at an angle of 2Θ° = 27.2°. The intensity of both maximum peaks was extremely low—about 200 cps (counts per second); all the remaining peaks only slightly exceeded the X-ray background, indicating either a massive dissociation of their crystal lattices due to the mechanical and chemical processes of iron ore processing or due to the rapid cooling of this volcanic lava during its outflow.
XRD pattern of concrete waste (Fig. 1B) showed the presence of the not fully hydrated mineral of Portland cement carbonate brownmillerite (4CaO·Al2O3·FeO3), portlandite Ca(OH)2, calcite CaCO3, and dolomite CaMg(CO3)2, and quartz SiO2.
Mineral composition of LPW (Fig. 1C) confirmed the correct classification of this material as a waste of lime production since no lime crystal lattice peaks were found regardless of concurrence with other minerals. Simultaneously, the most intense peaks are those of portlandite (as hydrated lime) and calcite and dolomite (as portlandite, which reacted with atmospheric CO2).
The cellulose production wastes (CPW) showed the highest crystallinity level among the raw materials (Fig. 1D) since the highest calcite peak at 2Θ° = 29.5° reached 3000 cps and many other peaks approached 500cps. CPW included calcite CaCO3, dolomite CaMg(CO3)2, pirssonite Na2Ca(CO3)2·2H2O, and quartz SiO2. This mineral composite of CPW was in line with the research by Machado et al. [18].
2.4.4 Morphological microstructures of the raw materials (by SEM method)
Almost all particles of the raw materials, studied by the SEM method at the micro-level (Fig. 2), presented different shapes with sizes between 1 and 50 µm. A significant amount of SW and LPW (Fig. 2B and C) was in the form of separate spherical or irregular rounded particles or their adhering aggregates. Only CPW particles (Fig. 2D) were represented by fragments of crystal-like combinations of prism and pyramid forms. Inside these cellulose crystals, channels of different diameters, characteristic of the wood structure, were neatly visible.
3 Mechanical and physical properties of developed materials
In order to study the feasibility of using the developed compositions as construction materials, the changes in their main properties were investigated throughout the hydration and hardening process of the test samples, particularly, their axial resistance, linear expansion, shrinkage, water absorption, and water resistance.
3.1 Axial resistance of the test samples
Composition 7 with almost maximum IOW (40%) and minimum CPW content (10%) showed the maximum resistance values at all ages (Table 4), followed by compositions 8, 6, and 5. The 30% difference in IOW and CPW amounts between compositions 6 and 7 or 25% between compositions 7 and 8 demonstrated IOW’s beneficial role regarding CPW. This conclusion was corroborated by comparing the resistance of compositions 1 and 2, or 3 and 4, or compositions 1 and 3, or 2 and 4; samples with higher IOW content, with equal amounts of other components, always exhibited higher resistance.
The IOW content reduction in composition 6 compared to compositions 1 and 5 was offset by the increase in LPW to 10% and 5%, correspondingly. Zuccheratte et al. [34] evaluated concrete production using IOW as aggregate, obtaining a 7-day compressive resistance for concrete of approximately 9.0 MPa. The maximum resistance of the 7-day-age samples reached 5.75 MPa (Table 3), but these authors’ Portland cement’s cementitious properties [34] are difficult to compare to the same properties of lime production waste.
The positive role of the LPW was especially evident when replacing 10% CPW with an equal amount of LPW in compositions 2 and 7, leading to an almost double increase in resistance at 720 days (11.47 versus 6.26 MPa). The compositions’ resistance values increased with the curing time and directly proportional to LPW and IOW contents.
According to the Brazilian standard NBR 7170 [35], solid bricks are classified into three classes: Class A > 1.5 MPa; Class B > 2.5 MPa; and Class C > 4.0 MPa. On the third curing day, the resistance significantly exceeded the demands for Class A; on the 28th day, four compositions have beaten the demand for Class C. At the age of 1 year, all compositions considerably surpassed the requirements for solid bricks Class C.
3.2 Coefficient of water resistance (CWR) of the test samples (TSs)
The water resistance values of the TSs (Table 5) were calculated using Eq. (1) [28]. The analysis of the study results showed (Table 5) an increase in CWR with the hydration and hardening time from 28 to 90 days.
As the increase in resistance (Table 4), the dependence of the materials’ CWR with the content of the component used as a binder (LPW) and as a compaction agent (IOW) was plainly visible. Therefore, the highest CWR values were detected for composition 7 on the 28th and 90th days (0.92 and 0.94, correspondingly), followed by composition 6 with CWR = 0.79 and 0.82 and compositions 5 and 8. Composition 3 exhibited the lowest CWR values (CWR = 0.55 and 0.57), followed by composition 1 (CWR = 0.60 and 0.62) and composition 2 (CWR = 0.62 and 0.66).
The maximum allowable decrease in resistance for TSs fully saturated with water on the 90th day is 35% for class 3; 30% for class 2; and 20% for class 1 [28]. Therefore, regarding water resistance, composition 2 met the requirements of class 3; compositions 4, 5, and 8 of class 2; and compositions 6 and 7 of class 1.
3.3 Water absorption (WA) of the TSs
The water absorption (WA) values (Table 5) were determined by Eq. (2) [29] on the 28th and 90th days of cure. The reduction regularly observed on the 90th day was due to the new formations that started filling the pore space of the TSs. The lowest results were shown by composition 7 with WA = 9.62 and 8.33 on the 28th and 90th days correspondingly, followed by compositions 5, 6, and 8. This fact might be the best explication for their best axial and water resistance values (Tables 3 and 4).
Zuccheratte et al. [34] used IOW as aggregate for concrete production with water absorption between 13.6 and 14.9% at 7 days. Similar water absorption values allowed Malaiskiene et al. [36] to conclude that 5% CPW might be used to replace part of the cement content. NBR 6136 [37] establishes a WA value between 10 and 16% as average WA values for concrete sealing blocks.
3.4 Coefficient of expansion (CEXP) and shrinkage of the test samples
In the test samples’ hydration and hardening process, there were two antagonistic processes—expansion up to 7 days inclusive, followed by their shrinkage up to 720 days (Table 7). Mymrin [38] observed and investigated in detail this transition from expansion to shrinkage at different ages of samples up to 60 and even 90 days, depending on the chemical and mineral composition of raw materials.
Both phenomena are explained initially by the active sol synthesis in the samples’ porous space due to the alkaline corrosion of the solid particle surfaces and their volume expansion by these new formations (in this case, up to 7 hydration days), followed by the transition from sol to gel. Between 7 and 14 days, the gel’s aging process (syneresis) has begun, expressing itself by its compaction with a decrease in the TSs volume up to 720 days or more. The most considerable magnitude of expansion (CEXP = 3.55–3.92%) in 7 days was observed for compositions 6–8 with the highest chemically active LPW content (25%) and, among them, composition 7 with 40% IOW (CEXP = 3.92%). These values confirmed once more the dependence of the properties of the materials on the amount of binder LPW and the IOW. Compositions 1–4 exhibited significantly lower CEXP values because they contained 15% LPW, and, therefore, lower alkalinity of the hydrated initial mixes with less quantity of sol formation.
On the 720th day, composition 7 remained the one with the highest CEXP = 1.08%, and composition 4 the one with the lowest CEXP = 0.84%, followed by compositions 3 and 1 (CEXP = 0.96%).
4 Physical–chemical processes of the structure’s formation
To better explain the results obtained for the mechanical and physical properties and their changes during the 720 hydration days, composite 7 was chosen, as it presented the best values at all ages of cure, thus manifesting the processes more intensely, making it easier its observation. Besides, composite 7 held almost the highest IOW content (40 wt.%), the most contaminant component, and, therefore, the most important to prevent environmental impact.
4.1 Changes in mineral compositions during materials hydration
Mineral composition of the composite 7 after 3 hydraition days (Fig. 3A) consisted of Portland cement mineral brownmillerite Ca2(Al,Fe)2O5; hydrated lime mineral portlandite Ca(OH)2; calcite CaCO3; clay mineral kaolinite Al4[Si4O10](OH)8; three iron ore waste minerals—hematite Fe2O3, magnetite Fe3O4, and goethite FeO(OH); and quartz SiO2. The XRD pattern also had a rather high X-ray background, indicating a large amount of amorphous phase due to both geological and industrial processes that occurred with each of the components.
Therefore, the crystalline structures of all minerals were extremely destructed, and the intensities of their peaks were very low. Only one of the quartz crystalline peaks exceeded 2000 cps (counts per second).
During the 180 days of composite 7 hydration in a highly alkaline environment, the complete destruction of kaolinite and portlandite weak crystal lattices occurred (Fig. 3B). Despite the samples’ expansion by 3.92% (Table 7), the samples of composite 7 hardened to 4.94 MPa (Table 4). Only the calcite peak significantly increased at 2Θ° = 29.5°, but it cannot explain such increase of resistance.
On the 720th, the material resistance doubled (up to 10.47 MPa) due to the complete hydration of portlandite, brownmillerite, and goethite. The chemical dissociation of these minerals in the form of new amorphous formations in the test samples’ pores led to a decrease in water absorption (Table 6) and an increase in water resistance (Table 5).
Such phenomena could only happen thanks to the synthesis of a significant amount of sol from the dissociation of kaolinite, brownmillerite, portlandite, and goethite in an alkaline environment, and the sol transformation and compaction to the gel state with completion of the linear expansion process and the continuity of the almost 6-month shrinkage process of the samples from 3.57 to 1.34% (Table 7). There was also the highly visible emergence of new calcite peaks at angles 2Θ° = 23.2°, especially 29.5°, 43.5°, 48.5°, 54.0°, 57.5°, and 61.5°.The synthesis of new calcite bodies or the improvement of their structure increased significantly during the 720 days of test samples hydration (Fig. 3D) compared to 180 days (Fig. 3C). Such a new crystalline phase amount in the materials’ porous space might become a significant factor in the enhancement of all the above-studied properties, along with the ongoing solidification of the amorphous phase.
4.2 Morphological microstructures of the TSs on the 365th day of cure
The images used to study the changes in the microstructure (Fig. 4) of the samples of composition 7 during their hydration and hardening, carried out by SEM with a magnification of 6000 times, can be seen in Fig. 4. In the initial dry mix (Fig. 4A), particles of different shapes with sizes from 0.5 to 30 µm were observed. The smaller particles were predominantly rounded, while the larger ones were mostly angular. The pores between the particles were also very diverse, both in size and shape. It was noticed that the particles were not bonded to each other by any bonding materials and did not have a common structure.
After 3 days (Fig. 4B) of hydration and compacting in an alkaline environment, the appearance of many new needle-shaped formations, druse-like crystals growing from one point is clear-cut. When compared to Fig. 4A, there was a marked reduction in the number of small particles due to their aggregation. This aggregation process was significantly intensified in samples at 180 days (Fig. 4C), and large monolithic fields appeared in the samples’ structure at 720 days (Fig. 4D). The vast predominance of such monolithic sections might explain the changes in the most important mechanical and physical properties of the samples at this age, such as water absorption, water resistance, axial resistance, linear expansion, and shrinkage.
4.3 Microchemical compositions of the new formations
The study of the microchemical composition of the new formations that strengthen the developed compositions was performed by the EDS method on samples from composition 7 on the 720th day (Fig. 4D and Table 8). All points, including those closest to each other (1 and 2; 3 and 4), exhibited very different chemical compositions. None of these points were similar in chemical composition to the total area. Such heterogeneity is due to the practical impossibility of achieving ideal homogeneity at the micro level when mixing dry components before hydration. Therefore, sol formed during alkaline corrosion of different solid particles, which then chemically reacted before turning into a gel, had significantly different compositions at the micro-level in this study. Nevertheless, such heterogeneity of the gel did not prevent it from playing a decisive role in the material’s hardening.
4.4 Isotopic composition of the new formations
Isotopic composition (Fig. 5) of the new formations from composition 7’s TSs after 720 hydration days was studied by laser micro mass analyses (LAMMA). The set of isotopes and their intensity at each of the new formations’ six points were significantly different from each of them (Fig. 5). In addition, the presence of heavy metal isotopes (Cr, Cu, and Zn) in most of the plotted points of new formations was established by the LAMMA method.
4.5 Environmental impact of the developed materials
The recognition of heavy metal isotopes in composition 7 by the LAMMA method made it necessary to study the solubility and leaching of metals in composition 7 and IOW as the only possible source of their appearance in the initial mixtures. The analyses using the AAS method (Table 9) showed that the metal content (Al, Cr, Fe, Cd, Pb, and Zn) in IOW was significantly less than Brazilian toxicity standards [39]. The leaching and solubility of composition 7 by the AAS method demonstrated the possibility of decreasing these values far below the NBR 10,004 demands [39]. Therefore, the developed materials are environmentally clean and can be used as natural aggregates at the end of their service life.
4.6 Economic efficiency of the application of the developed materials
It is impossible to estimate the economic efficiency of using industrial wastes without information on the application in specific places with the local price of natural raw materials, distances and delivery prices, and many other data sources. Therefore, the calculation of economic efficiency was not included in the objectives of this study. Nevertheless, in terms of common sense, free industrial wastes instead of relatively expensive natural materials must be very cost-effective.
Also, considering that the tailings are materials that have already been extracted, crushed, and processed (iron ore tailing and waste of cellulose-pulp), the real beneficiation costs for their reuse are significantly lower compared to the primary ore tailings that need to go through the entire mining beneficiation process, since processing represents about 40 to 60% of the total mineral processing cost [40].
5 Conclusions
According to this study’s objectives, building materials were developed from four industrial wastes—pulp and cellulose production, iron ore processing, concrete production and demolition waste, and lime production wastes—without the use of traditional natural materials. The structures and properties of the industrial wastes used as raw materials were characterized, and the changes in the properties of the test samples during hydration and cure were studied, as well as the physical–chemical processes of the developed composite structure formation. The samples’ axial resistance reached values until 4.94 MPa after 3 curing days, 5.75 MPa after 7 days, and 11.47 MPa at 720 days of outdoor curing. The water resistance coefficient of composite 7 on the 28th and 90th days of cure was 0.92 and 0.94, respectively; the water absorption values, 9.62–8.33%; and linear expansion on the 90th day reached 1.24% and on 720th, 1.08%. All properties of the developed composites met the requirements of Brazilian standards for the production of construction materials, such as road and airfield runways, levee cores, industrial and municipal dumps, building foundations, bricks, and blocks. The results of solubility and leaching studies of the metals in acid solutions confirmed their environmental friendliness.
All raw materials and developed materials were studied by the XRD, SEM, EDS, and LAMMA methods to explain their properties. The sol solution’s chemical interaction process, formed during the alkaline corrosion of solid particles’ surfaces of the initial components, was investigated. The gradual densification of the sol and its transition into a hardening gel provided the strengthening of the developed materials over time. The only crystalline form increased in number or perfection was the calcite crystal, which was also a positive factor in strengthening the materials’ structure. So high values of mechanical and environmental properties of the developed materials permit to use them for construction of road and airfield runways bases, levee cores, industrial and municipal dumps, building foundations, bricks, and blocks. The feasibility of using four types of industrial wastes, currently stored in dumps that poison the environment, to completely substitute traditional natural raw materials, whose careers irreversibly destroy the environment, has been experimentally proven.
Data availability
No data, models, or code were generated or used during the study.
References
DNPM (2015) Sumario mineral National Department of Mineral Production - DNPM, Brasília, 2016
SNISB (2018) National Dam Registration Systems, National Dam Safety Information System - SNISB, Brasília
Liu H et al (2005) Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). J Sci Total Environ 339:153–166. https://doi.org/10.1016/j.scitotenv.2004.07.030
Williams DV (2017) Tailings storage facilities. J Waste Manage 60:230–246. https://doi.org/10.1016/B978-0-444-63658-4.00037-2
Wang C et al (2014) Current state of fine mineral tailings treatment: a critical review on theory and practice. J Miner Eng 58:113–131
Stroeven P et al (2011) Research on cementitious materials to promote sustainable developments in Vietnam. Int Conf Conc Dev 1 Tehran. Proceedings. J Build Housing
IBAMA (2015) Technical report on the impacts of the Fundão dam rupture, Mariana, MG, Brazilian Institute of the Environment and Renewable Natural Resources - IBAMA, Brasília
Nuss P, Blengini GA (2018) Towards better monitoring of technology critical elements in Europe: coupling of natural and anthropogenic cycles. J Sci Total Env 613–614:569–578. https://doi.org/10.1016/j.scitotenv.2017.09.117
Do HS, Mun PH (2017) A study on engineering characteristics of asphalt concrete using filler with recycled waste lime. Waste Man 28:191–199
Monte MC et al (2009) Waste management from pulp and paper production in the European Union. J Waste Manage (Oxford) 29:293–308
Paper industry statistics & facts (2017) https://www.statista.com/topics/1701/paper-industry/
Iyaras A (2020) Combined effects of paper mill sludge and carbonation sludge on characteristics of fired clay bricks. J Con Build Mat 249:118722. https://doi.org/10.1016/j.conbuildmat.2020.118722
Hussain Z et al (2020) Production of bacterial cellulose from industrial wastes: a review J Cellulose 26:2895–2911
Gemelli E et al (2009) Evaluation of paper industry wastes in construction material. J Mat Res 4:297–304
Sebogodi KR et al (2020) Beneficiation of pulp mill waste green liquor dregs: applications in treatment of acid mine drainage as new disposal solution in South Africa. J Clean Prod 246:118979. https://doi.org/10.1016/j.jclepro.2019.118979
Silva RV et al (2014) Properties and composition of recycled aggregates from construction and demolition waste suitable for concrete production. J Con Build Mat 65:201–217
Hu X et al (2020) Utilization of fly ash and waste lime from pulp and paper mills in the argon oxygen decarburization process. J Clean Prod 261:121182. https://doi.org/10.1016/j.jclepro.2020.121182
Machado PJC et al (2020) Characterization and properties of cementitious composites with cellulose fiber, silica fume and latex. J Con Build Mat 257:119602. https://doi.org/10.1016/j.conbuildmat.2020.119602
Mokhena TC, John MJ (2020) John Cellulose nanomaterials: new generation materials for solving global issues. J Cellulose 27:1149–1194
European Commission (2018) EU Construction and Demolition Waste Protocol and Guidelines <https://ec.europa.eu/growth/content/eu-construction-anddemolition-waste-protocol-0_en> Accessed 17 July 2019
AGI (2014) Aggregate and the environment. American Geological Institute. Disponibilizado em: https://www.agiweb.org/environment/publications/aggregate.pdf Acessado em agosto de 2018
Islam R et al (2019) An empirical study of construction and demolition waste generation and implication of recycling. J Waste Manag 95:10–21
Bassani M et al (2019) Alkali-activation of recycled construction and demolition waste aggregate with no added binder. J Con Build Mat 205:398–413
Garside M (2020) Production of lime worldwide 2010–2019. https://www.statista.com/statistics/1006040/production-of-lime-worldwide/
NBR 6453 (2003) Lime for civil construction - requirements. Rio de Janeiro
Mymrin V (2019) Structure formation processes of sustainable construction material from hazardous sewage sludge with additions of wood ash, and lime production waste. J Advanced Manuf Tech. https://doi.org/10.1007/s00170-019-04408-4
Mymrin V et al (2017) Environmentally clean materials from hazardous red mud, ground cooled ferrous slag and lime production waste. J Clean Prod 161:376-381. https://doi.org/10.1016/j.jclepro.2017.05.109
CN-25–74 (2020) Guidelines for application of soils strengthened with binders for use in beds and pavements of roads and airfields. Moscow
NBR 9778 (2009) Hardened mortar and concrete - determination of water absorption, voids index and specific mass. Rio de Janeiro
NBR 248 (2003) Aggregates - determination of particle size composition. Rio de Janeiro
Foelkel C (2013) Practical and conceptual aspects on the manufacture of market cellulose of the kraft type bleached from eucalyptus wood. Chapter 31: The Kraft Pulp Making Process. Eucalyptus Online Book
Manskinen K et al (2011) Total and extractable non-process elements in green liquor dregs from the chemical recovery circuit of a semichemical pulp mill. J Chem Eng 166:954–961
Piotto ZC (2013) Eco-efficiency in the pulp and paper industry - case study. São Paulo
Zuccheratte A et al (2017) Synthetic gravel for concrete obtained from sandy iron ore tailing and recycled polyethyltherephtalate. J Con Build Mat 151:859–865. https://doi.org/10.1016/j.conbuildmat.2017.06.133
NBR 7170 (1983) Tijolo maciço cerâmico para alvenaria. Rio de Janeiro
Malaiskiene J et al (2018) The impact of primary sludge from paper industry on the properties of hardened cement paste and mortar. J Con Build Mat 172:553–561
NBR 6136 (2006) Blocos vazados de concreto simples para alvenaria – Requisitos. Rio de Janeiro
Mymrin V (1986) Theoretical bases of clay soils strengthening by dumped ground cooled ferrous slags for road base construction, Dr. of Sc. thesis, Moscow State University
NBR 10004 (2004) Solid waste classification, Rio de Janeiro, 2004
Zhao S et al (2014) Utilization of iron ore tailings as fine aggregate in ultrahigh performance concrete. J Con Build Mat 50:540–548. https://doi.org/10.1016/j.conbuildmat.2013.10.019
Acknowledgements
The authors would like to thank the Laboratory of Minerals and Rocks (LAMIR), the Ceramics Laboratory at UFPR, and the Multi-User Material Characterization Center (CMCM) at UTFPR, Curitiba, Brazil, for their dedicated technical assistance.
Funding
We included appropriate funding statements in the manuscript.
Author information
Authors and Affiliations
Contributions
All co-authors meet criteria for authorship and ensure appropriate acknowledgements made in the manuscript: Vsévolod Mymrin—author of the idea, developer of the plan of the experiments, participant of all stages of research, and author of the first manuscript’s version. Cleber L. Pedroso—performer of all principal laboratory studies and XRD analyses. Daniela E. Pedroso—bibliographical revision and performer of SEM analyses. Paulo H.B. Rolim—performer of all laboratory experimental works and co-author of this manuscript. Rodrigo E. Catai—performer of all laboratory experimental works and co-author of this manuscript. Karina Q. Carvalho—bibliographical revision and performer and co-author of this manuscript.
Corresponding author
Ethics declarations
Research involving human and animal participants
We show informed consent and provide assurances that participants’ rights are protected. We had no clinical trials and research using animals. We can sign a copyright agreement.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We declare that our manuscript was not published elsewhere.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mymrin, V., Pedroso, C.L., Pedroso, D.E. et al. Eco-friendly cementless concrete from hazardous iron ore tailing and waste of cellulose-pulp, concrete demolition, and lime production. Int J Adv Manuf Technol 122, 933–945 (2022). https://doi.org/10.1007/s00170-022-09811-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00170-022-09811-y