Abstract
Plasma electrolytic polishing (PeP) is an innovative technology used to obtain metal surfaces with low roughness and a high gloss. Its advantages, which include high efficiency, no structural selectivity, and low pollution, have recently attracted much attention. PeP is widely used in aerospace, biomedical, precision instrumentation, and 3C electronics industries. This paper primarily aims to introduce basic principles of PeP technology from both the macro- and micro-mechanism viewpoints. Accordingly, the typical characteristics and phenomena of the polishing process are summarized. The primary control parameters which affect the surface quality and material removal rate are discussed in detail. These include treatment time, electrolyte, electrical source, and voltage. Furthermore, the electrolyte jet polishing methods applicable to parts with various geometries are also analyzed. Finally, the PeP surface treatment of selected difficult-to-finish materials is reviewed, followed by the authors’ insights into the prospects of PeP technology. This review can serve as a suitable and effective guide for researchers to understand the PeP technology systematically.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the areas of aerospace, medical instruments, precision instrumentation, and 3C electronics, traditional polishing methods have been widely used to generate a low roughness and high gloss surface. However, with the rapid development of society and through innovation, traditional polishing technology aims to achieve higher polishing efficiency, higher polishing quality, and environmental protection. Traditional surface polishing techniques mainly include mechanical polishing, chemical polishing, and electrochemical polishing.
The mechanical polishing provides a way to obtain a smooth surface via plastic deformation through material surface cutting and grinding. Disadvantages include higher demand for manual labor, limited surface quality, and inability to process parts with complex geometries. On the other hand, chemical polishing is a process that removes the workpiece material by controlled chemical dissolution by using a chemical solution. Similarly, electrochemical polishing also uses a chemical solution to dissolve the work material, but the process is performed under current. The metal workpiece is the anode, while the tool electrode is the cathode. Using direct current (DC), the workpiece as anode is dissolved into metallic ions and removed atom by atom. However, harmful gases are generated during both chemical and electrochemical polishing using strong acid or alkali chemical reagent. Additionally, harmful waste liquid is generated after polishing. Both the gases and liquid are damaging to workers’ health and environmental protection [1,2,3].
The increasing number of complex structural parts and the growing environmental awareness make it is harder for traditional polishing methods to meet the current industrial polishing requirements. Therefore, in recent years, there is a rapid development of special polishing methods, mostly aiming to solve the problems mentioned above.
For abrasive jet polishing, abrasive particles are driven by the high-pressure liquid to impact the workpiece surface to achieve the micro-removal of the workpiece material [4]. Moreover, laser polishing is used to achieve exceptional surface finish levels through laser-material interactions, remodeling its topographic profile [5]. However, there are evident problems with both the abrasive jet and laser polishing methods. The former has low polishing efficiency for some of the hard and brittle materials, whereas the latter can cause surface ablation. Detailed characteristics of each polishing method are listed in Table 1.
Among the novel polishing methods, plasma electrolytic polishing (PeP) is an innovative technology characterized by high polishing efficiency and no pollution. Figure 1 presents the development of PeP technology, clearly showing its growth in popularity and advances. The PeP procedure was initiated by Duradzhi et al. in 1979 [7]. In 1986, Hans et al. [8] systematically proposed the PeP process parameters to improve the surface gloss of both single and multiphase alloys, such as brass, wrought alloys, and carbon-rich iron alloys. Stanishevsky et al. [9] developed the PeP method for metallic materials, including a variety of steels—stainless, tool, and low-carbon steels—aluminum, and copper and their alloys, which further popularized its application. In 1999, the apparatus and process for polishing metal surfaces using PeP technology were presented by Ryabkov [10]. The PeP method for smoothing pieces made of titanium and zirconium, including their alloys was introduced by Mirzoyev et al. [11]. Nevyantseva et al. [12] confirmed that PeP technology could also be used to remove coatings. Furthermore, the systematized PeP equipment was invented in 2008 [13], and a cobalt-chromium alloy was polished by PeP technology for the first time [14]. Subsequently, the technology was successfully applied to various metals, including magnesium alloys, amorphous alloys, gold, and platinum [15,16,17].
Theoretically, PeP technology can be used to polish any metal part structure. When compared to strong acid and caustic alkali solutions used in chemical and electrochemical polishing, the PeP technology uses a low-concentration salt solution electrolyte. Therefore, no harmful gases are generated. Furthermore, compared to mechanical polishing, abrasive jet polishing, and laser polishing, PeP technology has several distinct advantages. The advantages primarily include good surface quality, no mechanical stress, and no thermal distortion/damage. Therefore, PeP can be widely applied for manufacturing machinery, medical instruments, in the aviation industry, meter manufacturing, 3C electronics, shipbuilding, food industry, and chemical industry, among others.
This review attempts to provide a more comprehensive understanding of the fundamental principles, typical characteristics and phenomena, and key parameters of plasma electrolytic polishing. Additionally, it also covers the polishing method using electrolyte jet for different shaped parts and applications in various difficult-to-finish materials.
2 The principle of PeP technology
Plasma electrolyte polishing is a composite reaction process that removes surface materials through plasma-physical and electrochemical reactions. As such, it is quite similar to common electropolishing. The standard PeP equipment is shown in Fig. 2. The setup includes a bath containing the polishing solution, in which the workpiece is immersed and connected as an anode. During the PeP process, both plasma and electrochemical reactions occur. The former includes the vapor gaseous envelope ionization, discharge bombardment, hydrothermal reactions, and metal dissolution by the metal-water reaction, while the latter includes oxide formation, metal dissolution, alkalization, hydrogen, and oxygen formation [18]. It should be pointed out that the physical reaction of plasma discharge bombardment is dominant and is the main cause of surface material removal. The voltage required for PeP is much larger compared to that of common electropolishing and is typically up to several hundred volts. Therefore, the material removal rate is high. Finally, it can be concluded that PeP is an efficient technology that can achieve metal surfaces with low roughness and high smoothness in a timely manner.
Typical arrangement of the PeP equipment [19]
2.1 Macro-mechanism
The PeP macro model is described as a “power supply-electrolyzer-treated surface” system with lumped parameters [20]. The PeP layout schematic is shown in Fig. 3. Workpiece under DC voltage is immersed into a bath comprised of a specially formulated, temperature-controlled electrolyte solution and is connected to the plus pole of the DC power sources. Thus, the workpiece is the anode, while the cathode is connected to the minus pole of the DC power source. The polishing solution is electrolyzed at the start of the PeP. The electrolytic reaction occurs near the anode, including the oxygen evolution (Eq. (1)) and metal oxidation (Eq. (2)), which is expressed as follows:
where Me is the metal workpiece element.
Due to high voltage, an insulating gas layer is formed between the workpiece surface and the electrolyte, meaning that the electric circuit gets disconnected. When the voltage is high enough (between 200 and 400 V), the gas layer becomes ionized due to the high electric field within. The breakdown and discharge in the gas layer remove surface peaks, polishing the workpiece surface. Since the discharge removal rate is faster than oxidation, it can effectively remove surface peaks and achieve smoother surfaces [21].
2.2 Micro-mechanism
In-depth understanding of the PeP micro-mechanism can help us further analyze the macro-process. The streamer theory is suitable to explain the micro-mechanism of PeP [22]. The streamer theory of PeP is summarized in three stages [21, 22], as illustrated in Table 2. It shows the theoretical material removal process by plasma discharge during the PeP process.
-
The first stage is the avalanche formation and development. At first, the polishing solution is electrolyzed, accumulating a certain number of electrons and positive ions between the polishing solution and the workpiece. Since the electron mass is rather low, electrons move faster than the positive ions. After forming the gas layer, electrons form an avalanche head, while the positive ions remain in the back practically stationary as an avalanche tail (Table 2a). Furthermore, secondary electrons are formed as a result of photoionization, as shown in Table 2b.
-
The second stage is characterized by the formation of the plasma discharge channel. Electrons first reach the anode and form a primary plasma discharge channel with a part of positive ions. Simultaneously, secondary electrons are accelerated, forming secondary avalanches. These secondary avalanches are attracted by the primary plasma discharge channel, mainly due to the field distortion (Table 2c). When the process tends to stabilize, a complete plasma channel is formed (Table 2d).
-
The third stage includes gas explosion removal profile peak. Numerous collisions between the electrons and positive ions moving at high velocities produce a large amount of heat. Therefore, the plasma channel temperature increases, causing the gas to expand. The magnetic field of the electric current in the plasma channel restricts the gas expansion (magnetic compression). As shown in Table 2e, the result of the opposing processes (i.e., gas expansion and compression) is an explosion. After the gas explosion, the plasma channel collapses, removing the surface material (Table 2f).
The PeP micro-mechanism explains the process of reducing the workpiece surface roughness very well. There are three key factors in determining the rate of decrease of workpiece roughness during polishing—the rate of material removal (per discharge), the formation position of the discharge channel (in relation to the microscopic peak portion), and the discharge pit depth [23]. The PeP material removal rate is directly proportional to the anode current density [21]. The heat flux density on the workpiece surface increases with the increase in current density. This causes the workpiece surface layer to melt, making it easier to remove [24]. During the polishing process, the current density in the cavities is lower than that at the peaks, resulting in faster removal of the material at peaks [25]. Vaňa et al. [26] reported that the material removal in PeP technology starts at the top of the surface peak, as plasma discharges melt away the workpiece surface. As shown in Fig. 4, each plasma discharge removes the same amount of material (S1 = S2). Thus, the thickness of removed layer h2 is much lower compared to the h1 layer thickness. Such behavior leads to rapid removal of the workpiece surface peaks, achieving a smooth surface effect.
Surface profile of the treated specimen according to Vaňa et al. [26]
2.3 Current-voltage characteristic
Typical current-voltage characteristics of the PeP anode process [27] are shown in Fig. 5. The curve shows several sections which correspond to various polishing process voltages. The section of current-voltage characteristics between points A and B represents the common electropolishing process. The A–B section is described by Ohm’s law and Faraday’s law, and a positive correlation is found between current and voltage. When the voltage is at point B, a film consisting of vapor and gas is periodically formed around the active electrode. However, the vapor-gas film oscillates on the active electrode until the voltage value increases to point C. The B–C section is described as the unstable PeP process. Since point C, the gas layer of the anode is stable, but the luminescence phenomenon is still not obvious. Until point D spark discharges create the glow, the emergence and disappearance of gas layer are accompanied by crash and noise. Thereby, the C–D section and the following section of the current-voltage characteristic are the stable PeP processes. Among them, the C–D section is the heating zone, and the anode temperature in the area after point D gradually decreases. This area is described as the electro-hydrodynamic process mode, which is the only one used for PeP. Furthermore, in the C–D section, the workpiece polishing generally takes place at voltages between 200 and 350 V, with a current density between 0.2 and 0.5 A/cm2.
Typical current-voltage characteristic of the PeP anodic process [27]: A–B, the common electropolishing process; B–C, the unstable plasma electrolyte polishing process; C–D and D–, the stable plasma electrolyte polishing process
It should be noted that the heating zone only exists under a specific set of circumstances. Point C position depends on the electrolyte composition, concentration, and temperature [28]. Additionally, the current density has a strong negative correlation to the temperature; the current density decreases as the temperature increases [29].
2.4 The typical phenomena: gas layer and plasma discharge
In the polishing process, a gas layer is formed between the anode and the electrolyte due to a high-voltage value. The gas layer consists of vapor and other gases, which is nonconductive and separates the metal surface from the electrolyte. Kellogg [30] indicated that the gas layer surrounding the anode during the aqueous anode effect is maintained by the electrolyte vaporization. The gas layer is also known as the gaseous vapor envelope (VGE) since its fundamental component is water vapor. Based on the simulation and experimental results, Danilov et al. [31] concluded that the most significant voltage drop in PeP occurs in the gas layer.
The voltage was found to have an important influence on the gas layer formation; the energy gain on the workpiece surface increases with the voltage. Moreover, the energy gain on the workpiece surface per time unit plays an important role in the gas layer thickness—the larger the energy gain, the thicker the gas layer. It should also be added that a thicker gas layer is more stable [32]. The high electrolyte temperature is also critical to the gas layer formation. The continuous gas layer surrounding the sample does not appear at low-temperature electrolytes [29]. Duradji and Kaputkin [33] suggested that the layer thickness should not be lower than 0.05 mm for all electrolytic solutions. The generation of gas bubbles on both the anode and cathode is observed—O2 at the anode and H2 at the cathode [34]. It should be pointed out that the remaining gas in the layer is predominantly O2.
Plasma discharges occur in the gas layer during the polishing process, resulting in the high-temperature energy being released, removing the excessive workpiece material. The gas layer temperature can locally reach as high as 2000 °C [35]. A luminous glow can be seen on the plasma discharge gas layer. The luminous glow color varies depending on the type of metal ions present in the electrolyte. For example, an orange-colored glow is regularly seen in a solution containing sodium ions, while a blue-colored one is seen when using a solution consisting of Zn ions [35]. The presence of the gas layer is essential for sustaining anodic dissolution on the workpiece surface, providing both the cleaning and polishing effects [36]. Figure 6 shows a schematic diagram covering the gas layer evolution stages between the electrolyte and the working electrode [20, 37].
3 Key parameters affecting the PeP process
The factors related to plasma electrolytic polishing include electrolyte, electrical source, process parameters, pretreatment, posttreatment, cathode, hanger, and workpiece. The surface quality and material removal rate are affected by a number of PeP parameters, as shown in Fig. 7. Since many PeP process parameters are complex and should be optimized, it is difficult to find the polishing parameters suitable for each metal. Researchers generally select key parameters to optimize the other process parameters. Such parameters have a great impact on the removal rate and surface roughness, as well as treatment time, electrolyte, electrical source, and voltage.
3.1 Treatment time
The treatment time is an important parameter in the plasma electrolyte polishing process. Rajput et al. [38] reported the experimental details for confirming the relationship between the current and decrease in surface roughness with increase in treatment time; the voltage was held constant. Figure 8 shows the surface image of the copper material after PeP with tPeP = 15 s and tPeP = 120 s. It is evident that longer treatment time can result in a smoother surface [39]. Dobrynin [40] studied the effect of PeP on the surface roughness of samples made of titanium alloys VT6 and VT8M-1. When the polishing time reaches a specific time node, the sample surface roughness remains unchanged. Furthermore, Podhorský et al. [41] investigated the possibilities of using the PeP technology to pretreat the low-carbon steel surface before galvanic chrome plating. As shown in Fig. 9, the authors observed that the resulting surface roughness rapidly decreased in a brief time period. However, with long polishing times, the surface roughness was found to increase slightly. In a later study, Vana et al. [42] further studied this phenomenon. They evaluated the change in surface gloss level and roughness of the austenitic stainless steel surfaces after PeP through time. They found that a very short polishing time has a radical effect on the increase in the surface gloss level. However, the secondary surface relief is created by increasing the treatment time and decreasing the surface gloss level. Similar to the gloss level change, the surface roughness increases with the polishing time.
Copper material surfaces: a untreated, b after 15 s PeP, and c after 120 s PeP [39]
Relationship between surface roughness of the specimens and the treatment time (the serial numbers 1, 2, 3, and 4 in the figure are the sample numbers) [41]
As shown above, there is a limit to workpiece surface roughness that can be achieved by using PeP. The effective polishing time, described as the period between the polish start and reaching the stable surface roughness, is critical to achieve the minimum surface roughness. In addition, minimum achievable roughness is also associated with the initial workpiece roughness. The relationship between the workpiece surface roughness parameter Ra and treatment time t can be described as [43]:
where A is the maximum decrease in roughness, k is the coefficient accounting for the polishing conditions and the physical workpiece properties, and C is the minimum achievable roughness.
As described in Section 2, PeP is similar to the commonly used electropolishing process. The largest differences are found in the electrolyte type and the voltage level. When compared with electropolishing, for given current densities, the use of PeP can realize both lower surface roughness and higher gloss in a shorter polishing time. To achieve comparable results by using electropolishing, process time would be eight times higher [39].
3.2 Electrolyte
The electrolyte composition, concentration, and temperature have a significant impact on the machining efficiency and surface roughness. The selection of the PeP electrolyte is highly dependent on the pre-polished metals and alloys. For example, the low-concentration salt solution with ammonium sulfate as the main component is used for polishing steels. The polishing performance can be further improved by including additives to the electrolyte. Additives such as surfactant and glycerol [44] can significantly affect the electrolyte surface tension, kinematic viscosity, and conductivity properties. The change in electrolyte concentration will also affect the polishing process. Kashapov [45] indicated that increasing the electrolyte concentration will affect both the anodic dissolution and the occurrence of anodic micro-discharges. Wang et al. [24] found that stainless steel removal rates increase with the increase in ammonium sulfate solution concentration up to 6% and then decrease when the concentration is more than 6%, as shown in Fig. 10. Such behavior occurs since increasing concentration can improve the electrolytes’ electrical conductivity, electric field strength, and collision ionization coefficient. In the collision, electrons bombard the workpiece surface, increasing the removal rates. However, too high electrolyte concentration will negatively affect the polishing reaction, thereby reducing the removal rate.
Material removal rate at different concentrations [24]
The electrolyte temperature is another serious concern during the polishing process. A high-quality workpiece surface can be achieved only in the limited range of electrolyte temperatures. As the temperature increases, the evaporation rate of water within the electrolyte also increases. This makes the gas layer more stable, which results in improved polishing surface quality. However, if the temperature is too high, it can destroy the chemical components of the electrolyte [31]. Wang et al. [43] compared the effects of temperature, electrolyte concentration, and diving depth on the surface roughness. They found that temperature is the most important factor when considering surface roughness. Mihal et al. [29] observed a strong negative correlation between the current drop of steel AISI 304 and increase in the electrolyte temperature. The physical and chemical properties of the electrolyte solution change with the increase in temperature. Additionally, the pH and dissolved electrolyte oxygen also affect the polishing efficiency and its effect. Valiev et al. [46] pointed out that the electrolyte pH not only affects the anode current density but also the workpiece surface roughness. To conclude, the electrolyte composition has the most significant influence on PeP for different metals.
3.3 Electrical source and voltage
Electrical source and voltage parameters are critical to surface quality and removal rates. PeP technology uses a unique high-power electrical processing source with either regular or pulsed DC sources. The latter can control the process interruption and arc duration. Unlike regular DC sources, the pulse sources rely on the pulse effect to accelerate electrolyte flow and renewal during the polishing process. Thus, the workpiece finish and polishing are achieved with higher efficiency and better surface performance [16].
On the other hand, the most significant PeP limitation is high energy consumption. In order to reduce energy consumption, a novel polishing mode was developed—described as two-stage polishing. The voltage is called step DC voltage. The first stage is polished at a higher voltage, while the second stage is processed at a lower voltage [47]. Compared to DC constant voltage polishing, the proposed two-stage polishing mode can reduce polishing time and improve the polished effect. The schematic illustration of the voltage waveform is shown in Fig. 11. Moreover, researchers have found that the source frequency also has a notable influence on the polishing effect. A study by Yerokhin et al. [48] confirmed that the PeP process is more effective at a higher frequency source. In addition, the process energy source determines the maximum polishing area for one loading.
Selecting a very low-voltage value has a negative impact on the PeP process since VGE cannot be formed under the low-voltage treatment conditions. Parfenov et al. [36] reported the effects of VGE on the electric field distribution and surface layer removal during stainless steel PeP. The authors carried out both theoretical and experimental studies. Low-voltage treatments without VGE may cause the workpiece to passivate, with resulting oxide layers blocking the surface polishing (Fig. 12b). However, VGE formation during the high-voltage PeP processing promotes anodic metal dissolution and provides a smooth surface (Fig. 12c). Wang et al. [32] indicated that the voltage has an important role in the surface roughness value and the material removal rate. If the voltage is either too large or too small, the minimum achievable surface roughness value will increase. On the other hand, rising voltage increases the amount of heat released near the anode, making the gas layer thicker and more stable and thus decreasing the current. The material removal rate decreases while the roughness decrease rate becomes slower. Due to the short circuit at a low voltage, the smallest achievable surface roughness value increases.
Surface plane SEM images of stainless steel before treatment (a), after PeP for 15 min at 9 V (b), and after PeP for 15 min at 250 V (c) [36]
3.4 Other effects
Other factors that influence the plasma electrolyte polishing process include metal surface pretreatment, posttreatment, workpiece immersion depth, workpiece orientation (in the bath), cathode material, hanger, electrode spacing, and bath age, among others.
The pretreatment is an essential precondition to achieve the highest surface quality [50]. Before PeP, metal parts are mechanically prepared. The metal surface is generally covered by an oxide layer (or any other dirt), thus requiring the pretreatment process for the workpieces to be polished. The examples of pretreatment processes are surface cleaning and mechanical abrasive grinding. Pretreatment will reduce both the PeP polishing process time and increase resulting surface quality in case of burrs and other defects found on the end product. The posttreatment process is also essential to avoid chemical residues on the workpiece surface after polishing. The workpiece immersion depth indicates the solution pressure on the workpiece surface. The deeper the workpiece immersion depth, the greater the workpiece surface pressure, which can adversely affect the formation of a stable gas layer on the workpiece surface. To ensure the necessary current density for plasma formation at the workpiece (anode) surface, the cathode surface area must be larger than the workpiece.
The key factors have a significant impact on metal treatment surface quality. Basic PeP quality indices, which include the surface roughness and gloss, depend on the principal process parameters—treatment time, composition, concentration, electrolyte temperature, electrical source, and voltage on the electrodes. Depending on the metal, the most suitable polishing electrolyte must be developed. Additionally, high temperature is an important factor when forming a stable gas layer and ensuring the polishing quality. However, the electrolyte temperature should not be too high as it will destroy the electrolyte composition. High voltage is beneficial to the gas layer formation, allowing the workpiece to achieve a higher polished surface quality.
4 Polishing method using electrolyte jet for various shaped parts
Plasma electrolyte polishing successfully enables metal product finishing. However, there are a number of unresolved technical issues restricting its application. Due to the working bath size limitation, PeP equipment cannot process large parts, including large flat workpieces, large cylindrical workpieces, and long-length workpieces. Moreover, since the electrolyte does not flow in the working bath, it cannot reach workpieces with deep holes, making it difficult to polish such surfaces effectively.
For such cases, electrolyte jet PeP is recommended. PeP with electrolyte jet is a method that uses a pressure pump to convert the electrolyte into a liquid using pressure. The pressure propels the electrolyte to the nozzle to form a jet to spray the workpiece surface and polish it. Compared with the common immersion polishing method, electrolyte jet polishing is not limited by the bath size and can process parts of any shape and size (Fig. 13).
Schematic diagram of immersion (left) and electrolyte jet (right) polishing methods [51]
4.1 Large flat workpiece
Large flat workpieces are parts whose dimensions in the horizontal and vertical directions exceed the bath size, for example, large sheet metal parts. As shown in Table 3a, the bath (cathode) is connected to the negative side of a DC source, while the positive DC source side is attached to the workpiece (anode). The workpiece is driven by a movable mechanism to achieve translation in two directions. In order to close the electrolytic cell, the electrolyte is sprayed through the nozzle by the pump, forming the jet and covering the workpiece surface. When the DC source is turned on, the workpiece surface material is removed.
4.2 Large cylindrical workpiece
The term large cylindrical workpiece refers to a rotating body part whose diameter exceeds the bath size (i.e., aeroengine or cylindrical thin-walled parts). The polishing method for a large cylindrical workpiece with electrolyte jet is shown in Table 3b. The cylindrical workpiece is rotated around the central axis. A pump feeds the electrolyte from the bath through a flexible hose to the workpiece surface, closing the electrolytic circuit. The slow-speed reversible motor drives the spindle to move the nozzle along the central workpiece axis, allowing the entire workpiece to be polished.
4.3 Long-length workpiece
Long-length workpieces include tapes, tubes, and wires. The device setup for PeP of such workpieces is shown in Table 3c. A slow-speed reversible motor is connected to the long-length workpiece, driving it along the axial direction. A pump conveys the electrolyte from the bath, through a flexible hose, to the storage tank. The nozzle is connected to the drain at the bottom of the storage tank. Under the action of gravity, the electrolyte within the storage tank is sprayed through the nozzle to cover the workpiece surface, forming a closed loop required for processing.
4.4 Workpiece with deep holes or cavities
As implied by their name, workpieces with deep holes or cavities have holes or cavities of much larger depth than their cross-sectional size (i.e., cylinder hole, axial shaft oil hole, hollow main shaft hole, and hydraulic valve hole). The installation diagram of the PeP workpiece with deep holes or cavities using an electrolyte jet is shown in Table 3d. The workpiece is immersed in a bath filled with electrolyte. The hoisting mechanism is connected to the workpiece, allowing it to achieve vertical movement. The pump blades rotate, accelerating the electrolyte and thus filling inner surfaces of the workpiece cavity. When the DC source is turned on, the inner workpiece surface with a cavity is effectively polished.
The electrolyte jet PeP is also a recommended choice for polishing geometrically complex workpieces. Ablyaz et al. [53] demonstrated its applicability to polishing complex components produced by selective laser melting. In recent years, industrial robots are widely used in industrial production due to their flexibility, affordable price, and mechanical reconfigurability [54]. Wang et al. [55] proposed an industrial robot with a free electrolyte jet as the basis for a novel PeP technology automation. Such a solution is effective and economical for surface treatment of both geometrically complex and large-size workpieces.
The electrolyte jet PeP can be used to polish large flat workpieces, large cylindrical workpieces, long workpieces, workpieces with deep holes or cavities, and other parts of complex geometry. The electrolyte jet pressure needs to be controlled in a suitable range (0.02 to 0.05 MPa). A very low pressure will prevent the electrolyte from entering the inner surface, meaning that the inner surface cannot be polished. On the other hand, a very high pressure will affect the gas layer formation, making the process unstable [53].
5 Surface treatment of different difficult-to-finish materials
Currently, scholars have successfully polished steel, aluminum, copper, titanium, and their alloys, as well as several other metals and surface coatings. Additionally, PeP can be used to process some of the additive manufactured metals [56, 57]. A summary of the primary PeP process parameters (electrolyte, voltage, initial temperature, and roughness) used to polish various metals is shown in Table 4.
5.1 Stainless steel
Several studies have been carried out on stainless steel PeP, focused on the process parameter optimization to achieve the minimum surface roughness and high gloss. The polishing solution for such process is generally an ammonium sulfate electrolyte with a concentration of 3% to 5%, while also requiring the higher polishing voltage. Podhorský and Bajčičák [34] found that a higher surface gloss level is achieved at lower electrolyte concentrations. However, the ammonium sulfate electrolyte concentration cannot be too low, as it could reduce the process stability. After analyzing experimental results, Mihal et al. [29] determined that a low concentration of ammonium sulfate solution leads to the highest polishing quality.
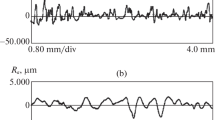
The optimum parameters for AISI 304 steel in an ammonium sulfate solution (mass fraction of 3%) are processing time below 390 s and temperature above 60 °C. Stainless steel was also polished by Wang et al. [43], and the optimal processing parameters were proposed. The ideal ammonium sulfate concentration range needs to be as low as 4% to 5%, with the temperature of 80°C and 5 mm diving depth. Volenko et al. [73] studied the influence of electrolyte composition and multistage polishing on the austenitic stainless steel process technology parameters and surface roughness. The sulfuric acid concentration range was between 4 and 5%, with the addition of 0.05% to 0.45% hydrogen chloride to reduce the working electrolyte temperature. When compared to traditional polishing methods, the multistage polishing decreases the surface roughness by 20% to 25%. In addition to low electrolyte concentration, the surface quality can also be improved by applying high voltage. As shown in Fig. 14, Cornelsen [74, 75] found that the initial inner pipe surface with irregularities and fractures can be processed into a smooth surface. The Sa surface roughness reduced from 0.678 to 0.029 μm at the voltage of 320 V. Finally, Starovoytov et al. [76] stated that PeP reduces the stainless steel surface roughness several times without affecting the original modified layer.
The top view of an unpolished and a polished tube inner surface at the voltage of 320 V [74]
5.2 Alloy steel
The majority of the literature pointed out that PeP technology can reduce the alloy steel surface roughness while also having a beneficial effect on both the surface wear and corrosion resistance. The electrolyte used for alloyed steels is a salt solution with either ammonium sulfate or ammonium chloride as the main component. Ablyaz et al. [60] confirmed that the PeP technology is feasible to improve the surface quality of 38KH2N2MA steel after electrical discharge machining (EDM). The workpiece surface following the EDM was characterized by numerous stacked holes (Fig. 15a), which were eliminated after polishing. Cheng et al. [77] reported that a nanocrystalline surface layer with a thickness of approximately 2 μm formed on the 4340 steel surface after the PeP treatment. The nanocrystalline surface layer formation is triggered by the rapid freezing of the local melting caused by the process. It should also be noted that internal compressive stress remains in the surface layer. However, Yerokhin et al. [48] found that by applying pulsed PeP technology, it is possible to reduce both the compressive residual surface stress and surface roughness, using a 10% sodium bicarbonate solution at a temperature of 70 °C (Fig. 16). The crater appears on the treated surface due to the discharges occurring during the polishing process. The sample d (f = 10 kHz; δ = 0.2) micrograph surface has visible blackened segments, which can be associated with thermally induced surface oxidation processes, meaning that the plasma discharge conditions were unstable. Furthermore, Zhuo et al. [78] confirmed that PeP technology could be applied to surface polish high-carbon micro-alloyed steel materials. It not only effectively removed the oxide scale from the metal surface but also improved the gloss level and wear resistance. Belkin [61] found that both the corrosion and wear resistance of nitriding carbon steel were improved after PeP. The original oxide layer containing micro-cracks and pores was removed after polishing.
Microscopic observation: a the surface after EDM; b the surface after PeP (conditions: voltage of 270 V, temperature of 90°C, and processing time of 5 min) [60]
SEM micrographs showing the surface morphologies: a untreated sample after grinding finishing process; b DC treated; c f = 10 kHz; δ= 0.8; d f = 0.1 kHz; δ = 0.2; where f is the frequency and δ is the duty cycle of source [48]
5.3 Aluminum and its alloys
Most of the reported studies on the polishing of aluminum and its alloys are concerned with the optimization of processing parameters. Duradji et al. [64, 79] concluded that the best effects for aluminum polishing could be achieved using the aqueous solution containing 10% NH4Cl, 4% KCl, and 3% oxalic acid, employed at a voltage of 300 V and temperature of 60°C to 80°C. It is evident that the aluminum anode polishing and the copper coating removal (from the cathode) coincide, but the mechanism remains unclear. Zakharov and Korotkikh [51] analyzed the literature, collected statistics on the composition of PeP electrolytes used in aluminum alloy D16 process, and found that the polishing only occurs in nitrate-based electrolytes.
At the same time, Zakharov and Korotkikh [51] developed an electrolyte composed of 4% to 5% KNO3, 2% to 3% C6H8O7, and 0.5% to 1% C3H8O3, which prevents surface etching and provides the required surface quality. Xue [80] found that the optimal aluminum alloy polishing parameters are 5-mm dive depth, 5 min processing time, and 80°C temperature. The 3D sample surface topography before and after the polishing using optimal process parameters is shown in Fig. 17. Qi [81] polished Al alloy products using the optimal process parameters and observed improved product gloss and reduced surface roughness (from the initial 5.4 to 1.6 μm), as shown in Fig. 18.
3D topography of aluminum alloy: a before PeP; b after PeP (conditions: 80°C temperature, 5-mm workpiece depth, and 4 min treatment time) [80]
Examples of Al alloy products: a before PeP; b after PeP (conditions: 80°C temperature and 1 min treatment time) [81]
5.4 Copper and its alloys
Plasma electrolytic polishing can reduce the surface roughness and improve surface gloss while removing the oxide layer found on the surface of copper and its alloys. In addition, it can modify the copper metal surface by improving its corrosion resistance. Bottger-Hiller et al. [82] transferred the PeP technique to copper polishing and applied it to copperized carbon fibers. The corroded copper sheet layer was completely removed, while the gloss was significantly increased as it was reported that the part was reflecting. Nestler et al. [39] also investigated the application of PeP on copper and its alloys. As shown in Fig. 19, PeP removes the surface materials and achieves a very smooth surface with unprecedented gloss at lower processing time. Duradji and Kaputkin [33] found that the copper material removed during the polishing process is converted into copper ions and transferred to the cathode under the influence of electric field force, forming a copper coating. Through experimental research, Huang [83] concluded that PeP could be used to reduce the brass surface roughness from 0.683 to 0.173 μm, enhancing the polished surface corrosion resistance. Reinhardt [84] confirmed that PeP could be used to improve the corrosion resistance of copper surfaces significantly. Two key reasons for such behavior were identified—the improvement of the surface flatness and the surface layer chemical modification.
Brass part and microscopic images: a before PeP; b after PeP [39]
5.5 Titanium and its alloys
A fluorine salt-based electrolyte is the most often used polishing solution for the PeP of titanium and its alloys. Due to a dense and thin oxide layer formed on the surface of titanium alloys during polishing, hydrofluoric acid (HF) is used to cut the oxide layer and smooth the surface. Many researchers have confirmed that PeP can be used for titanium alloy polishing in both the medical and aviation fields. Aliakseyeu et al. [68] proposed using PeP for polishing titanium alloy while using simple electrolyte composition based on an aqueous ammonium fluoride solution. As shown in Fig. 20a and b, the titanium sample surface scratch is removed, and the surface gloss is significantly improved after polishing. Based on the obtained results, the PeP processes of several products made of titanium alloy BT6 (Grade 5) were applied in the design of medical equipment and aircraft, as indicated in Fig. 20c.
Surface macro- and micro-photographs of titanium samples before and after PeP: a before PeP; b after PeP at a voltage of 300V and initial temperature of 90°C; c examples of PeP products made of titanium alloy ВТ6 [68]
Smyslova et al. [69] discussed the perspectives of applying PeP technology to treat the surfaces of medical implants. They found that PeP in electrolytes containing fluoride components changes the chemical composition of the Ti-6Al-4V alloy surface. After PeP, a titanium fluoride (TiF) layer forms on the surface, having positive effects on its biocompatibility. However, Smyslov et al. [85] confirmed that the oxide layer on the titanium alloy surface reacts with electrolytes containing ammonium fluoride and forms titanium tetrafluoride (TiF4) during the PeP process. At the same time, titanium tetrafluoride is removed due to high-temperature evaporation. Therefore, the surface roughness is reduced. Zhang [86] used the PeP technology to remove the burrs found in titanium alloy blades and found that the mean surface roughness value Ra was reduced from 1.29 to 0.6 μm.
5.6 Niobium, magnesium alloys, and cobalt-chrome alloys
Plasma electrolyte polishing technology can also be used to process other metals, such as niobium, magnesium alloys, and cobalt-chrome alloys. Aliakseyeu et al. [68] found that the largest PeP polishing efficiency and surface quality for niobium can be obtained at a voltage between 280 and 300 V and current density over the range of 0.18 to 0.2 A/cm2. Hoche et al. [15] indicated that the application of PeP procedures for magnesium alloy surface pretreatment leads to a significant improvement in the surface quality (as shown in Fig. 21). However, compared to mechanical polishing, the plasma polished surface exhibits a higher roughness. Researchers have provided examples [87] of the effective use of PeP to polish medical parts made of various alloys, including cobalt-chrome alloys. Using PeP technology, it is possible to achieve both defect-free and smooth surfaces within minutes.
SEM images of the magnesium alloy: a untreated; b after PeP; c after mechanical polishing [15]
5.7 Coating removal
Coatings are widely applied to protect various surfaces of cutting and forming tools, as they are highly resistant to temperature, wear, and corrosion. However, coating removal is necessary if the coating deposition process was incorrect or when the coating properties deteriorate during the operation. Based on the literature, the PeP technology can also be used in renovation technologies, primarily to remove coatings with deteriorated properties [12, 88, 89].
The remaining tool base contamination will affect the adhesion of the subsequent coating. Uhlmann et al. [90] proposed applying the PeP technology to clean coated tools, showing that PeP process removed the turning tool contamination completely (Fig. 21). It should be added that the contamination could not be cleaned by the wet-chemical cleaning technology, which caused the rake face and cutting edge coatings to flake off (please see Fig. 22a). However, the turning tool coating remained when treated using PeP, showing that the coating layer adhesion was increased (Fig. 22b). Finally, it was found that PeP does not influence tool micro-geometry, thereby not affecting the subsequent performance.
The cutting tool and SEM images of cutting edge: a contaminated tool, b contaminated tool after PeP [90]
6 Conclusions
The plasma electrolyte polishing technology improves the surface quality of metallic materials by providing enhanced mechanical and corrosion resistance properties, making it useful in various industrial areas. From an industrial viewpoint, the process offers an economical and environmentally friendly method for polishing metallic surfaces. Based on the literature reviewed in this paper, the following conclusions can be drawn:
-
1.
The underlying principle of PeP technology is that PeP mainly removes materials by forming a gas layer on the surface of the metallic part, followed by generating a plasma discharge, and surface bombarding.
-
2.
High electrolyte voltage and temperature are key factors affecting the formation of a stable gas layer and the occurrence of the plasma discharge.
-
3.
Many parameters influence the polishing process and the subsequent polishing results, such as electrolyte type, electrical source, process parameters, pretreatment, cathode, hanger, and workpiece, among others. The most significant factors affecting the surface roughness are treatment time, electrolyte, and voltage.
-
4.
Plasma electrolyte polishing with electrolyte jet is a suitable method to polish workpieces with complex geometries and configurations, such as large flat workpieces, large cylindrical workpieces, long-length workpieces, and workpieces with deep holes or cavities.
-
5.
PeP was successfully applied to steel, aluminum, copper, and titanium and their alloys, as well as several other metals and surface coatings. Furthermore, it was shown to effectively improve both the surface quality and performance of parts.
7 Prospects
The following PeP prospects are proposed based on this review paper:
-
1.
Studies on polishing theory are mainly experimental and lacking in theoretical formulas, making it hard to achieve the actual engineering application. Furthermore, specific composition, function, and luminescence mechanism of the gas layer during polishing remain unclear. Online monitoring of changes in phenomena and process parameters during polishing is still unexplored, but provides a plethora of possibilities for future theoretical research. Thereby, the systematic theoretical research of the polishing process should become an important research direction in PeP technology.
-
2.
Additional modeling and simulation studies should be emphasized to enable the prediction of optimum process parameter values, as it would enable achieving the expected workpiece improvements and reduce the time and cost of experiments. This work can be carried out by using commercial finite element programs such as COMSOL or ANSYS.
-
3.
Plasma electrolytic polishing has no harmful effects on cell life, paving the way for its application in the surface finish machining of medical parts. Introducing PeP technology into the industrial bio-manufacturing process chain is certainly among future development trends.
-
4.
Plasma electrolytic polishing technology coupled with either the ultrasonic or magnetic fields is still an unexplored area, presenting valuable future research opportunities.
Availability of data and materials
Not applicable here.
Code availability
Not applicable.
References
Liang SY, Shih AJ (2016) Electrochemical machining, chemical machining, and chemical mechanical polishing processes. Analysis of Machining and Machine Tools MA:181–191. https://doi.org/10.1007/978-1-4899-7645-1_12
Li Y, Liu JQ, Li JS (2006) Metal surface polishing technology. Chemical Industry Press, Beijing
Fang JL (2007) Metal material polishing technology. National Defense Industry Press, Beijing
Chen F, Miao X, Tang Y, Yin S (2017) A review on recent advances in machining methods based on abrasive jet polishing (AJP). Int J Adv Manuf Technol 90(1-4):785–799. https://doi.org/10.1007/s00170-016-9405-7
Bordatchev EV, Hafiz AMK, Tutunea-Fatan OR (2014) Performance of laser polishing in finishing of metallic surfaces. Int J Adv Manuf Technol 73(1-4):35–52. https://doi.org/10.1007/s00170-014-5761-3
PlasmaCraft (2019) Metal polishing industrial methods of metal surface final polishing. [Online document] http://plasmacraft.net/metal-polishing-industrial-methods-metal-surface-final-polishing. Accessed 12 February 2020
Duradzhi VN, Bryantsev IV, Tokarov AK (1979) Investigation of erosion of the anode under the action of an electrolytic plasma on it. Elektronnaya Obrabotka Materialov 5:15–19
Hans H, Eckart R Klaus R, Egbert K, Jan P (1986) Method for highly glaining power-conductive workstuffs in anodic electrolyte plasma. DD238074A1
Stanishevsky VK, Parshuto AE, Kosobutsky AA, Semenenko LM, Tikhonovsky VN, Khlebtsevich VA, Slepnev GE (1988) Method of electrochemical machining of articles made of conducting materials. US5028304
Ryabkov DV (1999) Process and apparatus for cleaning and/or coating metal surfaces using electro-plasma technology. RU99116537
Mirzoyev RA, Styrov MI, Stepanova NI, Mayorov AI (1999) Process of electrochemical smoothing of metal articles. RU2168565C1
Nevyantseva RR, Gorbatkov SA, Parfenov EV, Bybin AA (2001) The influence of vapor–gaseous envelope behavior on plasma electrolytic coating removal. Surf Coat Technol 148(1):30–37. https://doi.org/10.1016/S0257-8972(01)01334-2
Beckmann-lnstitut fuer technologieentwicklung, de (2008) Device for plasma polishing using a liquid electrolyte. DE202008011646U1
Stefan F, Martin H, Lothar V, Weisensel (2008) Process for the polishing of metallic dental prostheses. US8444914B2
Hoche H, Gross S, Foerster R, Schmidt J, Adamitzki W (2009) Development of decorative and corrosion resistant coatings for the surface refinement of magnesium alloys by plasma-based methods. Plasma Process Polym 6(S1):S671–S677. https://doi.org/10.1002/ppap.200931708
Cao CB (2012) A polishing solution and polishing method for amorphous alloys. CN102453444A
Polak, Martin, Weltmann, Klaus-Dieter, Ihrke, Roland F, Maik Q, Antje (2017) Method for polishing conductive metal surfaces. PCT/EP2017/066768
Zeidler H, Meyer W, Loeser C, Adamitzki W, Nestler K (2013) Changes on surfaces of electrodes in aqueous electrolytic solutions at high voltages. The 9th International Symposium on Electro Chemical Machining Technology INSECT. Chemnitz, November 12–13
Yerokhin AL, Nie X, Leyland A, Matthews A, Dowey SJ (1999) Plasma electrolysis for surface engineering. Surf Coat Technol 122(2-3):73–93. https://doi.org/10.1016/S0257-8972(99)00441-7
Parfenov EV, Yerokhin A, Nevyantseva RR, Gorbatkov MV, Liang CJ, Matthews A (2015) Towards smart electrolytic plasma technologies: an overview of methodological approaches to process modelling. Surf Coat Technol 269:2–22. https://doi.org/10.1016/j.surfcoat.2015.02.019
Wang J, Suo LC, Guan LL, Fu YL (2012) Analytical study on mechanism of electrolysis and plasma polishing. Adv Mater Res 472-475:350–353. https://doi.org/10.4028/www.scientific.net/AMR.472-475.350
Cornelsen M, Deutsch C, Seitz H (2017) Electrolytic plasma polishing of pipe inner surfaces. Metals 8(1):1–12. https://doi.org/10.3390/met8010012
Wang J, Suo LC, Guan LL, Fu YL (2013) Regularity of surface roughness with polishing time in electrolysis and plasma polishing. J Harbin Inst Technol 02:227–232. https://doi.org/10.3969/j.issn.1006-7043.201203060
Wang J, Suo LC, Fu YL, Guan L (2012) Study on material removal rate of electrolysis and plasma polishing. 2012 IEEE International Conference on Information and Automation. Shenyang, China. IEEE917-922. https://doi.org/10.1109/ICInfA.2012.6246913
Danilov I, Hackert-Oschätzchen M, Schaarschmidt I, Zinecker M, Schubert A (2018) Transient simulation of the removal process in plasma electrolytic polishing of stainless steel. Proceedings of the COMSOL Conference, Lausanne
Vaňa D, Podhorský Š, Hurajt M, Hanzen V (2013) Surface properties of the stainless steel x10 crni 18/10 after application of plasma polishing in electrolyte. Int J Modern Eng Res 3(2):788–792
Kalenchukova OV, Nagula PK, Tretinnikov DL (2015) About changes in the chemical composition of the electrolyte in the process of electrolytic-plasma treatment of materials. Mater Methods Technol 9(1):404–413
Podhorský Š (2015) Utilisation of plasma discharges in electrolyte for surface finishing of stainless steels. Hochsch, Anhalt (FH)
Mihal OV, Moroz OV, Starovoytov RI, Lytovchenko SV, Mazilin BA, Iliushyn LO (2018) Dynamics of the plasma electrolytic polishing process of austenitic steel AISI 304 in a solution of ammonium sulfate. Вопросы атомной науки и техники ISSN 1562-6016: p126-131. http://dspace.nbuv.gov.ua/handle/123456789/147717
Kellogg HH (1950) Anode effect in aqueous electrolysis. J Electrochem Soc 97(4):133–142
Danilov I, Hackert-Oschätzchen M, Zinecker M, Meichsner G, Edelmann J, & Schubert A (2019) Process understanding of plasma electrolytic polishing through multiphysics simulation and inline metrology. Micromachines 10(3): 214(1-22). https://doi.org/10.3390/mi10030214
Wang J, Zong XM, Liu JF, Feng S (2017) Influence of voltage on electrolysis and plasma polishing. ICMEIM 2017. Atlantis Press: 2352-5401. https://doi.org/10.2991/icmeim-17.2017.3
Duradji VN, Kaputkin DE (2016) Metal surface treatment in electrolyte plasma during anodic process. J Electrochem Soc 163(3):E43–E48. https://doi.org/10.1149/2.0011603jes
Podhorský Š, Bajčičák M (2018) Plasma polishing of stainless steels–the electrolyte concentration vs. gloss level. Res Pap - Fac Mater Sci Technol Slovak Univ Technol 26(42):171–176. https://doi.org/10.2478/rput-2018-0021
Gupta P, Tenhundfeld G, Daigle EO, Ryabkov D (2007) Electrolytic plasma technology: science and engineering—an overview. Surf Coat Technol 201(21):8746–8760. https://doi.org/10.1016/j.surfcoat.2006.11.023
Parfenov EV, Farrakhov RG, Mukaeva VR, Gusarov AV, Nevyantseva RR, Yerokhin A (2016) Electric field effect on surface layer removal during electrolytic plasma polishing. Surf Coat Technol 307:1329–1340. https://doi.org/10.1016/j.surfcoat.2016.08.066
Beck U, Lange R, Neumann HG (2007) Micro-plasma textured Ti-implant surfaces. Biomol Eng 24(1):47–51. https://doi.org/10.1016/j.bioeng.2006.05.009
Rajput AS, Zeidler H, Schubert A (2017) Analysis of voltage and current during the plasma electrolytic polishing of stainless steel. Proceedings of the 17th International Conference European Society Precision Engineering Nanotechnology. EUSPEN: 2-3
Nestler K, Böttger-Hiller F, Adamitzki W, Glowa G, Zeidler H, Schubert A (2016) Plasma electrolytic polishing–an overview of applied technologies and current challenges to extend the polishable material range. Procedia CIRP 42:503–507. https://doi.org/10.1016/j.procir.2016.02.240
Dobrynin YES (2017) Electrolytic-plasma polishing of VT6 and VT8M-1 titanium alloys. Proceedings of BNAM 7(55). https://doi.org/10.18577/2307-6046-2017-0-7-2-2
Podhorský Š, Malík A (2010) The possibilities of plasma polishing of the steel DIN 1.0570 in electrolyte. Proceedings of the 19th Conference Metal. Roznov pod Radhostem, Czech Republic 5:18–20
Vana D, Podhorský S, Suba R, Hurajt M (2013) The change of surface properties on tested smooth stainless steel surfaces after plasma polishing. Int J Eng Sci Invent 2(6):7–11
Wang J, Suo LC, Guan LL, Fu YL (2012) Optimization of processing parameters for electrolysis and plasma polishing. Applied Mechanics and Materials. Trans Tech Publications 217:1368–1371. https://doi.org/10.4028/www.scientific.net/AMM.217-219.1368
PlasmaCraft (2019) The use of electrolyte plasma for polishing metal products. http://plasmacraft.ru/primenenie-elektrolitnoy-plazmy-dlya-polirovki-metallicheskih-izdeliy. Accessed 13 February 2020
Kashapov LN, Kashapov NF, Kashapov RN (2013) Investigation of the influence of plasma-electrolytic processing on the surface of austenitic chromium-nickel steels. J Phys Conf Ser 479(1):012003. https://doi.org/10.1088/1742-6596/479/1/012003
Valiev RI, Khafizov AA, Shakirov YI, Sushchikova AN (2015) Polishing and deburring of machine parts in plasma of glow discharge between solid and liquid electrodes. IOP Conf Ser: Mater Sci Eng 86(1):012026. https://doi.org/10.1088/1757-899X/86/1/012026
PlasmaCraft (2019) Ways to reduce the energy intensity of the process of electrolyte-plasma polishing. [Online document] http://plasmacraft.ru/puti-snizheniya-energoemkosti. Accessed 13 February 2020
Yerokhin A, Pilkington A, Matthews A (2010) Pulse current plasma assisted electrolytic cleaning of AISI 4340 steel. J Mater Process Technol 210(1):54–63. https://doi.org/10.1016/j.jmatprotec.2009.08.018
Wang YN, Li NT, Wang Y (2016) An electrolytic plasma polishing power system and its control method. CN105827121A
Yang G, Wang B, Tawfiq K, Wei H, Zhou S, Chen G (2017) Electropolishing of surfaces: theory and applications. Surf Eng 33(2):149–166. https://doi.org/10.1080/02670844.2016.1198452
Zakharov SV, Korotkikh MT (2017) Electrolytic plasma processing of complex products from aluminum alloy D16. Вестник Концерна ВКО Алмаз-Антей 3(22)
PlasmaCraft (2019) Plasma treatment of metal in an electrolyte: how it works. [Online document] http://plasmacraft.ru/plazmennaya-obrabotka-metalla-v-elektrolite-kak-eto-rabotaet#1. Accessed 13 February 2020
Ablyaz TR, Muratov KR, Radkevich MM, Ushomirskaya LA, Zarubin DA (2018) Electrolytic plasma surface polishing of complex components produced by selective laser melting. Russ Eng Res 38(6):491–492. https://doi.org/10.3103/S1068798X18060035
Mohammad A, Wang D (2016) Electrochemical mechanical polishing technology: recent developments and future research and industrial needs. Int J Adv Manuf Technol 86:1909–1924. https://doi.org/10.1007/s00170-015-8119-6
Wang CY, Huang Y, He XL, Zheng LJ, Ding F (2019) A robot auxiliary electrolyte plasma polishing device and polishing method. CN110125734A
Zeidler H, Boettger-Hiller F, Wunderlich A (2017) Surface finish of additively manufactured parts using plasma electrolytic polishing. In Joint Special Interest Group meeting between euspen and ASPE on Dimensional Accuracy and Surface Finish in Additive Manufacturing. KU Leuven, BE, October. https://doi.org/10.3850/978-981-11-2728-1_42
Seo B, Park HK, Kim HG, Kim WR, Park K (2021) Corrosion behavior of additive manufactured CoCr parts polished with plasma electrolytic polishing. Surf Coat Technol 406:126640. https://doi.org/10.1016/j.surfcoat.2020.126640
Ding ZJ (2016) Plasma electrolyte polishing process for precision structural parts of stainless steel. CN105220218A
Dyblenko MJE (2013) Fabrication of reinforced layer on treaded surface of part from alloyed steels. RU2013138998A
Ablyaz T, Muratov K, Ushomirskaya L, Zarubin D, Sidhu S (2019) Electrolytic plasma polishing technique for improved surface finish of ED machined components. Eng Solid Mech 7(2):131–136. https://doi.org/10.5267/j.esm.2019.3.003
Belkin PN, Silkin S A, Dyakov IG, Tambovskiy IV, Korableva SS, Kusmanov SA (2019) Plasma electrolytic polishing of nitrided steel under force convection condition. IOP Conference Series: Materials Science and Engineering. IOP Publishing, 672(1): 012020.
Dyblenko MJE (2013) Method of electrolite-plasma processing of metal surface. RU2013129493A
Rakhcheev VG, Pashentsev AB, Luk Janov KJ, Filin AN, Rakhcheeva EV (2003) Method of combined treatment of parts made from aluminum and its alloys. RU2003134218A
Duradji VN, Kaputkin DE, Duradji AY (2017) Aluminum treatment in the electrolytic plasma during the anodic process. J Eng Sci Technol Rev 10(3):81–84. https://doi.org/10.25103/jestr.103.11
Lingath K, Zeidler H, Parshuta A (2002) Plasma polishing of objects made of titanium or titanium alloys comprises applying a voltage to the object positioned in a warm aqueous electrolyte solution, followed by processing using plasma polishing. DE10207632A1
Damir PT (2011) Method of electrolytic-plasma grinding of parts from titanium and its alloys. RU2461667C1
Smyslov AM, Smyslova MK, Mingazhev AD (2007) Method of electrolytic-plasma polishing of metals works. RU2355829C2
Aliakseyeu YG, Korolyov AY, Niss VS, Parshuto AE, Budnitskiy AS (2018) Electrolyte-plasma polishing of titanium and niobium alloys. Science & technique 17(3):211–219
Smyslova MK, Tamindarov DR, Plotnikov NV, Modina IM, Semenova IP (2018) Surface electrolytic-plasma polishing of Ti-6Al-4V alloy with ultrafine-grained structure produced by severe plastic deformation. IOP Conf Ser: Mater Sci Eng IOP Publishing 461(1):012079. https://doi.org/10.1088/1757-899X/461/1/012079
Mingazhev AD, Krioni NK, Yakupov IT (2017) Method of processing of turbomachine blades made of iron-chromium-nickel alloys. RU2649128C1
Zhang G (2009) A surface polishing method for magnesium alloys. CN101845662A
Wang CY, He XL, Li NT, Gao K, Zhang T, Zheng LJ (2018) A electrolyte and polishing method of amorphous alloy. CN108660504A
Volenko AP, Boychenko OV, Chirkunova NV (2016) Introduction of technology of electrolytic-plasma polishing of metal goods. Vektor nauki Tol'yattinskogo gosudarstvennogo universiteta 1:11–16. https://doi.org/10.18323/2073-5073-2016-1-11-16
Cornelsen M, Deutsch C, Seitz H (2017) Electrolytic plasma polishing of pipe inner surfaces. Metals 8(1):12. https://doi.org/10.3390/met8010012
Cornelsen M, Deutsch C, Seitz H (2018) Influence of the velocity and the number of polishing passages on the roughness of electrolytic plasma polished pipe inner surfaces. Metals 8(5):330. https://doi.org/10.3390/met8050330
Starovoytov RI, Mihal OV, Moroz OV, Mazilin BA, Donets SE, Lytvynenko VV, Lonin YF, Ponomarev AG, Uvarov VT (2018) Electrolysite-plasma smoothing of relief on targets modified by a high-current relativistic electron beam. Voprosy Atomnoj Nauki i Tekhniki 237-240. http://dspace.nbuv.gov.ua/handle/123456789/148872
Cheng YH, Gupta P, Meletis EI (2010) Surface characteristics of 4340 steel treated by electrolytic plasma processing. J Mater Sci 45(2):562–565. https://doi.org/10.1007/s10853-009-4037-z
Zhuo CZ, Lu Z, Liu GQ, Ji XD, Dong J (2018) Application of plasma electrolyte polishing to high carbon microalloyed steel. Surf Technol 047(004):115–119. https://doi.org/10.16490/j.cnki.issn.1001-3660.2018.04.017
Duradji VN, Kaputkin DE (2016) Processing of aluminum in plasma electrolyte during the anodic process. Russian Internet J of Ind Eng 3.3:38–41. https://doi.org/10.24892/RIJIE/20150307
Xue H (2013) Research on process parameters and equipment of aluminum electrolyte-plasma polishing. Dissertation, Harbin Institute of Technology
Qi Q (2015) Research on optimization and application of aluminium formula based on the small electrolysis plasma polishing machine. Dissertation, Harbin Institute of Technology
Bottger-Hiller F, Nestler K, Zeidler H, Glowa G, Lampke T (2016) Plasma electrolytic polishing of metalized carbon fibers. AIMS Mater Sci 2016 3:260–269. https://doi.org/10.3934/matersci.2016.1.260
Huang ZP (2012) Copper alloy electrolyte-plasma polishing method. Dissertation, Harbin Institute of Technology
Reinhardt F, Böttger-Hiller F, Kranhold C, Schulze HP, Kröning O, Zeidler H, Lampke T (2019) Surface modification for corrosion resistance of electric conductive metal surfaces with plasma electrolytic polishing. AIP Conference Proceedings AIP Publishing LLC, 2113(1): 110009. https://doi.org/10.1063/1.5112652
Smyslov AM, Smyslova MK, Mingazhev AD, Selivanov KS (2009) Multi-stage electrolyte-plasma processing of products from titanium and titanium alloys. Bulletin of the Ufa State Aviation Technical University13(1)
Zhang X (2016) Research on electrolyte-plasma deburring technology of aircraft engine blade. Dissertation, Harbin Institute of Technology
Zeidler H, Boettger-Hiller F, Edelmann J, Schubert A (2016) Surface finish machining of medical parts using plasma electrolytic polishing. Procedia CIRP 49:83–87. https://doi.org/10.1016/j.procir.2015.07.038
Parfenov EV, Nevyantseva RR, Gorbatkov SA (2005) Process control for plasma electrolytic removal of TiN coatings. Part 1: duration control. Surf Coat Technol 199(2-3):189–197. https://doi.org/10.1016/j.surfcoat.2004.10.143
Parfenov EV, Nevyantseva RR, Gorbatkov SA (2005) Process control for plasma electrolytic removal of TiN coatings: part 2: voltage control. Surf Coat Technol 199(2-3):198–204. https://doi.org/10.1016/j.surfcoat.2004.10.144
Uhlmann E, Riemer H, An S, Fröhlich M, Paschke H, Petersen M (2019) Ecological and functional optimization of the pretreatment process for plasma based coatings of cutting tools. Procedia Manuf 33:618–624. https://doi.org/10.1016/j.promfg.2019.04.077
Funding
This study was funded by the Key Program of the National Natural Science Foundation of China (Grant No. 51735003) and the Key Basic and Applied Research Program of Guangdong Province, China (Grant No. 2019B030302010).
Author information
Authors and Affiliations
Contributions
All authors contributed to the research, writing, and reviewing of the paper; Yu Huang contributed significantly to performing the analyses and writing the paper; Yu Huang, Chengyong Wang, and Feng Ding helped perform the analysis with constructive discussions.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, Y., Wang, C., Ding, F. et al. Principle, process, and application of metal plasma electrolytic polishing: a review. Int J Adv Manuf Technol 114, 1893–1912 (2021). https://doi.org/10.1007/s00170-021-07012-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00170-021-07012-7