Abstract
Purpose
Total knee arthroplasty (TKA) is often performed sequentially on both sides during a single hospital stay. Patients who experience postoperative nausea and vomiting (PONV) after the first operation are concerned about PONV recurrence after the second operation. However, there are few studies regarding the incidence of PONV in staged bilateral TKA with a ≥ 1-week interval. This study aimed to identify the differences in (1) PONV incidence, (2) use of rescue antiemetics, and (3) the amount of opioid consumption between the first and second operations for staged bilateral TKA with a 1-week interval. Based on our anecdotal experience, the hypothesis of this study was that during staged bilateral TKA at a 1-week interval, the PONV incidence and rescue antiemetic requirement after the second operation will be lower than those after the first operation, regardless of opioid consumption.
Methods
Fifty-eight consecutive patients who underwent staged bilateral TKA with a 1-week interval were retrospectively reviewed. All second-stage operations were performed with the same anaesthesia protocol and perioperative patient management protocol as the first-stage operation. PONV incidence was the primary outcome. The requirement for rescue antiemetic drugs and the amount of opioid consumption were secondary outcome variables. The outcome variables were recorded during three postoperative days (Days 0–2) for each stage and were compared between the first and second operations.
Results
The incidence rates of nausea and vomiting on Day 0 (p = 0.001 and p = 0.004, respectively) and nausea on Day 1 (p = 0.008) were significantly lower after the second operation. Rescue antiemetic use on Day 0 was significantly lower after the second operation (p = 0.001). The total opioid consumption 72 h after surgery was significantly higher after the second operation (61.76 vs. 34.28 mg, p < 0.001).
Conclusion
During staged bilateral TKA with a 1-week interval, PONV incidence was lower after the second operation, even with increased opioid consumption.
Level of evidence
III.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative nausea and vomiting (PONV) causes considerable distress to patients [18], contributes to delayed recovery after surgery [18], and may cause unsatisfactory surgical outcomes [8]. Many studies have reported methods for preventing PONV. Ramosetron and palonosetron, 5-hydroxytryptamine type 3 receptor antagonists, have been reported to be effective in preventing PONV in high-risk patients based on a highly simplified Apfel score [2, 15, 23]. It is also known that combination therapy, such as the administration of pre-emptive dexamethasone in addition to ramosetron, is more effective than monotherapy [16, 19]. More recent reports have shown that multiple doses of perioperative dexamethasone are more effective than a single dose in reducing postoperative pain and PONV [12, 26]. Recent studies have reported a PONV prevalence of 22–73% with improved management [12, 16, 23]. Although there are differences between these studies, a significant number of patients still experience PONV even with contemporary management.
Total knee arthroplasty (TKA) is sometimes performed sequentially on both sides during a single hospital stay. According to our experience, patients who experience PONV after the first operation worry that they will have the same experience after the second operation. Therefore, surgeons occasionally stop pain medications, such as intravenous (IV) patient-controlled analgesia (PCA) or oral opioids, to reduce the risk of PONV. In staged bilateral TKA, the second operation is known to be more painful than the first operation [13, 23]. Nevertheless, concerns about PONV have led to patients not being able to properly control their pain. Studies on the incidence of PONV in staged bilateral TKA are scarce, and to the best of our knowledge, there has been only one study on this topic [23]. However, our experience differs from the results of the previous study.
Therefore, this study aimed to (1) identify the difference in PONV incidence, (2) compare the requirement for rescue antiemetics, and (3) compare the amount of opioid consumption, a well-known cause of PONV, between the first and second operations of staged bilateral TKA. Based on our anecdotal experience, the hypothesis of this study was that during staged bilateral TKA at a 1-week interval, the PONV incidence and requirement for rescue antiemetics after the second operation will be lower than those after the first operation, regardless of opioid consumption.
Materials and methods
Study subjects
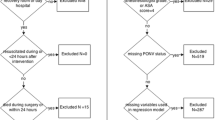
Among patients who underwent primary TKA by a single experienced surgeon at our hospital (a tertiary referral centre) between May 2020 and March 2021, consecutive patients who underwent staged bilateral TKA at intervals of 1 week were selected as study subjects.
The exclusion criteria were as follows: (1) patients who underwent staged bilateral TKA at intervals of more than 1 week; (2) patients with a history of alcohol or opioid dependence; (3) patients with renal or hepatic impairment; (4) patients with intolerance or severe allergy to any drug used in the perioperative pain management protocol; (5) patients with acute systemic complications or problems after surgery.
Ultimately, 58 patients were enrolled in this study, and the patients’ demographic data were collected, including age, sex, height, weight, body mass index, and medical history. History of smoking and motion sickness were evaluated and recorded for all patients who underwent TKA before surgery, and these data were collected. This retrospective study was based on a prospectively collected database.
Among the 58 patients, 10 (17.2%) were men and 48 (82.8%) were women. The mean age of the patients was 69.8 ± 5.4 years. The numbers of patients with one, two, three, or four risk factors for PONV described by Apfel et al.[2] were 2, 8, 38, and 10, respectively. The patient demographic data are summarized in Table 1.
Surgical technique and perioperative management protocol
All patients underwent the same perioperative care protocol. Multimodal oral analgesic drugs (200 mg celecoxib, 75 mg pregabalin, 650 mg acetaminophen) and 5 mg of IV dexamethasone were administered 1 h before the operation for pre-emptive analgesia on an on-call basis. All patients received 2.0 g of cefazolin for antimicrobial prophylaxis.
Spinal anaesthesia was performed in all patients using 10–15 mg of 0.5% bupivacaine and 10–20 μg of fentanyl based on the patient’s height and weight. After checking the level of anaesthesia and until tourniquet inflation, 1000 and 500 mg of tranexamic acid were administered intravenously to patients over 50 kg and under 50 kg, respectively, only in the absence of contraindications. Propofol or dexmedetomidine was continuously infused to induce sedation during the surgery. Oxygen was provided through a facial mask at a flow rate of 5 L/min.
All surgeries were performed by a single experienced surgeon using the same surgical protocol. All surgeries were performed using the medial parapatellar approach, modified gap technique, and mechanically aligned TKA. All prostheses were posterior-stabilized implants with a fixed bearing system and were fixed with cement.
All patients were administered periarticular injections of a multimodal drug cocktail containing 300 mg ropivacaine, 10 mg morphine sulphate, 30 mg ketorolac, 300 µg 1:1000 epinephrine, 750 mg cefuroxime, and 38.7 mL normal saline [17] during the operation. After closing the joint capsule, 1500 mg of tranexamic acid was injected intraarticularly in all patients.
At the end of the surgery, patients were administered IV 0.3 mg ramosetron. IV PCA was used in 31 patients in the first half of the study period; IV PCA was not used in 27 patients who underwent surgery in the second half of the study period. The PCA was programmed to administer 1 mL each dose when the patient pressed the button, with a 10 min lockout period without a basal infusion. The PCA formulation was a 100 mL solution containing 1500 μg fentanyl for patients aged 60–80 years and 1000 μg fentanyl for other patients.
When requested by the patient, IV tramadol (100 mg) was administered as a first-line rescue analgesic for acute pain, and IV morphine sulphate (2.5 mg) was a second-line rescue analgesic. The rescue antiemetic drug for PONV was 10 mg IV metoclopramide. All patients received oral medications from the day after surgery as follows: celecoxib 200 mg once daily, pregabalin 75 mg once before sleep, tramadol 50 mg or oxycodone/naloxone 5/2.5 mg every 12 h, and an extended release form of acetaminophen 650 mg every 8 h. The day after surgery, dexamethasone was administered intravenously at 5 mg in the morning and 2.5 mg in the evening.
Outcome assessment
The incidence of PONV was evaluated as the primary outcome. In addition, the requirement for rescue antiemetics and the amount of opioid consumption were evaluated as secondary outcome variables. Episodes of nausea and vomiting, administration of rescue antiemetic drugs, and administration of pain control medications were recorded during the three postoperative days (Day 0, Day 1, and Day 2) of each stage by reviewing the medical records. Day 0 was defined as a period of 0–24 h after surgery, Day 1 as 24–48 h after surgery, and Day 2 as 48–72 h after surgery.
The requirement for rescue antiemetics was measured by calculating the proportion of patients who were administered antiemetic medication during the three postoperative days. Total opioid consumption for 72 h postoperatively was calculated as the IV morphine equivalent dose (MED) (mg) [24] and included the administration of oral tramadol, oral oxycodone, IV PCA (fentanyl), IV morphine, and IV tramadol.
The primary and secondary outcomes were compared between the first and second operations.
Statistical analysis
A post hoc power calculation test was performed using G-power software (version 3.1). As a paired sample, data were analysed using the McNemar test for nausea and vomiting on Day 0 after the first and second operations. The statistical power was calculated with a two-sided alpha of 0.05. The calculated power values were 94.24% for nausea on Day 0 and 80.42% for vomiting on Day 0.
Descriptive statistical analysis was performed, and data normality was evaluated using the Kolmogorov–Smirnov test. To compare the differences in categorical variables, such as incidence of PONV and requirement for rescue antiemetics between the first and second operations, the chi-square and Fisher exact tests were employed. Among the patients who experienced nausea on Day 0, the linear-by-linear association method was used to determine whether the proportion of patients according to the number of antiemetic drugs administered was different between the first and second operations. Continuous variables, such as the amount of opioids consumed after the first and second operations, were compared using a paired t test. All statistical analyses except for the power calculation were conducted using SPSS software (version 25.0; IBM Corp., Armonk, NY, USA). P values of < 0.05 were considered statistically significant.
Results
The incidence rates of nausea and vomiting after the first and second operations are presented in Table 2. The 72 h overall incidence of PONV, the incidence of nausea and vomiting on Day 0, and the incidence of nausea on Day 1 were significantly lower after the second operation (Table 2).
The use of rescue antiemetic requirements on Day 0 and the overall requirement for 72 h after surgery were significantly lower after the second operation (Table 3). Among the patients who experienced nausea on Day 0 after the first operation, 12% did not take antiemetic medication, 76% received 1 dose, 8% received 2 doses, and 4% received 3 doses of antiemetics. Among the patients who experienced nausea on Day 0 after the second operation, 22.2% of patients did not take antiemetic medication, and 77.8% received only one dose of antiemetic drug. None of the patients received more than two doses of antiemetic drugs after the second operation. However, the differences in these ratios were not statistically significant (p > 0.05).
The total opioid consumption 72 h after surgery was significantly higher after the second operation (p < 0.001), with an average of 34.28 mg MED after the first operation and 61.76 mg MED after the second operation (Fig. 1).
Total opioid consumption within 72 h after the first and second operations of staged bilateral TKA. The values were converted to IV morphine equivalent doses (MED). Patients consumed significantly more opioids after the second operation than after the first. Values with significant differences (p < 0.05) are marked with asterisks. Circles denote outliers with > 1.5 times the upper quartile
Discussion
According to our study, the incidence of PONV was significantly lower after the second operation than after the first operation during staged bilateral TKA at a 1-week interval, especially on Days 0 and 1. Furthermore, the significantly higher opioid consumption after the second operation was an indicator of decreased PONV. In particular, the difference in the vomiting rate and the rescue antiemetic administration rate between the first and second surgeries was more pronounced than the difference in the nausea ratio between the two surgeries. These results indirectly show a decrease in the severity of PONV after the second operation. Efforts have been made to reduce PONV after TKA [7, 10, 12, 15, 16, 23]; however, there have been no studies on how to manage PONV after the second operation in patients who show PONV after the first operation for staged bilateral TKA with a 1-week interval between the two stages. Therefore, our study is the first to provide information on preparing for the second operation in patients with PONV after the first operation during stage bilateral TKA at a 1-week interval. In other words, to reduce PONV after the second operation, it is not necessary to stop the pain medications, which causes the patient to suffer.
We propose two possible causes of the lower PONV incidence after the second operation than after the first operation. The first is the activation of the sympathetic nervous system after the first surgery. The second is the activation of the hypothalamic–pituitary–adrenal (HPA) axis and the elevation of serum cortisol levels after the first operation.
Previous studies have shown that surgical stress activates the sympathetic nervous system [3, 6, 20, 22]. The nausea and vomiting reflex is mainly controlled by the vagus nerve [18], which is a component of the parasympathetic nervous system, but studies on the role of the sympathetic nervous system in nausea and vomiting are few and controversial [14]. However, since the sympathetic and parasympathetic systems are known to have opposite but complementary effects, it is assumed that the same will be true for nausea and vomiting. Some studies have explained that increased sympathetic activity in motion sickness-induced emesis suppresses nausea and vomiting [11, 25]. In other words, the sympathetic nervous system is thought to suppress nausea and vomiting. Studies on the duration of elevated catecholamine levels after surgery are scarce. Previous studies have reported that plasma epinephrine levels increase with surgery and return to basal levels within 1–2 days after major surgery, although this depends on the type of surgery [4, 9, 20]. Plasma norepinephrine levels are reported to increase after surgery and remain at higher concentrations than basal levels for more than 3–5 days [4, 9, 20]. Therefore, we estimated that a condition in which plasma norepinephrine levels do not decrease to baseline by a week after surgery could be a factor in reducing the risk of PONV after the second operation.
When the postoperative HPA axis is activated, there is an increase in plasma cortisol, which is a stress hormone [3, 4, 6, 22]. Glucocorticoids, including cortisol and dexamethasone, are effective antiemetic and anti-inflammatory agents [5, 12, 16, 19, 26]. Therefore, elevated levels of steroid hormones in the blood as a result of a stress reaction after the first operation could reduce the occurrence of PONV after the second operation. Studies have shown that plasma cortisol levels remain above basal levels even on the 7th day after moderate to highly invasive surgery [1, 21]. These results support our assumptions.
This study has several limitations. First, we did not measure the severity of PONV. Thus, we may have omitted some cases if patients had symptoms of nausea, but the severity was mild and they did not complain to the medical staff. In addition, on the day of the second operation, patients often expressed that they were more comfortable and in much better condition than they were on the day of the first operation. However, this finding cannot be objectively shown because the severity of PONV was not assessed. The severity of PONV was only estimated indirectly via the incidence of vomiting or the number of rescue antiemetics administered. Second, although it was certain that the incidence of PONV was lower after the second operation, we could not precisely identify the cause. Further research is needed to confirm our hypothesis. Third, the pain management protocol was changed during the study. PCA was removed to reduce its side effects and to enhance patient recovery after surgery. Our study would have been clearer if all the patients had received the same management protocol. However, there was no case of a patient who received a different pain management protocol after the first and second operations. In addition, since our study compared the first and second operations in each patient, changing the pain management protocol during the course of the study may not have significantly affected the outcome.
Conclusions
When staged bilateral TKA is performed at a 1-week interval, the incidence of PONV decreases after the second operation compared to the first, even with increased opioid consumption. By informing the patient of this possibility in advance, it is possible to reduce the patient’s concerns and prevent inadequate pain control after the second TKA operation.
Abbreviations
- HPA:
-
Hypothalamic–pituitary–adrenal
- MED:
-
Morphine equivalent dose
- PCA:
-
Patient-controlled analgesia
- PONV:
-
Postoperative nausea and vomiting
- TKA:
-
Total knee arthroplasty
References
Amar D, Fleisher M, Pantuck Carol B, Shamoon H, Zhang H, Roistacher N et al (1998) Persistent alterations of the autonomic nervous system after noncardiac surgery. Anesthesiology 89:30–42
Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N (1999) A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 91:693–700
Burton D, Nicholson G, Hall G (2004) Endocrine and metabolic response to surgery. Contin Educ Anaesth Crit Care Pain 4:144–147
Chernow B, Alexander HR, Smallridge RC, Thompson WR, Cook D, Beardsley D et al (1987) Hormonal responses to graded surgical stress. Arch Intern Med 147:1273–1278
Chu CC, Hsing CH, Shieh JP, Chien CC, Ho CM, Wang JJ (2014) The cellular mechanisms of the antiemetic action of dexamethasone and related glucocorticoids against vomiting. Eur J Pharmacol 722:48–54
Desborough JP (2000) The stress response to trauma and surgery. Br J Anaesth 85:109–117
DiIorio TM, Sharkey PF, Hewitt AM, Parvizi J (2010) Antiemesis after total joint arthroplasty: does a single preoperative dose of aprepitant reduce nausea and vomiting? Clin Orthop Relat Res 468:2405–2409
Dorr LD, Chao L (2007) The emotional state of the patient after total hip and knee arthroplasty. Clin Orthop Relat Res 463:7–12
Engelman RM, Haag B, Lemeshow S, Angelo A, Rousou JH (1983) Mechanism of plasma catecholamine increases during coronary artery bypass and valve procedures. J Thorac Cardiovasc Surg 86:608–615
Hahm TS, Ko JS, Choi SJ, Gwak MS (2010) Comparison of the prophylactic anti-emetic efficacy of ramosetron and ondansetron in patients at high-risk for postoperative nausea and vomiting after total knee replacement. Anaesthesia 65:500–504
Himi N, Koga T, Nakamura E, Kobashi M, Yamane M, Tsujioka K (2004) Differences in autonomic responses between subjects with and without nausea while watching an irregularly oscillating video. Auton Neurosci 116:46–53
Kim JK, Ro DH, Lee HJ, Park JY, Han HS, Lee MC (2020) Efficacy of systemic steroid use given one day after total knee arthroplasty for pain and nausea: a randomized controlled study. J Arthroplasty 35:69–75
Kim MH, Nahm FS, Kim TK, Chang MJ, Do SH (2014) Comparison of postoperative pain in the first and second knee in staged bilateral total knee arthroplasty: clinical evidence of enhanced pain sensitivity after surgical injury. Pain 155:22–27
Koch KL, Hasler WL (2016) Nausea and vomiting: diagnosis and treatment. Springer
Koh IJ, Chang CB, Jeon Y-T, Ryu J-H, Kim TK (2012) Does ramosetron reduce postoperative emesis and pain after TKA? Clin Orthop Relat Res 470:1718–1727
Koh IJ, Chang CB, Lee JH, Jeon Y-T, Kim TK (2013) Preemptive low-dose dexamethasone reduces postoperative emesis and pain after TKA: a randomized controlled study. Clin Orthop Relat Res 471:3010–3020
Koh IJ, Kang YG, Chang CB, Do SH, Seong SC, Kim TK (2012) Does periarticular injection have additional pain relieving effects during contemporary multimodal pain control protocols for TKA?: a randomised, controlled study. Knee 19:253–259
Kovac AL (2000) Prevention and treatment of postoperative nausea and vomiting. Drugs 59:213–243
Kovac AL (2013) Update on the management of postoperative nausea and vomiting. Drugs 73:1525–1547
Oehmke MJ, Podranski T, Mann M, Frickey N, Kuhn DF, Hempelmann G (2008) Perioperative concentrations of catecholamines in the cerebrospinal fluid and plasma during spinal anesthesia. Acta Anaesthesiol Scand 52:487–492
Prete A, Yan Q (2018) The cortisol stress response induced by surgery: a systematic review and meta-analysis. Clin Endocrinol (Oxf) 89:554–567
Roth-Isigkeit A, Brechmann J, Dibbelt L, Sievers HH, Raasch W, Schmucker P (1998) Persistent endocrine stress response in patients undergoing cardiac surgery. J Endocrinol Invest 21:12–19
Ryu JH, Jeon YT, Min B, Hwang JY, Sohn HM (2018) Effects of palonosetron for prophylaxis of postoperative nausea and vomiting in high-risk patients undergoing total knee arthroplasty: a prospective, randomized, double-blind, placebo-controlled study. PLoS ONE 13:e0196388. https://doi.org/10.1371/journal.pone.0196388
Swarm RA, Paice JA, Anghelescu DL, Are M, Bruce JY, Buga S et al (2019) Adult cancer pain, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 17:977–1007
Uchino M, Ishii K, Kuwahara M, Ebukuro S, Tsubone H (2001) Role of autonomic nervous system for development and suppression of motion sickness in Suncus murinus. Auton Neurosci 94:46–5126
Xu H, Zhang S, Xie J, Lei Y, Cao G, Pei F (2018) Multiple doses of perioperative dexamethasone further improve clinical outcomes after total knee arthroplasty: a prospective, randomized, controlled study. J Arthroplasty 33:3448–3454
Funding
There are no sources of funding used in this study.
Author information
Authors and Affiliations
Contributions
The authors have made the following contributions: (1) the conception and design (NKL, SK, CBC), analysis and interpretation of the data (NKL, JSK, CJY, BEI, CBC), (2) drafting of the article (NKL), critical revision of the article for important intellectual content (SK, JSK, CJY, BEI, CBC), (3) final approval of the article (NKL, SK, JSK, CJY, BEI, CBC).
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing financial interests.
Ethical approval
This study was approved by the local ethical committee in Seoul national university Bundang hospital, Gyenggi-do, Korea (B-2109-707-101).
Informed consent
The requirement for informed consent was waived due to the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, NK., Kim, S., Kim, J.S. et al. Reduction of postoperative nausea and vomiting risk in the second stage during bilateral total knee arthroplasty with a 1-week interval. Knee Surg Sports Traumatol Arthrosc 30, 3114–3119 (2022). https://doi.org/10.1007/s00167-022-06902-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-022-06902-x