Abstract
Purpose
This study aimed to determine which preoperative factors affect the postoperative change in the joint line convergence angle (JLCA) by preoperatively quantifying soft tissue laxity.
Methods
Thirty-four patients who underwent medial open-wedge high tibial osteotomy (HTO) with a navigation were analysed. The JLCA change after HTO was calculated using standing long-bone anteroposterior radiographs taken preoperatively and 6 months postoperatively. Latent soft tissue laxity was defined as the amount of soft tissue that can be extended to valgus or varus from the weight-bearing position, and calculated by subtracting the JLCA on weight-bearing standing radiographs from that on stress radiographs. Multiple linear regression was performed to determine the preoperative factors that statistically correlated with the postoperative JLCA change.
Results
In multiple linear regression, JLCA change had a statistically significant correlation with latent medial laxity (R = 0.6) and a statistically borderline significant correlation with correction angle (R = 0.2). These imply that the postoperative JLCA change increased by 0.6° per 1° increase in latent medial laxity, and increased by 0.2° per 1° increase in correction angle. Latent medial laxity was the most crucial factor associated with postoperative JLCA changes.
Conclusion
The JLCA change could be larger in patients with large latent medial laxity or severe varus deformity requiring a large correction, which could lead to unexpected overcorrection in HTO. Postoperative JLCA change should be considered in preoperative surgical planning. Target point shifting within the hypomochlion point could be a strategy to prevent overcorrection, especially in patients with large latent medial laxity.
Level of evidence
Level IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medial open-wedge high tibial osteotomy (HTO) is an effective surgical option for medial compartment osteoarthritis that causes a shift of the weight-bearing axis from medial to lateral. Appropriate alignment in HTO is essential to achieve good surgical outcomes [4, 30]. To obtain accurate alignment, several techniques in preoperative planning [2, 17, 20, 28, 32] have been introduced. Nonetheless, unexpected correction errors have been reported in previous studies [5, 10, 16, 17, 20].
The factors associated with correction errors in HTO are unknown. Kyung et al. [16] reported that the extent of bony correction does not correlate with changes in alignment. They suggested that this lack of correlation is affected by soft tissue laxity. Other studies [11, 14, 16, 20, 27, 29] suggested that soft tissue laxity is associated with correction errors. Although several authors have reported that soft tissue laxity affects the changes in alignment and the correction error, no study has identified which preoperative factors are associated with changes in soft tissue laxity.
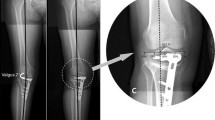
The most common method for quantifying soft tissue laxity is evaluating the joint line convergence angle (JLCA). Lee et al. [19] reported that the change in the JLCA was correlated with correction errors, and that a large JLCA change was correlated with overcorrection. Heijens et al. [11] also reported that bony correction in HTO is accompanied by additional JLCA changes in the range of 0°–5° by shifting the weight-bearing portion from medial to lateral, and stretching the medial soft tissue (Fig. 1). The postoperative change in the JLCA, which reflects soft tissue laxity, is a crucial factor for achieving appropriate alignment and preventing correction errors.
Perioperative change in the joint line convergence angle (JLCA). The medial joint space was opened and the lateral joint space was closed by shifting the weight-bearing axis in medial open-wedge high tibial osteotomy (a preoperative standing long-bone radiograph, b postoperative standing long-bone radiograph). The JLCA was also changed owing to the change in soft tissue tension
However, few studies have reported on preoperative factors associated with the postoperative change in the JLCA, and methods of preventing correction errors in HTO that could result in poor surgical outcomes [8, 12, 13, 30]. The purpose of this study was to determine the preoperative factors that affect the postoperative change in the JLCA after medial open-wedge HTO by quantifying soft tissue laxity. The hypotheses of this study were as follows: (1) the postoperative change in the JLCA can be predicted by evaluating the preoperative soft tissue laxity, and (2) the preoperative medial soft tissue laxity is associated with overcorrection in HTO.
Materials and methods
This study was a retrospective review of 40 patients who underwent medial open-wedge HTO from 2014 to 2016 for symptomatic medial compartment osteoarthritis with Kellgren–Lawrence grade III or IV and a varus alignment of > 5°. Of the 40 patients, 6 were excluded from the study owing to lack of navigation information or preoperative and postoperative radiographs. A total of 34 patients (7 men and 27 women) were thus included in the study. The postoperative hip–knee–ankle (HKA) angle was larger than valgus 2° (range, valgus 2°–10°) in all involved patients [11]. The patients’ demographic characteristics are listed in Table 1.
Data collection
Data were obtained from the navigation information and from preoperative and postoperative radiographs. Full-length standing anteroposterior radiographs of both legs were obtained preoperatively and at 6 months postoperatively. The Telos stress device (Metax GmhH, Hungen-Obbornhofen, Germany) was used in all patients for evaluating soft tissue laxity. The preoperative varus and valgus stress views were obtained in full extension and supine position with a varus and valgus force of 15 kgf to the knee joint at the joint line level, respectively.
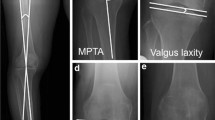
The correction angle was preoperatively determined using the Miniaci method [22] (Fig. 2a), and the target point was the weight-bearing line passing through 62.5% of the tibial plateau [32]. The osteotomy site was opened according to the preoperatively determined correction angle, which was confirmed from the difference between the preoperative and postoperative HKA angles in the navigation system (Fig. 2b, c).
Measurement of the correction angle in the navigation system. The correction angle was preoperatively determined using the Miniaci method. a The osteotomy site was opened according to the preoperatively determined correction angle, which was confirmed from the difference between the preoperative and postoperative hip–knee–ankle angle in the navigation system (b, c)
Latent soft tissue laxity was defined as the amount of soft tissue that can be extended to valgus or varus from the weight-bearing position. The latent soft tissue laxity was divided into latent medial laxity and latent lateral laxity, and was calculated by subtracting the JLCA on the weight-bearing standing radiograph from the JLCA on the valgus or varus stress radiograph (Fig. 3a, b).
The following radiographic parameters were considered: HKA angle (between the femoral and tibial mechanical axes); change in the HKA angle (difference of the HKA angle between preoperative and postoperative long-leg standing radiographs) [17, 18]; JLCA (angle formed by two articular lines of the distal femur and the proximal tibia) [24]; change in the JLCA (difference of JLCA between preoperative and postoperative long-leg standing radiographs) [24]; and correction error (difference of the postoperative HKA angle between the long-standing radiograph and the navigation system). These factors were measured in the picture archiving communication system of our institution. The measures were categorised as varus and valgus deformity and denoted by a negative (−) and positive (+) value, respectively [24].
Several factors, such as age, weight, correction angle in the navigation system, JLCA in the valgus and varus stress radiographs, latent medial and lateral laxity, and preoperative HKA angle in standing long-bone radiographs, were analysed to determine their effect on the postoperative change in the JLCA.
Surgical technique
A single surgeon performed biplane medial open-wedge HTO using the Orthopilot navigation system (HTO version 1.3, Orthopilot; Aesculap, Tuttlingen, Germany). Two bony transmitters on the distal femur and proximal tibia and a foot reference marker, which was wrapped around the foot by a rubber strap, were set up before the surgery. Anatomical landmarks of the distal femur, proximal tibia, patella, and ankle were registered with a pointer on the skin. The HKA angle could be monitored in real time using the HKA centres, which were defined as the centres of three-dimensional ranges of motion. During the procedure of the navigation-assisted HTO, the HKA angle from the navigation system was obtained before and after correction. The superficial medial collateral ligament (MCL) was cut transversely along the osteotomy line without detachment of the proximal portion of the MCL above the osteotomy line. The osteotomy site was opened according to the preoperatively determined correction angle, using a laminar spreader, and fixed with a Tomofix plate and locking screw (Synthes, Bettlach, Switzerland). The gap in the osteotomy site was filled with a cancellous bone chip allograft.
This study was approved by the institutional ethics committee at Seoul Samsung Medical Center and received institutional review board approval (SMC IRB No. 2016-07-114-001).
Statistical analysis
Two independent observers who are fellows in sport medicine (LBH, LDK) conducted the measurements. They were blinded to each other’s measurements and their prior measurements. To evaluate the intraclass correlation coefficients (ICCs), two independent orthopaedic surgeons measured each of the radiological parameters twice with a minimum interval of 2 weeks to determine the intraobserver and interobserver reliabilities. ICC values > 0.75 were considered good; values between 0.40 and 0.75, fair; and values < 0.40, poor [7]. For the reliability of the statistical analysis, ICC > 0.75 was adopted as the data-inclusion criterion. All measurements showed good correlation. The results of the interobserver and intraobserver reliabilities of each measurement are described in Online Resource 1.
A Pearson correlation analysis was performed to confirm the correlation between the change in the JLCA and the correction error, and to determine the factors affecting the JLCA change. Statistical significance was defined as a p value of < 0.05. Statistically correlated factors were selected and analysed in a multiple linear regression analysis to rule out confounding factors. Multiple linear regression with a stepwise method was performed with the selected statistically significant factors, and the eligibility criterion for the predicting model was a p value of < 0.10. If the p value of the variable was > 0.10, the variable was considered an explicative variable, and was excluded from the model. In multiple linear regression analysis, alpha (α) is a constant and the beta coefficient (β) denotes the affecting power of each variable. The regression equation [23] is expressed as follows:
The histogram of the residuals was evaluated for normal distribution of the established model. The discrepancy between the expected value and the observed value was evaluated using standardised residuals. Statistical analysis was performed using IBM SPSS version 20 (IBM, Armonk, NY, USA). A post hoc test was performed to determine the sample size, using a multiple linear regression test at a level of 0.05 and a power of 0.8. The calculations based on the sample size of 34 patients indicated adequate power (0.99) to detect a significant difference in the measurement outcomes in the present study.
Results
Correction error and JLCA change
The change in the JLCA was 2.2° ± 1.9°. The correction error was 2.6° ± 2.2° (Table 2). In the Pearson correlation analysis, the correction error was highly correlated with the change in the JLCA [correlation coefficient (R) = 0.7, p < 0.05].
Factors affecting the postoperative JLCA change
The statistically correlated factors for the postoperative change in the JLCA in the Pearson correlation test are shown in Table 3. In multiple linear regression analysis, the latent medial laxity had a statistically significant correlation and the correction angle had a statistically borderline significant correlation with the change in the JLCA (Table 4). The other factors (JLCA on the varus stress radiograph, preoperative HKA angle) were not statistically correlated with the change in the JLCA and were thus confirmed as confounding factors.
Prediction model for the JLCA change
The predicting equation was established with statistically correlated factors in multiple regression test as follows:
The observed data were highly correlated with the expected data for the change in the JLCA in the standardised residuals (Fig. 4). The predictive models showed that the latent medial laxity and the correction angle account for 49% of the change in the JLCA. These results indicate that the change in the JLCA was positively correlated with the latent medial laxity and the correction angle. For example, if the latent medial laxity was increased by 1°, the JLCA change was increased by 0.6°, and when the correction angle was increased by 1°, the JLCA change was increased by 0.2°.
Discussion
The first principal finding of this study was that correction errors were highly correlated with the JLCA change. This result suggests that the change in the JLCA could be the reason for the correction errors in HTO. Several studies [11, 16, 19, 24] have already reported similar results demonstrating that the correction error was related to the change in the JLCA. Ogawa et al. [24] reported that the change in the JLCA led to correction errors in HTO. Lee et al. [19] reported statistically significant differences in the JLCA change in the overcorrection, acceptable correction, and undercorrection groups. Heijens et al. [11] reported that bony correction in HTO followed the additional change in the JLCA in a range of 0°–5°, which is consistent with our results. A possible explanation for this phenomenon is that the shift of the weight-bearing axis with bony correction in HTO changes the tension in the soft tissue (Fig. 1). Furthermore, the release of the superficial MCL during medial open-wedge HTO could affect the tension in the medial soft tissue. Thus, unintended additional change in the JLCA can occur in medial open-wedge HTO.
The second principal finding is that the change in the JLCA was statistically correlated with the latent medial laxity in multiple linear regression analysis (Table 4). Previously, Ogawa et al. [24] reported that varus laxity was correlated with the change in the JLCA, and that the JLCA change increased by 0.59° for every 1° increase in the preoperative varus laxity; the change in the adduction moment was suggested as the reason in their study. Our results also showed that varus laxity was correlated with the change in the JLCA in the Pearson correlation test. However, varus laxity was found to be a confounding factor in multiple linear regression analysis. A possible explanation is that the latent medial laxity reflects not only the adduction moment but also the medial soft tissue laxity, as the latent medial laxity refers to the amount of the soft tissue that can be extended to valgus from the weight-bearing position. Ogawa et al. [24] also reported that the JLCA can be changed by shifting the weight-bearing axis from medial to lateral, and by stretching the medial soft tissue. Although the correction error can be larger in patients with medial soft laxity, varus laxity could not reflect the medial soft tissue laxity. Latent medial laxity that reflected medial soft tissue laxity and adduction moment turned out to be crucial factor for correction error in our results.
The third principal finding is that the change in the JLCA was statistically correlated with the correction angle in multiple linear regression analysis (Table 4). Lee et al. [19] reported that the change in the mechanical axis was statistically correlated with the change in the JLCA. In our study, the change in the JLCA was affected by the degree of correction, which was consistent with the previous study. The correlation between the correction angle and the change in the JLCA suggests that a larger correction angle is accompanied by a larger change in the JLCA. This is possibly because a larger correction angle is accompanied by a larger change in the weight-bearing axis, which increases the tension in the medial soft tissue. Although age and the preoperative HKA angle were correlated with the change in the JLCA in Pearson correlation analysis, no statistical correlation was found in multiple regression analysis. Considering the need for a larger correction angle in older patients because of their more severe varus deformity, the correlation between these indices and the change in the JLCA can be understood. Heijens et al. [11] also reported a similar result, in which the postoperative change in the JLCA tended to be larger depending on the severity of osteoarthritis.
Therefore, a large latent medial laxity and severe varus deformity requiring a large correction angle might be risk factors for overcorrection. In patients with high-risk factors, target point shifting within the coronal hypomochlion point (weight-bearing line passing through 57.5% of the total width of the proximal tibia; corresponding mechanical tibiofemoral angle [mTFA], 2°) [9] could be considered to prevent overcorrection. Recent biomechanical studies [21, 31] showed that overcorrection beyond 3° mechanical valgus has no advantage for unloading the medial peak pressure. Other studies [1, 26] also showed that even a neutral mechanical alignment (corresponding mTFA, 0°) could decrease the mean contact pressure of the medial compartment. Furthermore, a value greater than the valgus of 2° in the mTFA (coronal hypomochlion point) is also accompanied by additional change in the JLCA and alignment [11]. As our results showed, the postoperative change in the JLCA could be larger depending on the latent medial soft laxity and the correction angle, resulting in unexpected overcorrection. Considering that overcorrected HTO causes the sequential total knee arthroplasty to be technically demanding [3, 6, 15, 25] and the change in the JLCA could be unpredictable beyond the coronal hypomochlion point, target point shifting might be the solution to prevent overcorrection.
Our study had several limitations. The first limitation is the small sample size. Nevertheless, the calculations based on the sample size of 34 patients indicated adequate power in the post hoc test. Additional prospective studies with larger patient numbers are thus required to confirm the models. Second, surgical procedures [24] such as medial soft tissue release, different techniques for stress radiograph acquisition, and racial differences in soft tissue laxity may have affected the results. These discrepancies result in differences in the JLCA change. Lastly, navigation data were used as the reference; however, navigation could lead to mistakes if some of the references are not adequate.
Despite these limitations, this is the first study to analyse the preoperative factors associated with correction errors in HTO. Correction error and postoperative change of JLCA were correlated with latent medial laxity and correction angle in our results. In preoperative surgical planning, latent medial laxity should be considered to prevent overcorrection in HTO. Furthermore, in the above condition of the coronal hypomochlion point (weight-bearing line passing through 57.5% of the total width of the proximal tibia; corresponding mTFA, 2°), the change in the JLCA could be larger in patients with large latent medial laxity or needing a large correction. Therefore, in these cases, especially in patients with large latent medial laxity, target point shifting within the coronal hypomochlion point could be an optional strategy to prevent overcorrection after HTO.
Conclusion
The change in the JLCA could be larger in patients with a large latent medial laxity or severe varus deformity requiring a large correction, which could lead to unexpected overcorrection in HTO. Postoperative change in the JLCA should be considered in preoperative surgical planning, and target point shifting within the hypomochlion point could be an optional strategy to prevent overcorrection especially in patients with a large latent medial laxity.
References
Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P (2007) The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy 23:852–861
Akamatsu Y, Mitsugi N, Mochida Y, Taki N, Kobayashi H, Takeuchi R, Saito T (2012) Navigated opening wedge high tibial osteotomy improves intraoperative correction angle compared with conventional method. Knee Surg Sports Traumatol Arthrosc 20:586–593
Amendola L, Fosco M, Cenni E, Tigani D (2010) Knee joint arthroplasty after tibial osteotomy. Int Orthop 34:289–295
Amis AA (2013) Biomechanics of high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc 21:197–205
Brouwer RW, Bierma-Zeinstra SM, van Raaij TM, Verhaar JA (2006) Osteotomy for medial compartment arthritis of the knee using a closing wedge or an opening wedge controlled by a Puddu plate. A one-year randomised, controlled study. J Bone Joint Surg Br 88:1454–1459
Cerciello S, Vasso M, Maffulli N, Neyret P, Corona K, Panni AS (2014) Total knee arthroplasty after high tibial osteotomy. Orthopedics 37:191–198
Chilton NW, Fleiss JL (1986) Design and analysis of plaque and gingivitis clinical trials. J Clin Periodontol 13:400–410
El-Azab HM, Morgenstern M, Ahrens P, Schuster T, Imhoff AB, Lorenz SG (2011) Limb alignment after open-wedge high tibial osteotomy and its effect on the clinical outcome. Orthopedics 34:e622–e628
Feucht MJ, Minzlaff P, Saier T, Cotic M, Sudkamp NP, Niemeyer P, Imhoff AB, Hinterwimmer S (2014) Degree of axis correction in valgus high tibial osteotomy: proposal of an individualised approach. Int Orthop 38:2273–2280
Gaasbeek RD, Nicolaas L, Rijnberg WJ, van Loon CJ, van Kampen A (2010) Correction accuracy and collateral laxity in open versus closed wedge high tibial osteotomy. A one-year randomised controlled study. Int Orthop 34:201–207
Heijens E, Kornherr P, Meister C (2016) The coronal hypomochlion: a tipping point of clinical relevance when planning valgus producing high tibial osteotomies. Bone Joint J 98-B:628–633
Hernigou P, Medevielle D, Debeyre J, Goutallier D (1987) Proximal tibial osteotomy for osteoarthritis with varus deformity. A ten to thirteen-year follow-up study. J Bone Joint Surg Am 69:332–354
Ivarsson I, Myrnerts R, Gillquist J (1990) High tibial osteotomy for medial osteoarthritis of the knee. A 5 to 7 and 11 year follow-up. J Bone Joint Surg Br 72:238–244
Kendoff D, Board TN, Citak M, Gardner MJ, Hankemeier S, Ostermeier S, Krettek C, Hufner T (2008) Navigated lower limb axis measurements: influence of mechanical weight-bearing simulation. J Orthop Res 26:553–561
Kuwashima U, Tashiro Y, Okazaki K, Mizu-Uchi H, Hamai S, Murakami K, Iwamoto Y (2017) Comparison of the impact of closing wedge versus opening wedge high tibial osteotomy on proximal tibial deformity and subsequent revision to total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 25:869–875
Kyung BS, Kim JG, Jang KM, Chang M, Moon YW, Ahn JH, Wang JH (2013) Are navigation systems accurate enough to predict the correction angle during high tibial osteotomy? Comparison of navigation systems with 3-dimensional computed tomography and standing radiographs. Am J Sports Med 41:2368–2374
Lee DH, Han SB, Oh KJ, Lee JS, Kwon JH, Kim JI, Patnaik S, Shetty GM, Nha KW (2014) The weight-bearing scanogram technique provides better coronal limb alignment than the navigation technique in open high tibial osteotomy. Knee 21:451–455
Lee DH, Nha KW, Park SJ, Han SB (2012) Preoperative and postoperative comparisons of navigation and radiologic limb alignment measurements after high tibial osteotomy. Arthroscopy 28:1842–1850
Lee DH, Park SC, Park HJ, Han SB (2015) Effect of soft tissue laxity of the knee joint on limb alignment correction in open-wedge high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc 24:3704–3712
Marti CB, Gautier E, Wachtl SW, Jakob RP (2004) Accuracy of frontal and sagittal plane correction in open-wedge high tibial osteotomy. Arthroscopy 20:366–372
Mina C, Garrett WE Jr, Pietrobon R, Glisson R, Higgins L (2008) High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med 36:949–955
Miniaci A, Ballmer FT, Ballmer PM, Jakob RP (1989) Proximal tibial osteotomy. A new fixation device. Clin Orthop Relat Res 246:250–259
Nakazato T, Ikehira H, Imasawa T (2017) An equation to estimate the renal cortex volume in chronic kidney disease patients. Clin Exp Nephrol 22:603–612
Ogawa H, Matsumoto K, Ogawa T, Takeuchi K, Akiyama H (2016) Preoperative varus laxity correlates with overcorrection in medial opening wedge high tibial osteotomy. Arch Orthop Trauma Surg 136:1337–1342
Parvizi J, Hanssen AD, Spangehl MJ (2004) Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am 86-A:474–479
Riegger-Krugh C, Gerhart TN, Powers WR, Hayes WC (1998) Tibiofemoral contact pressures in degenerative joint disease. Clin Orthop Relat Res 348:233–245
Sabharwal S, Zhao C (2008) Assessment of lower limb alignment: supine fluoroscopy compared with a standing full-length radiograph. J Bone Joint Surg Am 90:43–51
Schroter S, Ihle C, Mueller J, Lobenhoffer P, Stockle U, van Heerwaarden R (2013) Digital planning of high tibial osteotomy. Interrater reliability by using two different software. Knee Surg Sports Traumatol Arthrosc 21:189–196
Sim JA, Kwak JH, Yang SH, Choi ES, Lee BK (2010) Effect of weight-bearing on the alignment after open wedge high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc 18:874–878
Sprenger TR, Doerzbacher JF (2003) Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Am 85-A:469–474
Van Thiel GS, Frank RM, Gupta A, Ghodadra N, Shewman EF, Wang VM, Bach BR, Verma NN, Cole BJ, Provencher MT (2011) Biomechanical evaluation of a high tibial osteotomy with a meniscal transplant. J Knee Surg 24:45–53
Yoon SD, Zhang G, Kim HJ, Lee BJ, Kyung HS (2016) Comparison of cable method and miniaci method using picture archiving and communication system in preoperative planning for open wedge high tibial osteotomy. Knee Surg Relat Res 28:283–288
Acknowledgements
We appreciate Dae-Hee Lee for his effort in providing medical advice in this study.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2017R1A2B3007362).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest in the authorship and publication of this contribution.
Ethical approval
Ethical approval for the present study was obtained from the institutional review board of Samsung Medical Center (Study no. 2016-07-114-001)
Informed consent
For this retrospective study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, D.k., Wang, J.H., Won, Y. et al. Preoperative latent medial laxity and correction angle are crucial factors for overcorrection in medial open-wedge high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc 28, 1411–1418 (2020). https://doi.org/10.1007/s00167-019-05502-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-019-05502-6