Abstract
Purpose
Previous studies reported sonography was inferior to MRI to predict hamstring tendon graft diameter for ACL reconstruction. This study aimed to investigate the correlation between intraoperative hamstring tendon graft diameter and its preoperative measurement using different sonographic scanning protocol from previous studies.
Methods
Two cadaveric knees were utilized for validation. Sonographically guided gracilis tendon (G) and semitendinosus tendon (ST) injections were performed at myotendinous junction of sartorius using colored latex and then dissection was performed. In the clinical studies, 28 patients underwent primary ACL reconstruction were enrolled. Cross-sectional area (CSA) of G and ST were measured at myotendinous junction of Sartorius. The diameter of doubled G (2G), doubled ST (2ST) and quadrupled ST + G (4STG) were intraoperatively measured using graft sizing devices with 0.5-mm increments.
Results
Cadaveric dissection showed the presence of latex on the surface of G and ST at myotendinous junction of Sartorius in all specimens. In the clinical studies, CSA of G, ST, and ST + G significantly correlated with diameter of 2G (r = 0.464, p = 0.039), 2ST (r = 0.712, p < 0.001), and 4STG (r = 0.792, p < 0.001), respectively. As a result of the simple linear regression analysis, 4STG diameter could be predicted by the following formula: 4.345 + 0.210 × CSA. The differences between calculated diameter by this formula and intraoperative 4STG diameter were within ± 0.5 mm in 89.3% (25/28) of subjects.
Conclusions
The diameter of 2ST and 4STG can be reliably predicted based on sonographic CSA measurement preoperatively. Sonography is a cost-effective alternate to repeat MRI to predict hamstring graft diameter preoperatively.
Level of evidence
Diagnostic study; Level II.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Autologous hamstring tendons, that is, semitendinosus tendon (ST) and gracilis tendon (G), are one of the most common graft choices for anterior cruciate ligament (ACL) reconstruction. For example, Scandinavian ACL reconstruction registries showed 84% of ACL reconstructions were performed using hamstring tendon autografts [11]. In Sweden, participating in the aforementioned registry, hamstring tendon autograft consisted of 95% of ACL reconstructions in 2012 [16]. A majority (73%) of Canadian orthopaedic surgeons indicated preference for hamstring autograft [19]. Ease of harvest, reduced donor site morbidity in comparison to bone–patellar tendon–bone, and no need for additional skin incision seem to be the reasons for its popularity [14].
As there are substantial variability in cross-section of these tendons [22, 24], many researchers have tried to predict graft size preoperatively using anthropometry [6, 15, 26, 29], radiograph [6], and magnetic resonance imaging (MRI) [1,2,3,4, 9, 12, 13, 18, 28]. Since sonography is a readily accessible and cost-effective imaging modality, sonography appeared to be a reasonable alternate to MRI for soft tissue imaging. Therefore, sonography began to attract attention for preoperative measurement of the hamstring tendons [9, 10, 20, 21, 25]. Intraoperative hamstring graft diameter has been reported to have a better correlation with preoperative MRI measurement than sonography [9, 10]. However, high-resolution sonographic equipment is reportedly comparative with MRI for the diagnosis of a rotator cuff tear [5] and meniscus abnormalities [8]. Different sonographic scanning protocol from previous studies and utilizing high-resolution sonographic equipment may improve prediction ability of hamstring tendon graft diameter for ACL reconstruction. If a new sonographic scanning protocol with high prediction ability is established, sonography can be considered as a cost-effective alternate to repeat MRI when MRI originally taken to evaluate the injured knee is poor in evaluating hamstring graft size, such as in the case of overwhelming inflammation or suboptimal slices.
Therefore, the purpose of this study is to investigate the correlation between intraoperative graft diameter of G and ST, and their preoperative measurement using high-resolution sonographic equipment and different sonographic scanning protocol from previous literatures. It was hypothesized that the diameter of doubled G (2G), doubled ST (2ST) and quadrupled STG (4STG) would show significantly strong correlation with preoperatively measured cross-sectional area (CSA) using sonography for G, ST and combination of both tendons (ST + G), respectively.
Materials and methods
Validation study for sonographic technique using cadaveric knees
Overall protocol
Two fresh frozen cadaveric knees (mean age 67.0 ± 1.4 years old, mean BMI 22.0 ± 2.6) from anatomic donation program were utilized for validation study. They were free of apparent trauma or postsurgical changes and thawed for 24 h at room temperature prior to the study. One fellowship-trained musculoskeletal sonography specialist, both in diagnostic sonography and sonographically guided interventions, performed sonographically guided G and ST injections using approximately 0.1 ml of diluted colored latex solution. At a minimum of 24 h post-injection, specimens were dissected, and the presence and distribution of latex on the surface of G and ST were assessed by an orthopaedic surgeon.
Visualization of G and ST
Cadaveric knee specimens were flexed at 20° and positioned to simulate a clinical scanning position with their pes anserinus region facing skyward. Both short axis views of G and ST were identified at pes anserinus region using a 12 − 3 MHz linear array transducer (LOGIQ S8; General Electric, Fairfield, CT, USA). Both tendons were followed proximally along their anatomic curvature to myotendinous junction of Sartorius, where measurement was performed.
Injection protocol
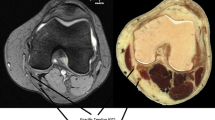
Injections were performed at both insertions and myotendinous junctions for each tendon. Once either G or ST was visualized at pes anserinus region (Fig. 1a), the operator advanced a 22-gauge, 40 mm stainless steel needle to each tendon using an in-plane approach, from caudal to cranial direction (Fig. 1b). After confirming the needle on the surface of G and ST (Fig. 1c), 0.1 ml of colored diluted latex (33% latex, 66% water) [27] was injected, while peritendinous flow was monitored with sonography (Fig. 1d). Dissection showed the presence of latex on the surface of G and ST in all subjects (Fig. 1e).
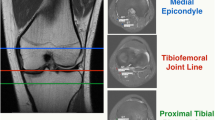
a Short axis view of G and ST at pes anserinus region. G gracilis tendon, ST semitendinosus tendon. b Sonographically guided G injection at pes anserinus region. c Confirming needle placement before injection. Arrowheads: needle. d Monitoring peritendinous flow of diluted colored latex. e Dissection after latex injection. G1: gracilis tendon at pes anserinus region, ST1: semitendinosus tendon at pes anserinus region, G2: gracilis tendon at myotendinous junction of Sartorius, ST2: semitendinosus tendon at myotendinous junction of Sartorius, *: patella. Three 0.8 mm K-wires (arrowheads) showed medial tibio-femoral joint line
Clinical study
Twenty-eight patients who underwent primary ACL reconstruction using 4STG autograft were enrolled. Exclusion criteria were hamstring tendon injuries, which could be diagnosed by preoperative sonography, history of neurological disease [10] and intraoperative graft amputation [9, 10]. Nobody was excluded and 28 patients (11 men and 17 women; mean age 21.9 ± 8.6 years old; mean body mass index 23.5 ± 3.6) were included. Two cases were chronic ACL tear (one case underwent surgery after confirming growth plate closure and the other case injured his ACL in his late forties) and the other cases were acute injury. Excluding these two cases, the mean time from injury to sonographic measurement was 3.8 ± 4.0 weeks. Mean time from sonographic measurement to surgery in all cases was 11.3 ± 9.9 days.
Preoperative measurement using sonography
The sonographic examination was preoperatively performed by one fellowship-trained musculoskeletal sonography specialist, same examiner in the cadaveric validation study, using an 18 − 4 MHz linear-array transducer (Samsung Medicine RS80 Prestige, Samsung Medicine, Seoul, South Korea). Subjects were in a supine position with their ipsilateral hip and tibia in maximum external rotation and knees at 20° of flexion. A pillow was positioned beneath the distal femur for the ease of knee positioning. Both G and ST were identified at pes anserinus region and scanned proximally, then measurements were performed at the myotendinous junction of Sartorius. Measurement items were CSA, width (long axis) and thickness (short axis) (Fig. 2). CSA was measured by freehand tracing the outline for each tendon using an electrical caliper.
Intraoperative measurement of hamstring tendon graft
Each hamstring tendon was harvested by tendon stripper after cutting and suturing the distal end of tendon substance. The diameter of 2G, 2ST, and 4STG were intraoperatively measured using graft sizing devices with 0.5-mm increments.
The institutional review board at the University of Pittsburgh approved the protocol of this study (IRB number: PRO14050110). All participants gave their written informed consent.
Statistical analysis
Intra- and inter-rater reliabilities for sonographic measurement were assessed using 10 healthy knees. Intra-rater reliability was assessed for primary examiner, who was a fellowship-trained musculoskeletal sonography specialist, using single measurement on 2 separate occasions. Inter-rater reliability was assessed using first measurements by primary examiner and single measurements by another examiner, who was an orthopaedic surgeon with 10 years’ experience of musculoskeletal sonography and blinded to CSA, width and thickness obtained by primary examiner. Intraclass correlation coefficient (ICC) with a 95% confidence interval (CI) and standard error of measurement (SEM) were calculated for intra- and inter-rater reliability.
Paired t test was used to compare each parameter between G and ST. Pearson correlation coefficients were calculated to determine the relationship between intraoperative graft diameter and width, thickness and CSA for both 2G and 2ST. Combined CSA was defined as sum of each CSA of ST and G. Pearson correlation coefficient was also calculated to evaluate the correlation between combined CSA and diameter of 4STG. Simple linear regression analysis was performed to obtain the formula to predict the 4STG graft diameter based on CSAs of G and ST measured by sonography. Statistical significance was set at p < 0.05. All analyses were performed using the statistical program SPSS version 23.0 (SPSS, Chicago, IL).
Power analysis for detection of correlation between intraoperative graft diameter and CSA measured by sonography was conducted using G*Power3 statistical analysis software package. An α value was set at 0.05, a correlation was set at 0.55, which was determined according to the results of the preliminary study, and a power (1-β) was set at 0.8. Power analysis suggested that 23 patients were needed for the total number of subjects.
Results
ICC, 95% CI, and SEM values for intra- and inter-rater reliabilities for sonographic measurements of G and ST are shown in Table 1. Although ICC values for inter-rater reliability of G-CSA was considered as moderate, the other measurements were considered as substantial or almost perfect [17].
Both CSA and thickness were significantly greater in ST than G although width was not significantly different (Table 2). Intraoperative doubled graft diameter was also significantly greater in ST than G.
In G, CSA and thickness significantly correlated with 2G graft diameter. In ST, only CSA significantly correlated with 2ST graft diameter while neither width nor thickness correlated with 2ST graft diameter (Table 3). The mean combined CSA was 17.3 ± 2.5 mm2 and mean 4STG graft diameter was 8.0 ± 0.7 mm. Combined CSA had the strongest and positive correlation with intraoperative 4STG graft diameter with significance (Table 3).
As a result of the simple linear regression analysis (Fig. 3), 4STG diameter could be predicted by the following formula:
4.345 + 0.210 × [Combined CSA], (coefficient of determination: 0.627).
The differences between calculated diameter by this formula and intraoperative 4STG diameter were within ± 0.5 mm in 89.3% (25/28) of subjects (Table 4).
Discussion
The most important finding of this study was that the formula could be found to precisely predict 4STG graft diameter from preoperative CSA measurement when hamstring tendons were measured at myotendinous junction of sartorius using high-resolution sonographic equipment. While the diameter of hamstring tendon grafts was hypothesized to show a significantly strong correlation with preoperatively measured CSA using sonography, that was validated for ST and STG. This is the first study to precisely predict the intraoperative graft diameter for ACL reconstruction, using new sonographic scanning protocol and high-resolution sonographic equipment.
Although unit of CSA (square millimeters) is different from diameter (millimeters), correlational analysis between them was performed in many studies [2,3,4, 9, 10, 12, 18, 28]. Previous MRI study demonstrated that CSA of elliptical G and ST significantly correlated with intraoperative graft diameter while neither thickness and width correlated [3], which was consistent with the present study. Correlation coefficients between CSA measured by our sonographic scanning protocol with high-resolution sonographic equipment and diameters of 2ST and 4STG were better than previous sonographic studies [9, 10, 20, 21, 25] and comparable with MRI [9, 10].
Possible reasons for better correlation between sonographic measurement and intraoperative graft diameter in this study would be (1) high-frequency sonographic transducer was utilized, (2) CSA was measured by freehand tracing, not ellipse tool, (3) different scan level and posture of subjects from previous studies. The frequency of sonographic transducer was 18 − 4 MHz in this study while between 12 − 7 MHz in previous studies [9, 10, 20]. As the subjects in this study were young, healthy and active, mean BMI (23.5) in this study cohort was lower than former studies (24.8–27.2) [9, 10, 20, 21], which enabled us to utilize high-frequency transducer. Another reason may be the way to measure the CSA: former sonographic studies measured CSA by ellipse tool [9] or mixture of ellipse tool and freehand tracing [10], while in this study every CSA was measured by freehand tracing. As cross-sectional shape of each tendon has variation [22, 24], ellipse tool would not be suitable for the accurate measurement. The other reasons may be the different scan level and positioning of subjects: previous researches utilized prone position and scan level was between medial joint line and medial femoral epicondyle [9, 10, 20, 21]. Previous sonographic studies have followed the same protocol of the MRI studies [9, 10]. Due to technological limitation, in MRI hamstring studies, medial tibio-femoral joint line or medial femoral epicondyle (physis or physeal scar of distal femur) was set as measurement level [4, 12, 13, 18, 28]. On the other hand, subjects were in supine position and CSA was measured at the level of myotendinous junction of the Sartorius in this study. The reasons why measurement was performed at the myotendinous junction of Sartorius tendon in this study were (1) relative location among G, ST and Sartorius are consistent and (2) surgically relevant. A cadaveric validation study was conducted to confirm if myotendinous junction of Sartorius could be visualized using sonography. Specimens were positioned in the same alignment and same sonographic scanning protocol was followed as human subjects in the clinical study. Therefore, both G and ST deep to the myotendinous junction of Sartorius were clearly differentiated.
Several studies performed simple linear regression analysis to obtain a formula for the prediction of 4STG graft diameter [9, 10, 20]. Previous studies have examined the prediction ability of MRI or sonographic measurements by identifying a certain cut-off point which discriminates a sufficient diameter of the final graft [9, 28]. This is because failure rates of a quadrupled STG autograft have reportedly increased with graft diameter of less than 8 mm [7]. The differences between calculated diameter and intraoperative 4STG diameter were within ± 0.5 mm in 89.3% (25/28) of subjects. If accepting underestimate, which can easily be dealt with by trimming the graft, concordance rate increased 96.4% (27/28). Based on the formula obtained in this study, combined CSA was required to be more than 18 mm2 to obtain 4STG graft with the diameter over 8.0 mm.
The formula to predict 4STG graft diameter was obtained from linear regression analysis of 28 subjects involved in this study. Therefore, real prediction ability need to be examined prospectively as a future study.
This study has a few limitations that should be acknowledged. First, only the diameters of 2G, 2ST and 4STG were analyzed in this study. Therefore, these results were not applicable to ACL reconstruction using tripled or quadrupled ST or G. Second, cadaveric validation study was performed using only two specimens.
Graft choice for ACL reconstruction is decided considering multiple factors such as tissue availability, prior surgery, patient preference, activity level, sports choice, and anatomic variance of each individual patient [14]. Accurate prediction of hamstring tendon graft diameter helps surgeons choose optimal graft as preoperative planning. A new sonographic scanning protocol of hamstring tendon, established in this study, increased prediction ability of 4STG and 2ST graft diameter compared to previous studies, and it shows sonographic predictability is as accurate as MRI. If available MRI leaves hamstring tendon visualization in questions due to concomitant injuries to surrounding tissues or due to slices that visualized tendons of interest optimally, sonography is far more cost- and time-sensitive imaging modality to be considered instead of repeating an MRI [23], allowing for more predictable operative flow on the day of the operation.
Conclusion
The diameter of doubled ST and quadrupled STG can be reliably predicted based on preoperative CSA measurement when hamstring tendons were measured at myotendinous junction of Sartorius using high-resolution sonographic equipment. Sonography is a cost- and time-effective alternate to repeat MRI to accurately predict hamstring tendon graft diameter preoperatively.
References
Agarwal S, de Sa D, Peterson DC, Parmar D, Simunovic N, Ogilvie R, Musahl V, Ayeni OR (2018) Can Preoperative magnetic resonance imaging predict intraoperative autograft size for anterior cruciate ligament reconstruction? A systematic review. J Knee Surg. https://doi.org/10.1055/s-0038-1666830
An VVG, Scholes C, Mhaskar VA, Parker DA, Fritsch BA (2017) Regression modelling combining MRI measurements and patient anthropometry for patient screening and prediction of graft diameter in hamstring autograft arthroscopic ACL reconstruction. Asia Pac J Sports Med Arthrosc Rehabil Technol 8:24–31
Beyzadeoglu T, Akgun U, Tasdelen N, Karahan M (2012) Prediction of semitendinosus and gracilis autograft sizes for ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 20(7):1293–1297
Bickel BA, Fowler TT, Mowbray JG, Adler B, Klingele K, Phillips G (2008) Preoperative magnetic resonance imaging cross-sectional area for the measurement of hamstring autograft diameter for reconstruction of the adolescent anterior cruciate ligament. Arthroscopy 24(12):1336–1341
Chauhan NS, Ahluwalia A, Sharma YP, Thakur L (2016) A prospective comparative study of high resolution ultrasound and MRI in the diagnosis of rotator cuff tears in a tertiary hospital of North India. Pol J Radiol 81:491–497
Chiba D, Tsuda E, Sasaki S, Liu X, Ishibashi Y (2017) Anthropometric and skeletal parameters predict 2-strand semitendinosus tendon size in double-bundle anterior cruciate ligament reconstruction. Orthop J Sports Med 5(8):2325967117720148
Conte EJ, Hyatt AE, Gatt CJ Jr, Dhawan A (2014) Hamstring autograft size can be predicted and is a potential risk factor for anterior cruciate ligament reconstruction failure. Arthroscopy 30(7):882–890
Cook JL, Cook CR, Stannard JP, Vaughn G, Wilson N, Roller BL, Stoker AM, Jayabalan P, Hdeib M, Kuroki K (2014) MRI versus ultrasonography to assess meniscal abnormalities in acute knees. J Knee Surg 27(4):319–324
Erquicia JI, Gelber PE, Doreste JL, Pelfort X, Abat F, Monllau JC (2013) How to improve the prediction of quadrupled semitendinosus and gracilis autograft sizes with magnetic resonance imaging and ultrasonography. Am J Sports Med 41(8):1857–1863
Galanis N, Savvidis M, Tsifountoudis I, Gkouvas G, Alafropatis I, Kirkos J, Kellis E (2016) Correlation between semitendinosus and gracilis tendon cross-sectional area determined using ultrasound, magnetic resonance imaging and intraoperative tendon measurements. J Electromyogr Kinesiol 26:44–51
Gifstad T, Foss OA, Engebretsen L, Lind M, Forssblad M, Albrektsen G, Drogset JO (2014) Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am J Sports Med 42(10):2319–2328
Grawe BM, Williams PN, Burge A, Voigt M, Altchek DW, Hannafin JA, Allen AA (2016) Anterior cruciate ligament reconstruction with autologous hamstring: can preoperative magnetic resonance imaging accurately predict graft diameter? Orthop J Sports Med 4(5):2325967116646360
Hamada M, Shino K, Mitsuoka T, Abe N, Horibe S (1998) Cross-sectional area measurement of the semitendinosus tendon for anterior cruciate ligament reconstruction. Arthroscopy 14(7):696–701
Hofbauer M, Muller B, Murawski CD, van Eck CF, Fu FH (2014) The concept of individualized anatomic anterior cruciate ligament (ACL) reconstruction. Knee Surg Sports Traumatol Arthrosc 22(5):979–986
Janssen RPA, van der Velden MJF, van den Besselaar M, Reijman M (2017) Prediction of length and diameter of hamstring tendon autografts for knee ligament surgery in Caucasians. Knee Surg Sports Traumatol Arthrosc 25(4):1199–1204
Kvist J, Kartus J, Karlsson J, Forssblad M (2014) Results from the Swedish national anterior cruciate ligament register. Arthroscopy 30(7):803–810
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Leiter J, Elkurbo M, McRae S, Chiu J, Froese W, MacDonald P (2017) Using pre-operative MRI to predict intraoperative hamstring graft size for anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 25(1):229–235
McRae SM, Chahal J, Leiter JR, Marx RG, Macdonald PB (2011) Survey study of members of the Canadian Orthopaedic Association on the natural history and treatment of anterior cruciate ligament injury. Clin J Sport Med 21(3):249–258
Mohd Asihin MA, Bajuri MY, Ahmad J, Syed Kamaruddin SF (2018) Pre-operative ultrasonographic prediction of hamstring autograft size for anterior cruciate ligament reconstruction surgery. Ceylon Med J 63(1):11–16
Momaya AM, Beicker C, Siffri P, Kissenberth MJ, Backes J, Bailey L, Rulewicz GJ, Mercuri JM, Shealy EC, Tokish JM, Thigpen CA (2018) Preoperative ultrasonography is unreliable in predicting hamstring tendon graft diameter for ACL reconstruction. Orthop J Sports Med 6(1):2325967117746146
Pagnani MJ, Warner JJ, O’Brien SJ, Warren RF (1993) Anatomic considerations in harvesting the semitendinosus and gracilis tendons and a technique of harvest. Am J Sports Med 21(4):565–571
Parker L, Nazarian LN, Carrino JA, Morrison WB, Grimaldi G, Frangos AJ, Levin DC, Rao VM (2008) Musculoskeletal imaging: medicare use, costs, and potential for cost substitution. J Am Coll Radiol 5(3):182–188
Pichler W, Tesch NP, Schwantzer G, Fronhofer G, Boldin C, Hausleitner L, Grechenig W (2008) Differences in length and cross-section of semitendinosus and gracilis tendons and their effect on anterior cruciate ligament reconstruction: a cadaver study. J Bone Joint Surg Br 90(4):516–519
Rodriguez-Mendez LM, Martinez-Ruiz JJ, Perez-Manzo R, Corona-Hernandez JL, Alcala-Zermeno JL, Sanchez-Enriquez S (2017) Preoperative ultrasonographic prediction of hamstring tendon diameter for anterior cruciate ligament repair. J Knee Surg 30(6):544–548
Schwartzberg RS (2014) Prediction of semitendinosus and gracilis tendon lengths and diameters for double bundle ACL reconstruction. Am J Orthop (Belle Mead NJ) 43(1):E1–E6
Smith J, Hackel JG, Khan U, Pawlina W, Sellon JL (2015) Sonographically guided anterior cruciate ligament injection: technique and validation. Pm r 7(7):736–745
Wernecke G, Harris IA, Houang MT, Seeto BG, Chen DB, MacDessi SJ (2011) Using magnetic resonance imaging to predict adequate graft diameters for autologous hamstring double-bundle anterior cruciate ligament reconstruction. Arthroscopy 27(8):1055–1059
Xie G, Huangfu X, Zhao J (2012) Prediction of the graft size of 4-stranded semitendinosus tendon and 4-stranded gracilis tendon for anterior cruciate ligament reconstruction: a Chinese Han patient study. Am J Sports Med 40(5):1161–1166
Acknowledgements
The authors sincerely appreciate the assistance of Orthopaedic Robotics Laboratory, University of Pittsburgh, for allowing the authors to execute the cadaveric portion of this investigation.
Funding
No financial support was provided for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The University of Pittsburgh Institutional Review Board reviewed and approved the requested modifications by the expedited review procedure authorized under 45 CFR 46.110 and 21 CFR 56.110.
Rights and permissions
About this article
Cite this article
Takenaga, T., Yoshida, M., Albers, M. et al. Preoperative sonographic measurement can accurately predict quadrupled hamstring tendon graft diameter for ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 27, 797–804 (2019). https://doi.org/10.1007/s00167-018-5101-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-018-5101-5