Abstract
Purpose
The aim was to compare the outcome of anterior cruciate ligament (ACL) reconstruction with bone-patellar tendon-bone (BPTB) autograft, with and without a poly(urethane urea) augmentation device.
Methods
Patients were randomized to BPTB reconstruction with a synthetic degradable augmentation device (n = 96) or without augmentation (n = 105). Follow-ups were made during 4 years after surgical treatment with the KT1000™ arthrometer for objective evaluation of sagittal stability. The Tegner scoring system for assessment of physical activity level and the Knee injury Osteoarthritis Outcome Score (KOOS) for assessment of knee-specific health were evaluated after 4 and 12 years.
Results
KT1000™ tests showed a significant decrease in mean manual maximum side-to-side difference after 4 years in both patients with and those without augmentation, without any statistical difference between the groups (n.s.). Pre-injury, 76 and 80 % of the patients, respectively, reported Tegner level 7–10. Pre-surgery, the corresponding figures were 6 and 5 %, and at 4 years, 33 and 30 %. Twelve years after ACL reconstruction, both groups had significantly higher KOOS scores in function in sports and recreational activities (p < 0.001) and knee-related quality of life (p < 0.001) compared to before surgical treatment. In 10 patients, the augmentation device was removed, in six of these because of insufficient screw fixation to femur and in four due to swelling/hydrops.
Conclusion
This study showed no significant difference in clinical outcome with use of an additional synthetic augmentation device in a single-bundle BPTB ACL reconstruction compared with non-augmentation, in short, intermediate, or long-term perspective.
Level of evidence
Therapeutic study, Level I.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical reconstruction of an anterior cruciate ligament (ACL) injury with single-bundle bone-patellar tendon-bone (BPTB) or hamstring autografts aims to restore the stability and function of the knee. However, if a restoration of normal knee biomechanics is not achieved by the ACL reconstruction, resulting in remaining rotational instability or laxity, this will contribute to the development of posttraumatic knee osteoarthritis [22, 29]. This is reported even more commonly if the ACL injury is associated with a medial meniscus tear or articular cartilage injury [18, 39, 45]. Patients who have been treated operatively with reconstruction have shown better stability compared to those with non-operative treatment, and fewer meniscus and cartilage injuries [9], but it is still unclear if surgical ACL reconstruction decreases or prevents the risk for future osteoarthritis [20, 22, 25].
A general disadvantage with avascular autografts, as well as allografts, is the decrease in strength and loss of elasticity starting a few weeks after surgery caused by tissue necrosis and resorption. This is followed by a period of ongoing revascularization, cell ingrowth, tissue regeneration, remodelling, and at the same time a gradual resorption of the avascular tendon tissue, lasting over a period of 9–12 months [6]. The reduced mechanical properties during this period increase the risk for stretching out and re-rupture of the autograft with increased laxity and instability, especially during early return to sports or hard labours.
Different types of augmentation materials for support of the autologous ligament graft in a single-bundle surgical procedure have been suggested to increase stability and to better protect the function of the native ACL repair or reconstruction. Also prostheses have been tried, but studies have failed to show a positive long-term effect [8, 26, 30, 32, 34, 46]. The materials in these studies are all non-degradable and have a high stiffness. For more than a decade, a degradable poly(urethane urea), Artelon®, has been used in orthopaedic surgery. One of the first developed products was an augmentation device designed to support the BPTB autograft during the rehabilitation period and when returning to sports and labour. The device was designed with the purpose to share the mechanical load with the graft during the sensitive initial healing and rehabilitation period and thus achieve long-term stability. The hypothesis was thus that augmentation of the autograft will reduce instability and thereby prevent the associated long-term complications.
The aim of this study was to compare the outcome of ACL reconstruction with BPTB autograft, with and without a poly(urethane urea) augmentation device.
Materials and methods
A prospective, randomized, controlled, double-blinded multicentre study was performed in patients with an isolated ACL injury. A guidance document by Food and Drug Administration (FDA) was used for the design of the study [12]. These guidelines prescribe a multicentre study including 100 patients in each group, or more, depending on if the statistical calculations request for more patients. Furthermore, the treatments should be performed by at least six surgeons with a minimum of 10–15 device implant procedures per surgeon.
The study included 201 patients with diagnosed ACL injury with duration of at least 4 weeks. For inclusion in the study, the patients had to be above 15 years of age and had subjectively experienced instability limiting their activity level. The instability had also been objectively evaluated. Exclusion criteria were concomitant posterior cruciate ligament injury and/or collateral ligament injury in the same knee, or bilateral ACL injury, reason to expect poor compliance in rehabilitation, serious illness, other joint or skeletal disease, or medical treatment which could affect the wound healing. The study involved six surgeons at four Swedish clinics, all experienced in the arthroscopic BPTB technique. The surgeons were individually instructed in the augmentation technique and assisted during the first operation by a surgeon experienced in the technique. The patients were randomized to BPTB autograft reconstruction with a synthetic augmentation device (test group, n = 96) or without augmentation (control group, n = 105) according to a randomization list and by using sealed envelopes. The patients were operated on between June 1999 and May 2000. The characteristics of the patients are given in Table 1.
The investigation plan was reviewed and approved by the Ethics Committees at the universities of Gothenburg and Örebro, and at Karolinska Institutet in Stockholm, according to the Declaration of Helsinki. All patients provided informed consent before participating.
Clinical evaluation
The pre- and postoperative examinations included both subjective and objective tests and were performed by experienced observers before treatment and after 3 and 6 months, and after 1, 2, 3, and 4 years. The number of patients followed for 4 years and the reasons for lost to follow-up are given in Fig. 1. The patients and observers were blinded with regard to the treatment group. Any clinical instability, swelling, bleeding, erythema, and infections were recorded at all visits. The status of the articular joint surfaces was graded from 0 to 4 during the arthroscopic surgery for evaluation of any secondary cartilage injuries [31, 41]. Knee ligament was tested with the KT1000™ arthrometer (MEDmetric®, San Diego, California) for objective evaluation of sagittal stability (primary outcome measure) at 15 lb (6.8 kg) and 20 lb (9 kg) active displacement and with a manual maximum force [38]. Only the latter measurement is presented, since it has been shown to be the strongest discriminant [2]. Knee joint stability was determined in both the healthy and injured knee and a side-to-side difference ≥3 mm was considered positive [7]. Also clinical stability tests such as the Lachman and the pivot shift tests were used to assess any sagittal or rotational instability. The International Knee Documentation Committee (IKDC) standard evaluation was used for a subjective assessment of knee function [15]. The IKDC final grade of normal (A), nearly normal (B), abnormal (C), or severely abnormal (D) was determined by the worst score in any of the four principal categories: subjective assessment, symptoms, range of movement, and ligament examination. The Tegner scoring system was used to assess the physical activity level [43]. The patients also estimated their grade of activity level before the injury occurred. Furthermore, the Knee injury Osteoarthritis Outcome Score (KOOS) was used for assessment of knee-specific health [35]. The five subscales of the self-administered KOOS cover pain, symptoms, activities in daily living (ADL), difficulty in sports and recreational activities, and quality of life, with scores ranging from 0 (worst) to 100 (best). After the completion of the 4-year follow-up, the patients could if they wished get information on to what group they belonged.
Long-term follow-up
After a mean follow-up time of 11 years and 8 months, the long-term status was evaluated by sending questionnaires to the participating patients. The number of patients who replied of those who could be reached was 71 (80 %) in the test group and 77 (78 %) in the control group (Fig. 1). Median age at long-term follow-up was for all patients 37 years (range 26–58). The KOOS questionnaire was used for assessment of knee-specific health [35]. In addition, the Tegner scoring system was used to assess the activity level [43]. There were no visits to the clinic at this long-term follow-up.
Surgical technique
A single-bundle BPTB autograft technique was used in the control group with non-resorbable metal screw fixation in the tibia and femur, respectively. A non-anatomical transtibial approach was used for drilling of the femoral tunnel. The same arthroscopic surgical technique was used in the test group with the addition of the augmentation device and with drill holes with the diameter of 11 mm to allow the space for the BPTB autograft and the device.
The augmentation device
The Augmentation Device ACL (Artimplant AB, Gothenburg, Sweden) is a woven structure made of Artelon®, which is a polycaprolactone-based poly(urethane urea) [13, 19]. The degradation of the material is slow with 50 % of the initial strength remaining after 4 years. The material degrades by hydrolysis, which results in a resorbable and a non-resorbable fraction. The resorbable fraction is eliminated from the body through the Krebs cycle (citric acid cycle), primarily as carbon dioxide and in urine. The non-resorbable fraction is incorporated in the surrounding host tissue without eliciting any inflammatory or foreign body response.
Preparation of the combined autograft/augmentation device
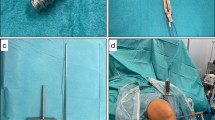
A 10-mm-wide BPTB graft was harvested in the central part of the patellar tendon. Two narrow drill holes were made in each bone plug in the end of the graft for pullout sutures and device fixation. The synthetic augmentation band was positioned on the anterior (cortical) side of the autograft. Interrupted sutures were used to fix and adapt the folded edges of the tendon on the anterior side covering the tendon, securing the augmentation band to the tendon (Fig. 2a–b). Sutures were also put through the two drill holes to anchor the augmentation band to the bony ends and also to be used as pull-out sutures for later insertion.
a The bone-patellar tendon-bone (BPTB) autograft and augmentation device was prepared in a customary-made graft holding device with the anterior (cortical) side up. The synthetic augmentation band was positioned on the anterior (cortical) side of the graft. b Illustration on how the suture was put through the augmentation band
Arthroscopic insertion and fixation
Cancellous bone acquired from drilling the bone tunnels, and from contouring the bone plugs during sizing of the combined construct, was replanted into the harvesting defect on the patella (and on the tibia if there was enough material). The defect in the patellar tendon was not closed in order to prevent tendon shortening, only the prepatellar fascia was closed using interrupted sutures.
The tibial and femoral tunnels were drilled using standard guiding devices. The combined construct (autograft and augmentation band) was pulled through the tibial and femoral tunnels using the pull-out sutures, positioning the patella tendon part in an anteromedial plane and the augmentation device in a posterolateral plane with the knee near extension (Fig. 3a). Typically, the bone plug tendon level of the construct was positioned flush with the entrance of the femoral tunnel. A guide wire was inserted via the anteromedial portal into the femoral tunnel, between the tunnel wall and the cancellous part of the plug, with the knee in adequate flexion. An interference screw was driven into the femoral tunnel over the guide wire, securing the plug of the combined construct in the lateral femoral condyle (Fig. 3b). The combined construct was conditioned by tensioning the graft and moving the knee repeatedly through a full range of motion. Thereafter, it was fixed by an interference screw in the tibial tunnel with the knee near full extension. Stability was tested and the arthroscope was again inserted into the knee for a thorough examination of the combined construct in situ. Tension was controlled as well as signs of impingement against lateral wall and roof of the notch, in flexion and in full extension. The surgical procedure was finalized.
Not all patients received antibiotic prophylaxis according to the study protocol, but there was no difference between the test (45 %) and control patients (46 %). The postoperative treatment was according to the routines of the clinics. Both treatment groups followed the same stepwise rehabilitation program with follow-up of a physical therapist. Immediate mobilization with range of motion and quadriceps training and weight bearing was allowed.
Statistical analysis
The patients were randomly allocated to test and control groups. In order to detect a clinical relevant difference of 15 % between the groups (p1 test = 90 % and p2 control = 75 %) in knee joint stability after 1 year, i.e., KT1000™ side-to-side difference <3 mm, with χ2 test at significance level 0.05, and with a power of 80 %, at least 100 subjects were needed in each group. Changes over time in manual maximum force within groups were analysed with the Wilcoxon signed rank test. For comparison between groups, the Mann–Whitney U test was used. Mean difference between groups is given with 95 % confidence intervals (CIs). The Fisher’s exact test was used to compare the differences in proportions between the two groups. The Mantel–Haenzel test was used to analyse the differences in distribution of cartilage surface pathology in relation to time from injury to surgical treatment. The analyses were performed with the last observation carry forward (LOCF) for the study subjects who were lost to postoperative follow-ups after the 1-year visit (Fig. 1) according to guidelines by European Medicines Agency [11]. All significance tests were two-tailed and conducted at the 5 % significance level. IBM SPSS Statistics was used for analyses.
Results
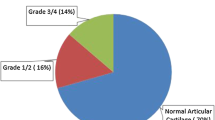
The median time from injury to surgery was for all patients 9 months (range 1–263). The ACL was reconstructed within 12 months in 66 % (63/96) of the test patients and in 64 % (67/105) of the control patients (Table 1). There was a significant difference in the status of the medial femur cartilage surface at surgery, including both test and control patients with <6 months from injury to surgery compared to the patients with >6 months delay to surgery (p = 0.001, Table 2). The total number of patients with grade 0 (medial femur), i.e., normal articular surfaces, was 78 (81 %) in the test group and 80 (76 %) in the control group.
KT1000™ tests showed a significant decrease in mean manual maximum side-to-side difference after 4 years in patients with ACL reconstruction with a synthetic augmentation device (mean difference 5.0 mm, SD 4.7, n = 84, p < 0.001) and also in those without (mean difference 5.9 mm, SD 4.7, n = 100, p < 0.001), without any statistical difference between the groups (n.s., 95 % CI −0.3–0.8; Fig. 4; Table 3). No significant differences were seen between the two groups with regard to negative Lachman test or negative pivot shift test after 1 and 4 years (Fig. 5; Table 3).
Both groups had a pre-injury Tegner activity median level of 7 (range 2–10). Fifty-three percent of the patients in the augmentation group returned to the same or higher activity level group as before the injury compared to 47 % in the group without augmentation (Table 4). Four years after surgical treatment, 27 % (23/85) of the test patients and 36 % (37/102) of the controls had decreased three or more activity levels as compared to before the injury. In both groups, the ACL reconstruction had most impact on the KOOS subscales of sports and recreational function and also knee-related quality of life (Fig. 6).
At least one follow-up surgical procedure, mainly arthroscopy, was performed in 30 % (29/96) of the patients with augmentation and in 22 % (23/105) of the controls. Pain was recorded in 13 patients with augmentation and in 12 of the controls during the 4-year postoperative follow-up. Swelling/hydrops was seen in 11 and 3 patients in the two groups, respectively. Furthermore, the clinical evaluation revealed meniscus/cartilage injuries in 9 patients with augmentation and in 13 of the patients without augmentation. A revision was performed in 3 of the 5 augmented ACL reconstructions with a re-rupture, and in one of the 4 controls with re-rupture, of which 2 of the latter were partial. Other recorded events were tibia tunnel widening in one patient and leakage tibia tunnel/synovitis in another of the patients with augmentation. No intra-articular infections were recorded.
The reasons for explantation of 10 augmentation devices were swelling/hydrops in four patients and insufficient screw fixation to femur in six patients, in five of these after new trauma. In one of these cases, there were no signs of rupture of the BPTB autograft, only the augmentation device had become loose. The 4-year cumulative survival rate was 89 % for the augmentation device.
Long-term follow-up
At 12 years after ACL reconstruction, approximately 50 % of the patients in both groups reported a Tegner activity level of 4–6 (Table 4). Both groups had significantly higher KOOS scores in pain (i.e. less pain, p < 0.001), function in activities of daily living (ADL, p = 0.005 and p = 0.003 respectively), function in sports and recreational activities (p < 0.001), and knee-related quality of life (p < 0.001) compared to before surgical treatment (Fig. 6).
Discussion
The principle finding of this study was that no difference could be seen in remaining instability after 4 years between ACL reconstructions with or without augmentation of the autograft, although both groups showed a significant improvement. Nearly 80 % of all patients reported a Tegner activity level between 7 and 10 before injury, i.e., were active athletes. A slight decline in activity level was seen in both groups as compared to before injury, as has also been presented by others [44]. The reason for this could partly be due to that many patients do not wish to return to their pre-injury activity level, even if possible, due to changed social priorities or caution in using the knee. There was no difference between the two groups in recorded meniscus/cartilage injuries after 4 years. However, in the total group of patients, more signs of cartilage injury were seen at time of surgical treatment in the patients with >6 months delay from ACL injury to surgery. This is in agreement with earlier presented data on the timing of surgery and the incidence of meniscal tears and degenerative change [5]. A number of additional surgical procedures were observed in both groups. In the augmentation group, the device had to be removed for various reasons in 10 patients. Six of these reconstructions had become loose, i.e., had insufficient screw fixation to femur. This could be due to that the technique for fixation was the same as commonly used for BPTB reconstruction and not adapted to the augmentation procedure. Re-ruptures were few (5 and 4 % respectively), which is in accordance with a large cohort study where 4.3 % suffered a new ACL injury [40].
Due to an increased interest in augmentation devices for ligament and tendon repair and reconstruction, it was decided to evaluate long-term status by using self-administered questionnaires. The results from KOOS knee-related questionnaire showed no significant long-term differences between the patients with ACL reconstruction with a synthetic degradable augmentation device and those reconstructions without. As could be expected, the scores for function in sports and recreational activities and also knee-related quality of life after 12 years were lower than compared to a reference material of individuals with no previous knee surgery and no meniscal or cruciate ligament injury [10]. However, the score values were somewhat higher than compared to a group of male soccer players 14 years after ACL reconstruction [45].
No reconstructions restore all functions of the ACL, including the single-bundle BPTB technique. It may restore the sagittal instability, but to a minor degree the rotational instability [33, 42]. Different types of augmentation of the autograft have been studied [17], but further developments of the ACL surgical technique have also been suggested. More recent “anatomical single-bundle ACL reconstruction” techniques try to find the ideal tunnel placement to restore the native ACL function [4]. A double-bundle technique for repair of ACL ruptures was first described in the early 1980s with the attempt to restore the native ACL anatomy [27, 47]. A further development of the technique has later been performed [37], which should theoretically give the patient better rotational stability.
Artificial ligaments may provide either an augmentation to an autologous graft or allograft, or a complete substitute in the ACL reconstruction. Most of these artificial non-degradable devices have, however, failed to show satisfactory long-term performance. The synthetic Kennedy Ligament Augmentation Device (LAD), made of polypropylene, has been the most commonly used augmentation in ACL reconstruction [16]. In a clinical study by Grøntvedt et al. [14], ACL chronic ruptures were reconstructed using the BPTB technique with and without reinforcement of the Kennedy LAD. The addition of augmentation gave no better results compared with BPTB alone after 2 and 8 years [8, 14]. The results of an arthroscopic and histological study by Asahina et al. [1] did not show any advantage in using LAD. In contrary, the augmentation device has added to the morbidity and severity of complications [3]. Also other materials have been used for augmentation. The use of carbon fibre has shown migration of carbon wear particles causing inflammatory synovitis in the knee joint [36]. The problem persisted after coating of the carbon fibre [26]. The Gore-Tex polytetrafluorethylene ligament was developed as a prosthetic ligament, but has also been used as an augmentation device. Clinical studies with longer follow-ups have, however, shown high rates of complications [34].
The Dacron and the Leeds-Keio artificial ligaments, both made of polyester, were designed as prostheses. Studies have shown high rate of ruptures and revisions in patients treated with the Dacron ligament [46] and also a narrowing of the joint space [23]. A high incidence of unstable knees was reported with the Leeds-Keio artificial ligament [32]. The latest artificial ligament that has been presented is the Ligament Advanced Reinforcement System (LARS), a polyethylene terephthalate graft. Evaluation with KOOS showed better results during the initial year with LARS ligament in comparison with BPTB autograft in a randomized study, but with no significant differences 2 years after surgery [28]. Although initial results have been encouraging [21], long-term studies are still required [30]. These materials are all non-degradable.
The advantage with a degradable augmentation device is that it allows for tissue ingrowth and tissue tensioning, i.e., allows for a natural biomechanical stimulation of the grafted tissue [19]. We know there is a decrease in strength and loss of the elasticity in the autograft starting a few weeks after surgery caused by necrosis and resorption, and a long unprotected period of regeneration [6]. The design of a degradable augmentation device aims to share the mechanical load of the biological graft during its weak phase, and then gradually increase the stress on the autograft. However, this stress shield distribution from the degradable augmentation device to the autograft needs to be investigated in future studies. The device used in the present study was the first step in the development of Artelon® degradable products for reinforcement in reconstruction or repair of ligaments and tendons [24].
A limitation of the present study is that the comparison between groups in sagittal stability had a power of 72 % at 4 years, i.e., less than 80 %. However, there was no tendency to a clinical relevant difference in the primary outcome variable. Furthermore, although the FDA guidelines prescribed at least 100 patients in each group [12], a study evaluating long-term complications as a consequence of instability would have required much larger groups. After the finalizing of the 4-year follow-up, the patients could, if they wished, be informed on what kind of surgical treatment they had had, i.e., augmentation of the BPTB autograft or not. This means that the 12-year follow-up with questionnaires was not blinded with regard to test or control. Also, since the patients did not visit the different centres after 12 years, no objective measurements could be made.
Conclusion
This randomized, controlled study showed no significant difference in clinical outcome with use of an additional poly(urethane urea) degradable augmentation device in a single-bundle BPTB ACL reconstruction compared with non-augmentation, in short intermediate or long-term perspective. Augmentation of the autograft did not add further benefits in this group of non-selected patients and had drawbacks such as prolonged surgical procedure and later explantation of the device in 10 of the patients, in six of these because of insufficient screw fixation to femur, and in four due to swelling/hydrops.
References
Asahina S, Yamamoto H, Muneta T, Ishibashi T, Furuya K (1995) Evaluation of anterior cruciate reconstruction reinforced by the Kennedy ligament augmentation device. An arthroscopic and histological study. Int Orthop 19:229–233
Bach BR Jr, Warren RF, Flynn WM, Kroll M, Wickiewiecz TL (1990) Arthrometric evaluation of knees that have a torn anterior cruciate ligament. J Bone Joint Surg Am 72:1299–1306
Barrett GR, Field LD (1993) Comparison of patella tendon versus patella tendon/Kennedy ligament augmentation device for anterior cruciate ligament reconstruction: study of results, morbidity, and complications. Arthroscopy 9:624–632
Bird JH, Carmont MR, Dhillon M, Smith N, Brown C, Thompson P, Spalding T (2011) Validation of a new technique to determine midbundle femoral tunnel position in anterior cruciate ligament reconstruction using 3-dimensional computed tomography analysis. Arthroscopy 27:1259–1267
Church S, Keating JF (2005) Reconstruction of the anterior cruciate ligament. Timing of surgery and the incidence of meniscal tears and degenerative change. J Bone Joint Surg Br 87:1639–1642
Clancy WG, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA (1981) Anterior and posterior cruciate ligament reconstruction in Rhesus monkeys. J Bone Joint Surg Am 63:1270–1284
Daniel DM, Stone ML, Sachs R, Malcom L (1985) Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med 13:401–407
Drogset JO, Grøntvedt T (2002) Anterior cruciate ligament reconstruction with and without a ligament augmentation device: results at 8-year follow-up. Am J Sports Med 30:851–856
Dunn WR, Lyman S, Lincoln AE, Amoroso PJ, Wickiewicz T, Marx RG (2004) The effect of anterior cruciate ligament reconstruction on the risk of knee reinjury. Am J Sports Med 32:1906–1914
Englund M, Roos EM, Lohmander LS (2003) Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis. A sixteen-year followup of meniscectomy with matched controls. Arthr Rheum 48:2178–2187
European Medicines Agency (2011) Guideline on missing data in confirmatory clinical trials. EMA/CPMP/EWP/1776/99 Rev. 1. http://www.ema.europa.eu/ema/
Food and Drug Administration. Guidance document for the preparation of investigational device exemptions and premarket approval applications for intra-articular prosthetic knee ligament devices 1987, revised 1993. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm081345.pdf
Gisselfält K, Edberg B, Flodin P (2002) Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules 3:951–958
Grøntvedt T, Engebretsen L, Bredland T (1996) Arthroscopic reconstruction of the anterior cruciate ligament using bone-patellar tendon-bone grafts with and without augmentation. J Bone Joint Surg Br 78:817–822
Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, Richmond JC, Shelbourne KD (2001) Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med 29:600–613
Kennedy JC, Roth JH, Mendenhall HV, Sanford JB (1980) Intraarticular replacement in the anterior cruciate ligament-deficient knee. Am J Sports Med 8:1–8
Legnani C, Ventura A, Terzaghi C, Borgo E, Albisetti W (2010) Anterior cruciate ligament reconstruction with synthetic grafts. A review of literature. Int Orthop 34:465–471
Lidén M, Sernert N, Rostgård-Christensen L, Kartus C, Ejerhed L (2008) Osteoarthritic changes after anterior cruciate ligament reconstruction using bone-patellar tendon-bone or hamstring tendon autografts: a retrospective, 7-year radiographic and clinical follow-up study. Arthroscopy 24:899–908
Liljensten E, Gisselfält K, Edberg B, Bertilsson H, Flodin P, Nilsson A, Lindahl A, Peterson L (2002) Studies of polyurethane urea bands for ACL reconstruction. J Mater Sci Mater Med 13:351–359
Linko E, Harilainen A, Malmivaara A, Seitsalo S (2005) Surgical versus conservative interventions for anterior cruciate ligament ruptures in adults. Cochrane Database Syst Rev 18:CD001356
Liu Z-t, Zhang X-l, Jiang Y, Zeng B-F (2010) Four-strand hamstring tendon autograft versus LARS artificial ligament for anterior cruciate ligament reconstruction. Int Orthop 34:45–49
Lohmander LS, Englund PM, Dahl LL, Roos EM (2007) The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med 35:1756–1769
Maletius W, Gillquist J (1997) Long-term results of anterior cruciate ligament reconstruction with a Dacron prosthesis. The frequency of osteoarthritis after seven to eleven years. Am J Sports Med 25:288–293
Marberry TA (2013) A synthetic reinforcement patch in repair of challenging two-tendon rotator cuff tears. Should Elbow 5:24–29
Meuffels DE, Favejee MM, Vissers MM, Heijboer MP, Reijman M, Verhaar JAN (2009) Ten year follow-up study comparing conservative versus operative treatment of anterior cruciate ligament ruptures. A matched-pair analysis of high level athletes. Br J Sports Med 43:347–351
Mody BS, Howard L, Harding ML, Parmar HV, Learmonth DJ (1993) The ABC carbon and polyester prosthetic ligament for ACL-deficient knees. Early results in 31 cases. J Bone Joint Surg Br 75:818–821
Mott HW (1983) Semitendinosus anatomic reconstruction for cruciate ligament insufficiency. Clin Orthop Relat Res 172:90–92
Nau T, Lavoi P, Duval N (2002) A new generation of artificial ligaments in reconstruction of the anterior cruciate ligament. Two-year follow-up of a randomised trial. J Bone Joint Surg Br 84:356–360
Nelson F, Billinghurst RC, Pidoux I et al (2006) Early post-traumatic osteoarthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthr Cartil 14:114–119
Newman SDS, Atkinson HDE, Willis-Owen CA (2013) Anterior cruciate ligament reconstruction with the ligament augmentation and reconstruction system: a systematic review. Int Orthop (SICOT) 37:321–326
Peterson L, Brittberg M (1998) Articular cartilage classification. International Cartilage Repair Society: Newsletter, Issue Spring, pp 6–8
Rading J, Peterson L (1995) Clinical experience with the Leeds-Keio artificial ligament in anterior cruciate ligament reconstruction. A prospective two-year follow-up study. Am J Sports Med 23:316–319
Ristanis S, Stergiou N, Patras K, Tsepis E, Moraiti C, Georgoulis AD (2006) Follow-up evaluation 2 years after ACL reconstruction with bone-patellar tendon-bone graft shows that excessive tibial rotation persists. Clin J Sports Med 16:111–116
Roolker W, Patt TW, van Dijk CN, Vegter M, Marti RK (2000) The Gore-Tex prosthetic ligament as a salvage procedure in deficient knees. Knee Surg Sports Traumatol Arthrosc 8:20–25
Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD (1998) Knee injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther 28:88–96
Rushton N, Dandy DJ, Naylor CPE (1983) The clinical, arthroscopic and histological findings after replacement of the anterior cruciate ligament with carbon-fibre. J Bone Joint Surg 65:308–309
Schreiber VM, van Eck CF, Fu FH (2010) Anatomic double-bundle ACL reconstruction. Sports Med Arthrosc 18:27–32
Sernert N, Kartus J, Köhler K, Ejerhed L, Karlsson J (2001) Evaluation of the reproducibility of the KT-1000 arthrometer. Scand J Med Sci Sports 11:120–125
Shelbourne KD, Gray T (2000) Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med 28:446–452
Shelbourne KD, Gray T, Haro M (2009) Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med 37:246–251
Smith GD, Taylor J, Almqvist KF et al (2005) Arthroscopic assessment of cartilage repair: a validation study of 2 scoring systems. Arthroscopy 21:1462–1467
Tashman S, Kolowich P, Collon D, Anderson K, Anderst W (2007) Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res 454:66–73
Tegner Y, Lysholm J (1985) Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 198:43–49
van der Hart CP, van den Bekerom MPJ, Patt TW (2008) The occurrence of osteoarthritis at a minimum of ten years after reconstruction of the anterior cruciate ligament. J Orthop Surg Res 3:24
von Porat A, Roos EM, Roos H (2004) High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis 63:269–273
Wredmark T, Engström B (1993) Five-year results of anterior cruciate ligament reconstruction with the Stryker Dacron high-strength ligament. Knee Surg Sports Traumatol Arthrosc 1:71–75
Zaricznyj B (1987) Reconstruction of the anterior cruciate ligament of the knee using a doubled tendon graft. Clin Orthop Relat Res 220:162–175
Acknowledgments
The study was sponsored by Artimplant AB, Gothenburg, Sweden. The authors gratefully acknowledge the physical therapist at each of the involved centres for professional work with the follow-ups.
Conflict of interest
The first author owns shares in the sponsoring company and is since 2011 a board member.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peterson, L., Eklund, U., Engström, B. et al. Long-term results of a randomized study on anterior cruciate ligament reconstruction with or without a synthetic degradable augmentation device to support the autograft. Knee Surg Sports Traumatol Arthrosc 22, 2109–2120 (2014). https://doi.org/10.1007/s00167-013-2636-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-013-2636-3