Abstract
Purpose
Extracorporeal membrane oxygenation (ECMO) is used as temporary cardiorespiratory support in critically ill patients. Little is known about population-level short- and long-term outcomes following ECMO, including healthcare use and health system cost across a wide range of sectors.
Methods
Population-based cohort study in Ontario, Canada (October 1, 2009–March 31, 2017) of adult patients (≥ 18 years) receiving ECMO for cardiorespiratory support. We captured outcomes through linkage to health administrative databases. Primary outcome was mortality during hospitalization, as well as at 7 days, 30 days, 1 year, 2 years, and 5 years following ECMO initiation. We analyzed health system costs (in Canadian dollars) in the 1 year following the date of the index admission.
Results
A total of 692 patients were included. Mean (standard deviation [SD]) age was 51.3 (16.0) years. Median (interquartile range [IQR]) time to ECMO initiation from date of admission was 2 (0–9) days. In-hospital mortality was 40.0%. Mortality at 1 year, 2 years, and 5 years was 45.1%, 49.0%, and 57.4%, respectively. Among survivors, 78.4% were discharged home, while 21.2% were discharged to continuing care. Median (IQR) total costs in the 1 year following admission among all patients were Canadian $130,157 (Canadian $58,645–Canadian $240,763), of which Canadian $91,192 (Canadian $38,507–Canadian $184,728) were attributed to inpatient care.
Conclusions
Hospital mortality among critically ill adults receiving ECMO for advanced cardiopulmonary support is relatively high, but does not markedly increase in the years following discharge. Survivors are more likely to be discharged home than to continuing care. Median costs are high, but largely reflect inpatient hospital costs, and not costs incurred following discharge.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Patients surviving to discharge following ECMO have low mortality up to 5 years later. While ECMO costs are significant, they largely reflect inpatient costs and not costs incurred following discharge. |
Introduction
Extracorporeal membrane oxygenation (ECMO) provides temporary respiratory and/or cardiac support to critically ill patients, and is often considered in patients who have failed initial, conventional treatment [1,2,3]. ECMO may be considered in patients with medically refractory respiratory failure, cardiac failure, or as an adjunct to cardiopulmonary resuscitation during cardiac arrest [4,5,6,7]. While major recent advances in extracorporeal technology have improved the risk–benefit profile of ECMO [3], not all patients may be deemed candidates for therapy, including patients with advanced age and multi-organ failure [8].
With the increasing use of ECMO [9], there is a growing need to understand its impact on short- and long-term outcomes. Short-term mortality following ECMO has been estimated to range between 30 and 70%, depending upon the indication for therapy [8]. The largest trial enrolling patients with acute respiratory distress syndrome (ARDS) demonstrated a 60-day mortality of 35%, associated with venovenous ECMO (VV-ECMO) [5]. In patients with cardiogenic shock receiving venoarterial ECMO (VA-ECMO), in-hospital mortality has been estimated to range between 50 and 70%, with many survivors experiencing significant long-term disability [10, 11]. Given the paucity of data, measuring long-term survival has been identified as an area of focus for future investigation in extracorporeal life support [12, 13].
Importantly, use of ECMO requires considerable resources, and is associated with high costs [14]. Although there have been some estimates of the high expense of ECMO, there is little information on long-term costs following discharge for survivors, including no information of costs outside of the acute care sectors. To investigate these questions, we conducted a population-based cohort study to evaluate the short- and long-term health outcomes and costs of critically ill adults receiving ECMO for cardiorespiratory support.
Methods
This study was approved by The Ottawa Health Science Network Research Ethics Board.
Data sources and setting
We conducted a population-based, retrospective cohort study using health administrative databases in Ontario, Canada. Ontario is Canada’s most populous province with approximately 13 million inhabitants. In Ontario’s single-payer healthcare system, all medically necessary health care services, physician, hospital, and demographic information for residents are recorded in these databases. These data are held and linked at ICES, an independent, non-profit custodian of health data. ICES is funded by an annual grant from the Ontario Ministry of Health and Long-term Care.
The following databases are linked, then de-anonymized, at the individual level at ICES: (1) The Ontario Health Insurance Plan (OHIP) Claims Database, to capture data on physician fee-for-service claims for inpatient and outpatient services; (2) The Canadian Institute for Health Information Discharge Abstract Database, to capture information on all acute care hospitalizations, including detailed diagnostic and procedural information; (3) The Registered Persons Database, for capturing all demographic information; (4) The Home Care Database, to capture publicly funded homecare services; (5) The National Ambulatory Care Reporting System, to capture information on Emergency Department use; (6) The National Rehabilitation Reporting System for inpatient rehabilitation programs; (7) The Continuing Care Reporting System, for data on continuing care and complex continuing care use; (8) Statistics Canada Census data, for income quintile and rurality through postal codes; and (9) The Ontario Drug Benefit Claims database, for tracking data on prescription medications dispensed among patients ≥ 65 years of age eligible for coverage.
Patients
We identified adult patients (≥ 18 years of age) receiving ECMO in Ontario between October 1, 2009, and March 31, 2017. We identified patients whose hospitalization records indicated the presence of an inpatient ECMO intervention code by searching through the Discharge Abstract Database (all codes displayed in Supplemental Table 1). This ECMO procedural code may also be applied to a select group of cardiac surgery patients undergoing particular elective procedures, to indicate the use of percutaneous or open cardiopulmonary bypass. For this reason, we also used the OHIP Claims Database to identify all individuals who had a code for ECMO billed (OHIP code Z788) by a physician caring for them. We first included patients who had both of the above codes (Cohort A).
To further capture relevant patients who may not have had an OHIP code applied, we utilized the International Classification of Diseases, 10th Revision (ICD-10) “most responsible diagnosis” from Cohort A, and included patients with these ICD-10 diagnoses, but who only had an ECMO inpatient code from the Discharge Abstract Database (Cohort B). As above, we again ensured these patients did not undergo elective cardiac surgery procedures.
For each subject, we calculated the Charlson Comorbidity Index (CCI) [15] using hospitalization data up to 2 years prior to the date of admission. Subjects who had not been hospitalized in the previous 2 years had their CCI score set to zero. We further identified the presence of complex chronic diseases among our cohort, using previously described methods (Supplemental Table 2) [16]. Where applicable, we used validated algorithms to ascertain cases. All other conditions were based on the presence of any one inpatient hospital diagnostic code, or two or more outpatient physician billing codes within a 2-year period, using relevant ICD, Version 9 (ICD-9) and ICD-10 codes.
As outcomes following ECMO initiation are strongly linked to indication (i.e., respiratory versus cardiac failure) [8], we conducted a secondary subgroup analysis based upon this variable. Since data related to ECMO configuration (e.g., VV-ECMO vs. VA-ECMO) were unavailable, patients were categorized as either ‘Respiratory Failure’ or ‘Cardiac Failure’, based upon the ICD-10 most responsible diagnosis (see Supplemental Table 1 for categorization). Patients whose most responsible diagnosis did not allow for clear delineation between these subgroups were categorized as ‘Other’. To identify factors associated with survival, we compared characteristics of ECMO patients who died during hospitalization against those who survived to hospital discharge.
Outcomes
The primary outcome was survival to hospital discharge. Secondary outcomes included survival at 7 days, 30 days, 1 year, 2 years, and 5 years following ECMO initiation. Time to ECMO initiation was determined by calculating the difference between the hospital admission date, and the date associated with the ECMO intervention code. Length of stay for the hospitalization was reported from the date of hospital admission to the date of discharge or in-hospital death. Discharge disposition was determined using a hierarchy approach (Supplemental Table 3). Ventricular assist device (VAD) implantation and transplant were determined using the relevant procedural codes in the Discharge Abstract Database.
For patients with available costing data (October 1, 2009–March 31, 2016), we examined the total and sector-specific direct healthcare costs accumulated in the 1 year following the date of the index hospital admission (including the admission itself). We obtained all records of health care use paid for by the Ministry of Health and Long-term care (MOHLTC) following hospital admission. We estimated the costs associated with each record using previously described methods developed for health administrative data [17]. For sectors that use global budgets (e.g., hospital, complex continuing care, rehabilitation), we used a top-down approach through case-mix methodology. Sectors where each use has an associated fee payment (e.g., drug costs, physician remuneration) had costs estimated directly. Further details regarding cost data acquisition are included in Supplemental Fig. 1. Private patient expenses are not included. All costs were expressed in 2016 Canadian dollars, and past costs were inflated using the healthcare-specific yearly Consumer Price Index reported by Statistics Canada.
Cell sizes with ≤ 5 patients are suppressed, as per ICES regulations, to protect patient confidentiality.
Statistical analyses
We conducted all statistical analyses using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC). We present data as mean values, with standard deviation (SD), or medians, with interquartile range (IQR), where appropriate. The Student’s t test (parametric values), Mann–Whitney test (non-parametric values), and χ2 (for categorical values), were performed to determine between-group differences. We used Kaplan–Meier survival curves to describe 1-year survival. We assessed variation in total hospital costs using a multivariable generalized linear model with gamma distribution and log link.
Results
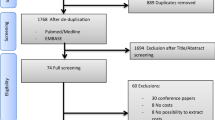
Study flow is depicted in Supplemental Fig. 2. In total, we included 692 patients in the primary analyses. Patient characteristics are displayed in Table 1. Mean age was 51.3 years (SD = 16.0), and 62.0% of patients were male. The median time to ECMO initiation from hospital admission was 2 days (IQR 0–9 days). In terms of primary indication, 321 (46.4%) patients receiving ECMO had a primary diagnosis of respiratory failure, 303 (43.8%) patients receiving ECMO had a primary diagnosis of cardiac failure, and 68 (9.8%) were categorized as ‘Other’. The CCI score was ≤ 2 in 76.7% of patients. Patient and institution Local Health Integration Networks (LHINs) (i.e., health funding regions) are displayed in Supplemental Table 4.
A total of 277 (40.0%) patients died in-hospital, while 176 (25.4%) and 252 (36.4%) died within 7 days and 30 days of ECMO initiation, respectively (Table 2). Mortality at 1 year, 2 years, and 5 years was 45.1%, 49.0%, and 57.4%, respectively. Median hospital length of stay was 22 days (IQR 9–45). In-hospital mortality was 30.5% in patients receiving ECMO with a primary respiratory diagnosis, and 50.5% in patients receiving ECMO with a primary cardiac diagnosis. Mortality after hospital discharge was higher among patients who initially required ECMO for cardiac failure than those with respiratory failure (53.8% vs. 37.1% at 1-year post-ECMO initiation). Kaplan–Meier survival curves are displayed in Fig. 1 and demonstrate a decline in survival over the first 40 days following ECMO initiation, followed by a relative plateauing of mortality rates.
During the index hospitalization, ECMO was followed by lung transplant in 193 (27.9%) patients, and heart transplant in 46 (6.6%) patients (Table 2). Of patients receiving lung transplant, 163 (84.5%) survived to hospital discharge, while 30 patients (65.2%) receiving heart transplant survived to discharge. ECMO was followed by VAD in 92 (13.3%) patients. Among survivors to hospital discharge, 174 (41.9%) patients were discharged home with no government-sponsored homecare services, 153 (36.8%) patients were discharged home with government-sponsored homecare services, and 88 (21.2%) were discharged to a continuing care facility. Within 1 year of discharge, 270 survivors (65.1%) had at least one Emergency Department visit, with 208 (50.1%) requiring at least one hospital admission.
As compared to those who died in-hospital, patients with respiratory failure who survived to discharge had a lower CCI, shorter time to ECMO initiation, and more commonly received ECMO followed by lung transplant (Table 3). Among those with cardiac failure, survival to discharge was associated with heart transplant following ECMO, and a longer time to ECMO initiation. Outcomes stratified by patient age are displayed in Supplemental Fig. 3.
Costs in the year following the index hospital admission for ECMO were available for 550 patients and are shown in Table 4. Median cost (IQR) was Canadian $130,157 (Canadian $58,645–Canadian $240,763). Following discharge, median costs for continuing care (complex continuing care, long-term care, rehabilitation, and homecare) were Canadian $0 (Canadian $0–Canadian $679). Median costs for outpatient clinics and physician billings were Canadian $2321 (Canadian $731–Canadian $5852) and Canadian $22,191 (Canadian $12,665–Canadian $33,656), respectively. Median outpatient laboratory and drug costs were Canadian $0 (Canadian $0–Canadian $314) and Canadian $0 (Canadian $0–Canadian $3953), respectively. Factors associated with increased costs include younger age, respiratory failure, increased length of stay, and transplant (Supplemental Table 5). Patients receiving heart or lung transplant had significantly higher costs than patients who were not transplanted (Supplemental Tables 6 and 7). Mean and median costs of patients, based upon the region in Ontario where they received care, are shown in Supplemental Tables 8 and 9.
Discussion
In our population-based cohort analysis of critically ill adult patients receiving ECMO for cardiorespiratory support, we found that in-hospital mortality was 40.0%, but did not substantially increase 1, 2 or 5 years following ECMO initiation. Mortality was higher among patients receiving ECMO for cardiac failure, as compared to respiratory failure. Over a third of patients received heart or lung transplant following ECMO, and the large majority of patients (78.5%) surviving to discharge were able to be discharged home. Finally, median total costs among patients in our cohort were significant at Canadian $130,157 in the 1 year following admission for ECMO, with the large majority attributed to inpatient costs during admission. This study provides important information regarding short- and long-term outcomes following the use of this novel therapy in the care of critically ill adults.
Worldwide utilization of ECMO for cardiorespiratory support is growing [9], but the use of this technology is outpacing the existing evidence. Several important questions have been identified with regard to the use of ECMO [12], and our study sought to provide insight into many of them. The first is the importance of patient selection. There are several patient factors that have been associated with worse outcomes, including advanced age and significant chronic comorbidities [8, 18]. The mean age of patients included in our cohort was 51.3 years, and 76.7% of patients had a CCI ≤ 2, suggesting few chronic conditions. We found that patients who died in hospital had higher CCI scores than those surviving to discharge. We also found that, among patients with respiratory failure, those who survived to discharge had earlier initiation of ECMO, while patients with cardiac failure showed the opposite relationship. The importance of these associations is unclear and may be due to unmeasured confounders. Finally, patients receiving heart and lung transplant following ECMO had higher survival, possibly reflecting a more meticulous selection process, given the additional need to fulfill candidacy for transplantation. Further research is required to determine the optimal patient population for ECMO, and the appropriate time for initiation.
We also investigated the long-term outcomes of patients receiving ECMO, since existing evidence on this topic is largely based upon single-center experience [19]. Chang et al. previously investigated long-term outcomes in a population-based cohort of patients receiving ECMO in Taiwan, and found a 30-day mortality of 59.8%, and 1-year mortality of 76.5% [20]. This study was limited by a variety of factors, including indication for ECMO (a large proportion of included patients had cardiac failure, and presumably received VA-ECMO for cardiac support) and patient selection (older cohort with a greater proportion of comorbidities). One-year mortality in our cohort was lower at 40.0%. While this value may seem high, the utilization of ECMO is often in patients who have been deemed to be refractory to medical therapy, and mortality without the use of ECMO would likely be higher, as was demonstrated in patients with ARDS [5, 21]. Furthermore, the large majority of deaths occurred during the index hospitalization, suggesting that patients in our cohort who survived to hospital discharge were likely to survive to 1 year. We show that mortality did not substantially increase from year 1 to year 2, or even year 5, suggesting fairly stable survival curves among patients surviving their ECMO hospitalization.
Disability following critical illness is a significant concern, with survivors often left with long-term impairment and reduced independence [22, 23]. This is frequently manifested by increased resource utilization following hospital discharge [24]. While we were unable to evaluate functional ability at the time of hospital discharge, the large majority of survivors in our cohort were discharged home. The proportion of patients in our cohort requiring discharge to continuing care facilities (e.g., nursing homes) was lower than what has been seen in other critical care populations [25, 26]. Though it is possible that survivors may still experience significant morbidity, the higher likelihood of disposition to home suggests at least an acceptable level of functioning at the time of hospital discharge in a high percentage of patients. Despite functional independence, existing evidence among ECMO survivors notes difficulties in important patient-oriented functional abilities, such as return to work [27], and increased incidence of neuropsychiatric comorbidities [28].
Finally, given the resource-intensive nature of ECMO [29], we evaluated its impact on total patient costs, both during hospitalization, as well as following discharge. We found that the median total cost in the year following hospital admission was Canadian $130,157. Of these costs, the large majority were accumulated during the inpatient stay. Importantly, very little cost was accrued due to continuing care (namely long-term care, rehabilitation, and homecare) post-discharge. These findings are in particular contrast to continuing care costs seen among other critical care populations in Ontario [24, 30]. The median total costs of patients in our cohort were high, but their inpatient costs are less than what has been found in the 90th percentile of high-cost ICU users in Ontario (median cost of Canadian $148,328 during hospital admission [31]). Other ICU patient populations (e.g., patients with subarachnoid hemorrhage) have been shown to accrue markedly higher median inpatient costs than what was seen in our ECMO cohort [32]. More importantly, such patient populations have also been shown to incur high costs following hospital discharge [24], which was not the case in our cohort. Therefore, while the inpatient costs of patients receiving ECMO are high, they are less than some other high-cost ICU populations, and survivors incur few costs following hospital discharge.
We utilized robust population-level data to identify long-term outcomes and costs among critically ill adults receiving ECMO in Ontario, Canada. To our knowledge, this study provides some of the first data related to long-term outcomes and costs in these high-risk patients. However, there are limitations to our study. Importantly, outcomes of patients receiving ECMO are largely dependent upon the indication, and the subsequent required configuration (i.e., VV- vs. VA-ECMO) [8]. We did not have sufficient granularity in data to determine the configuration, and instead used the most responsible diagnosis to make assumptions regarding the indication and configuration. Additionally, our cost data were not able to differentiate the specific cost of ECMO administration, (including the cost of the relevant components, e.g., cannulation, filters) from a patient’s total inpatient costs. Second, the patients in our cohort reflect a highly selected group, as indicated by the relatively younger age and low CCI. Caution should be exercised in extending our findings to all critically ill patients. Third, ECMO is not a benign treatment, and is associated with important complications, including hemorrhage, thrombosis, and infection [2, 3]. We did not have data related to these complications, and, therefore, are unable to comment on incidence and impact in our population. Fourth, in Ontario, ECMO is largely centralized and conducted at a few, specialized centers. Outcomes from ECMO are associated with center-specific frequency of use and volume [33], and, therefore, our results should be interpreted in that context. Finally, while ICES databases are robust and have been used extensively for large population-based studies [16, 34], there are inherent limitations, including data related to illness severity, quality-adjusted life years associated with cost, as well as costs and resources that are funded privately by patients.
Conclusion
In our population-based cohort, in-hospital mortality among critically ill adult patients receiving ECMO was 40.0%, and incremental increase in 1-year, 2-year, or 5-year mortality was minimal. The majority of ECMO patients who survived to hospital discharge were discharged home. Finally, while ECMO patients accrued significant inpatient costs, these costs were less than typical high-cost populations, and these patients had little healthcare-associated costs in the 1 year following ECMO initiation. This work provides novel data regarding the long-term outcomes and costs of critically ill patients receiving ECMO for cardiorespiratory support.
References
Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, Sadique MZ, Sekhon JS, McAuley DF, Firmin RK, Harvey C, Cordingley JJ, Price S, Vuylsteke A, Jenkins DP, Noble DW, Bloomfield R, Walsh TS, Perkins GD, Menon D, Taylor BL, Rowan KM (2011) Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 306:1659–1668
Brodie D, Bacchetta M (2011) Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 365:1905–1914
Abrams D, Combes A, Brodie D (2014) Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 63:2769–2778
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374:1351–1363
Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, Maury E, Levy B, Cohen Y, Richard C, Kalfon P, Bouadma L, Mehdaoui H, Beduneau G, Lebreton G, Brochard L, Ferguson ND, Fan E, Slutsky AS, Brodie D, Mercat A (2018) Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 378:1965–1975
Shekar K, Mullany DV, Thomson B, Ziegenfuss M, Platts DG, Fraser JF (2014) Extracorporeal life support devices and strategies for management of acute cardiorespiratory failure in adult patients: a comprehensive review. Crit Care 18:219
Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, Chen WJ, Huang SC, Chi NH, Wang CH, Chen LC, Tsai PR, Wang SS, Hwang JJ, Lin FY (2008) Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 372:554–561
Schmidt M, Brechot N, Combes A (2016) Ten situations in which ECMO is unlikely to be successful. Intensive Care Med 42:750–752
Karagiannidis C, Brodie D, Strassmann S, Stoelben E, Philipp A, Bein T, Muller T, Windisch W (2016) Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med 42:889–896
Combes A, Leprince P, Luyt CE, Bonnet N, Trouillet JL, Leger P, Pavie A, Chastre J (2008) Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med 36:1404–1411
Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, Hodgson C, Scheinkestel C, Cooper DJ, Thiagarajan RR, Brodie D, Pellegrino V, Pilcher D (2015) Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 36:2246–2256
Combes A, Brodie D, Chen YS, Fan E, Henriques JPS, Hodgson C, Lepper PM, Leprince P, Maekawa K, Muller T, Nuding S, Ouweneel DM, Roch A, Schmidt M, Takayama H, Vuylsteke A, Werdan K, Papazian L (2017) The ICM research agenda on extracorporeal life support. Intensive Care Med 43:1306–1318
Brodie D, Vincent JL, Brochard LJ, Combes A, Ferguson ND, Hodgson CL, Laffey JG, Mercat A, Pesenti A, Quintel M, Slutsky AS, Ranieri VM (2018) Research in extracorporeal life support: a call to action. Chest 153:788–791
Harvey MJ, Gaies MG, Prosser LA (2015) US and international in-hospital costs of extracorporeal membrane oxygenation: a systematic review. Appl Health Econ Health Policy 13:341–357
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Yarnell CJ, Fu L, Manuel D, Tanuseputro P, Stukel T, Pinto R, Scales DC, Laupacis A, Fowler RA (2017) Association between immigrant status and end-of-life care in Ontario, Canada. JAMA 318:1479–1488
Wodchis WP, Bushmeneva K, Nikitovic M, McKillop I (2013) Guidelines on person level cost using administrative databases in Ontario. Tor Health Syst Perform Res Netw
Abrams DC, Prager K, Blinderman CD, Burkart KM, Brodie D (2014) Ethical dilemmas encountered with the use of extracorporeal membrane oxygenation in adults. Chest 145:876–882
von Bahr V, Hultman J, Eksborg S, Frenckner B, Kalzen H (2017) Long-term survival in adults treated with extracorporeal membrane oxygenation for respiratory failure and sepsis. Crit Care Med 45:164–170
Chang CH, Chen HC, Caffrey JL, Hsu J, Lin JW, Lai MS, Chen YS (2016) Survival analysis after extracorporeal membrane oxygenation in critically ill adults: a nationwide cohort study. Circulation 133:2423–2433
Goligher EC, Tomlinson G, Hajage D, Wijeysundera DN, Fan E, Juni P, Brodie D, Slutsky AS, Combes A (2018) Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA 320:2251–2259
Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS (2003) One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348:683–693
Herridge MS, Batt J, Santos CD (2014) ICU-acquired weakness, morbidity, and death. Am J Respir Crit Care Med 190:360–362
Hill AD, Fowler RA, Pinto R, Herridge MS, Cuthbertson BH, Scales DC (2016) Long-term outcomes and healthcare utilization following critical illness—a population-based study. Crit Care 20:76
Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ, Clay AS, Chia J, Gray A, Tulsky JA, Cox CE (2010) One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med 153:167–175
Desai SV, Law TJ, Needham DM (2011) Long-term complications of critical care. Crit Care Med 39:371–379
Hodgson CL, Hayes K, Everard T, Nichol A, Davies AR, Bailey MJ, Tuxen DV, Cooper DJ, Pellegrino V (2012) Long-term quality of life in patients with acute respiratory distress syndrome requiring extracorporeal membrane oxygenation for refractory hypoxaemia. Crit Care 16:R202
McDonald MD, Sandsmark DK, Palakshappa JA, Mikkelsen ME, Anderson BJ, Gutsche JT (2019) Long-term outcomes after extracorporeal life support for acute respiratory failure. J Cardiothorac Vasc Anesth 33:72–79
Maxwell BG, Powers AJ, Sheikh AY, Lee PH, Lobato RL, Wong JK (2014) Resource use trends in extracorporeal membrane oxygenation in adults: an analysis of the Nationwide Inpatient Sample 1998–2009. J Thorac Cardiovasc Surg 148:416–421
Hill AD, Fowler RA, Burns KE, Rose L, Pinto RL, Scales DC (2017) Long-term outcomes and health care utilization after prolonged mechanical ventilation. Ann Am Thorac Soc 14:355–362
Reardon PM, Fernando SM, Van Katwyk S, Thavorn K, Kobewka D, Tanuseputro P, Rosenberg E, Wan C, Vanderspank-Wright B, Kubelik D, Devlin RA, Klinger C, Kyeremanteng K (2018) Characteristics, outcomes, and cost patterns of high-cost patients in the intensive care unit. Crit Care Res Pract 2018:5452683
Fernando SM, Reardon PM, Dowlatshahi D, English SW, Thavorn K, Tanuseputro P, Perry JJ, Rosenberg E, Wijdicks EF, Heyland DK, Kyeremanteng K (2018) Outcomes and costs of patients admitted to the ICU due to spontaneous intracranial hemorrhage. Crit Care Med 46:e395–e403
Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, Annich GM (2015) Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med 191:894–901
Jerath A, Laupacis A, Austin PC, Wunsch H, Wijeysundera DN (2018) Intensive care utilization following major noncardiac surgical procedures in Ontario, Canada: a population-based study. Intensive Care Med 44:1427–1435
Funding
None received for this study. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care.
Author information
Authors and Affiliations
Contributions
SMF, PT, and KK conceived the study idea. SMF, DQ, PT, EF, LM, BR, and KK participated in study design. DQ and RT gathered the data and performed data analyses. All the authors interpreted the data analyses. All the authors co-wrote and revised the manuscript for intellectual content. All the authors provided their final approval for manuscript submission. All the authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Eddy Fan is supported by a New Investigator Award from the Canadian Institutes of Health Research. Dr. Fan reports receiving personal fees from ALung Technologies, Abbot, and MC3 Cardiopulmonary, outside of the submitted work. Dr. Daniel Brodie reports providing expert advice to Hemovent, Baxter, BREETHE, and ALung Technologies. Dr. Brodie reports receiving grants from ALung Technologies, receiving personal fees from Baxter, and anticipated personal fees from BREETHE, outside of the submitted work. None of the other authors report any conflict of interest.
Ethical approval
The Ottawa Health Science Network Research Ethics Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fernando, S.M., Qureshi, D., Tanuseputro, P. et al. Mortality and costs following extracorporeal membrane oxygenation in critically ill adults: a population-based cohort study. Intensive Care Med 45, 1580–1589 (2019). https://doi.org/10.1007/s00134-019-05766-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05766-z