Abstract
Purpose
Acute kidney injury (AKI) is associated with the activation of the renin–angiotensin system. Whether angiotensin-converting enzyme inhibitors (ACEi) or angiotensin-receptor blockers (ARB) improve outcome in patients recovering from AKI remains unexplored. The purpose was to investigate the association between prescription of ACEi/ARB at intensive care unit (ICU) discharge and 1-year outcome in patients recovering from AKI.
Methods
Association between ACEi/ARB and 1-year mortality rate was explored in 1551 patients discharged from 21 European ICUs in an observational cohort. One-year all-cause mortality after ICU discharge was the primary endpoint. AKI was defined using the kidney disease improvement global outcome definition. Propensity score matching was used to consider the probability to receive ACEi/ARB at ICU discharge and included chronic heart failure, ACEi/ARB on ICU admission, Charlson Comorbidity Index, age, diabetes mellitus, chronic kidney disease, estimated glomerular filtration rate and arterial blood pressure at ICU discharge vasopressors and renal replacement therapy.
Results
Overall, 1-year mortality was 28 and 15% in patients with AKI (n = 611, 39%) and without AKI (n = 940), respectively. In patients with AKI, unadjusted, adjusted and propensity-score matched 1-year mortality rates were lower in patients treated with ACEi/ARB at ICU discharge [HR of 0.55 (0.35–0.89), HR of 0.45 (0.27–0.75), and HR of 0.48 (0.27–0.85, p < 0.001), respectively]. These results were consistent across sensitivity analysis. No association was observed in patients without AKI.
Conclusions
In patients discharged alive from the ICU after experiencing AKI, ACEi/ARB prescription at discharge is associated with a decrease in 1-year mortality.
Trial registration
ClinicalTrials.gov NCT01367093. Registered on 6 June 2011.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In patients discharged alive from the ICU after experiencing AKI, ACEi prescription at discharge is associated with a decrease in one-year mortality rate. |
Introduction
Acute kidney injury (AKI) is associated with activation of the renin–angiotensin–aldosterone system (RAAS) [1, 2]. Activation of the RAAS has been shown to be associated with long-term detrimental consequences, especially with chronic kidney disease (CKD) and cardiovascular damage [3,4,5,6,7]. In this line, angiotensin converting enzymes inhibitors (ACEi) and or angiotensin-receptor blockers (ARB) have been recognized as key drugs to protect the kidney and the heart in chronic conditions such as diabetes or heart failure [8]. Protective effects of ACEi/ARB are thought to lie in the prevention of organ fibrosis development [2, 9, 10]. On the other hand, ACEi/ARB have long been considered to be potential nephrotoxic drugs in acute settings. It is currently recommended to stop ACEi/ARB in the setting of AKI caused by hypovolemia or hypotension [11]. An unresolved question is whether an ACEi or ARB should be started in patients who recovered from AKI. Recently, potential protective effects of ACEi/ARB following acute injury have, however, been reported [1, 2, 9, 12]. The impact of ACEi/ARB in patients recovering from AKI still remains largely unexplored. In this study, we hypothesized that administration of ACEi/ARB in patients that had AKI during their ICU stay would be associated with lower 1-year mortality rate.
Materials and methods
Patients
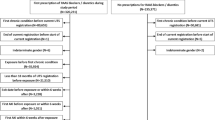
The outcome of patients experiencing AKI and discharged alive from the ICU was explored in the FROG-ICU cohort (trials.gov identifier: NCT01367093) [13]. The study was conducted in France and Belgium in accordance with Good Clinical Practice (Declaration of Helsinki 2002) and Ethical Committee approvals (Comité de Protection des Personnes—Ile de France IV, IRB no. 00003835 and Commission d’éthique biomédicale hospitalo-facultaire de l’hôpital de Louvain, IRB no. B403201213352). Patients were included from August 2011 to June 2013. The study was an international observational study including 2087 consecutive patients admitted to 21 ICUs receiving mechanical ventilation and/or vasopressors for at least 24 h. The protocol has previously been described elsewhere [14]. Among the 2087 included patients, 1570 (74%) were discharged alive from ICU and 1551 had data regarding ACEi or ARB treatment available representing our study population.
Definitions of acute kidney injury
The AKI definition was based on the KDIGO criteria using serum creatinine (Screat) or need for renal replacement therapy [15]. The baseline Screat was ICU admission Screat when eGFR was above 60 mL/min/1.73 m2 or based on the Modified and Diet Renal Disease formula (MDRD) equation in all other cases. Severe AKI was defined as AKI stage 2 or 3. Patients who had AKI during ICU stay were explored for non-recovery, defined as acute kidney disease (AKD) patients [16]. AKD was defined as Screat level at discharge > 1.5 times baseline Screat level among patients who developed AKI in the ICU.
Endpoint
The primary endpoint was 1-year all-cause mortality after ICU discharge. Outcome was collected by questionnaire and/or phone contact and/or civil registry examination.
Statistical analysis
The association between mortality and ACEi/ARB prescribed at ICU discharge was assessed using both univariate and multivariate analyses after adjustment for potential confounding factors (age, Charlson score, CKD, diabetes mellitus, chronic heart failure, ACEi/ARB intake previous to ICU admission, administration of vasopressors during ICU stay, RRT during ICU stay, systolic blood pressure at ICU discharge). The proportional hazards assumption of the Cox regression was tested as appropriate using the and Therneau approach [17]. Different sensitivity analyses were conducted. In particular, a potential center effect was included in the multivariate mode using a robust estimator of the variance, which takes into account potential intra-center correlation. Missing values were handled by multiple imputation by chained equations [18], and results of the association between the exposure and the main outcome measure after multiple imputation were reported as a sensitivity analysis (e-Table 1). Different variables were also added to the multivariate model: cardiogenic shock as the cause of ICU admission, and the level of NT-proBNP at ICU discharge. The log-linearity of NT–proBNP association with the outcome was checked using restricted cubic spline.
The association between ACEi/ARB at discharge and 1-year outcome was also considered after adjustment in various subgroups: patients treated or not by ACEi/ARB at ICU admission, after exclusion of patients with chronic renal disease, patients with non-severe versus severe AKI (defined by KDIGO class 2 or 3) and patients with or without acute kidney disease as previously defined [16].
Finally, the effect of ACEi/ARB on mortality was estimated using propensity score matching (PS-matching). Given the observational nature of the data, treatment allocation was not randomly allocated in the study population. The risk of allocation bias due to the presence of confounders was handled using PS-matching., which in our investigation took the probability into account that a patient with specific baseline characteristics had a prescription of ACEi/ARB at ICU discharge, thus allowing the comparison of patients with or without ACEi/ARB at discharge having similar characteristics. PS-matching characteristics included chronic heart failure, ACEi/ARB on ICU admission, Charlson’s score, eGFR at discharge (using the MDRD formula), systolic blood pressure at discharge, age, diabetes, CKD, and vasopressors or RRT during ICU stay. Variables included in the propensity score were selected when either major difference among treated and non-treated patients were observed or when they were identified as potentially true confounded (i.e. associated with both treatment allocation and prognosis). Each patient treated with ACEi was matched to one untreated control with similar PS using the nearest-/ARB neighbor approach, with no replacement and a calliper size of 0.2. Imbalance between treated and untreated patients before and after PS matching was assessed using a standardized difference, considering less than 10% acceptable to define the study patients’ characteristics balanced with respect to the previously described features. The association between outcome and ACEi/ARB prescription was further described in patients with or without AKD.
Data are expressed in median with interquartile range or count with percentage as appropriate. p < 0.05 was considered statistically significant. All analyses were performed using R statistical software (The “R” Foundation for Statistical Computing, Vienna, Austria, URL http://www.jstatsoft.org/v42/i08/).
Results
Patients characteristics
A total of 1551 patients survived during their ICU stay, of which 611 (39%) had an AKI episode during the ICU stay. Table 1 details patient characteristics at discharge according to outcome and Table 2 details patient characteristics according to treatment at discharge. Among patients who developed AKI during ICU stay, 186 were classified as AKI stage 1, 94 as stage 2, and 331 patients as stage 3. Overall, 1-year mortality of the AKI group was 28% (n = 173). The rate of ACEi/ARB introduction was slightly higher in post-AKI patients than in patients without AKI (6.3 vs. 5.7%).
Impact of ACEi/ARB prescription on outcome
Patients prescribed with ACEi/ARB at ICU discharge [n = 109 (18%)] revealed a lower mortality rate compared to those who were not (18 vs .31% respectively, p = 0.01; Fig. 1). Unadjusted mortality risk was greater in patients untreated when compared to those that were prescribed with ACEi/ARB at ICU discharge. Mortality risk remained significantly associated to non-prescription of ACEi/ARB after adjustment for prognostic variables (Fig. 2).
Propensity-based matching produced 82 matched pairs with standardized differences in patient characteristics of less than 10%, indicating a successful balance of potential confounders between treated and untreated patients (e-Table 2; e-Fig. 1). SAPS2 score was 55 (44–73) in patients treated with ACEi at discharge and 56 (40-68) in patients not treated. PS analysis confirmed the suggested protective effect of ACEi on 1-year mortality (18 vs. 35%, p = 0.01). In the PS-matched patients, 1-year mortality was lower in patients receiving ACEi compared to those who did not. All sensitivity analysis (including cluster effect, multiple imputation, NT-ProBNP at discharge and cardiogenic shock) showed consistent effects on the association between ACEi/ARB and outcome (Fig. 2). Associations between outcome and ACEi/ARB in sub-goups are presented in Fig. 2.
In contrast, mortality in ACEi and non-ACEi subgroups was similar in non-AKI patients in both adjusted analysis and PS matching [HR of 1.8 (1.21–2.68), HR of 0.87 (0.53–1.42), and HR of 1.00 (0.58–1.73) respectively; eFig. 2].
Impact of AKI severity and ACEi prescription on outcome
Among patients who experienced AKI, Figs. 2 and 3 show that benefits associated with ACEi treatment for 1-year mortality may be influenced by AKI severity or kidney function recovery at discharge. ACEi were associated with benefits on 1-year mortality in patients with both, high and low eGFR at discharge. When considering AKI severity, the association was found significant in non-severe AKI and in patients with AKD.
Discussion
In this ancillary study of FROG-ICU which enrolled the widest population of ICU patients at discharge, we observed an association between the prescription of ACEi/ARB at ICU discharge and 1-year survival in patients with AKI. Propensity analysis confirmed the suggested protective effect. This association suggests that treatment with ACEi in this setting might improve outcome after AKI.
Acute kidney injury is associated with worse long-term outcome in many ICU studies [19]. The reasons for such association remain largely unknown, but the impact of AKI on remote organ function and damage has been highlighted [20]. Activation of the RAAS triggers vasoconstriction and pro-fibrotic pathways involved in chronic organ damage and dysfunction [3, 9, 21, 22]. Blocking the RAAS using ACEi/ARB is currently recommended to prevent occurrence or heart damage and failure after acute myocardial infarction or to limit progression of chronic heart failure [19]. ACEi/ARB are also recommended for patients with diabetic nephropathy, hypertension and proteinuria to limit the progression towards CKD [23].
AKI leads to activation of the RAAS and may therefore lead to systemic chronic cardiovascular and renal damage. In this regard, AKI promotes renal fibrosis and chronic kidney damage [3]. AKI was further perceived to be a risk factor for chronic kidney disease and to be associated with a high incidence of cardiovascular complications [22]. While ACEi/ARB administration in chronic conditions is beneficial, they have, however, long been considered as potential nephrotoxic in altered intra-renal hemodynamics in acute condition. ACEi/ARB may buffer renal autoregulation and impair glomerular filtration rate in patients with systemic hypotension or hypovolemia [11], this is not, however, associated with kidney damage.
Acute kidney injury further carries a high risk of long-term mortality and cardiovascular events. Several pre-clinical data suggest that AKI may induce remote cardiovascular damage, which may partially be causal in the long-term outcome after AKI [16, 20]. Furthermore, AKI, chronic kidney disease and chronic heart failure are thought to share common pathophysiological pathways, including activation of the renin–angiotensin–aldosterone system and activation of pro-fibrotic pathways [21, 24]. Altogether, previous pre-clinical and clinical studies suggest that AKI activates the renin–angiotensin–aldosterone system and may promote the development of chronic renal and cardiac injuries through organs fibrosis. It is highly plausible that protective strategies targeting these pathways may improve long-term outcome after AKI. We therefore hypothesized that ACEi/ARB may prevent long-term consequences of AKI.
We acknowledge that this study suffers from several limitations. First, the observational nature of the data prevents the confirming of causality even though the association was also observed after adjustment for confounding variables and using PS-matching. We must, however, acknowledge that residual confounding factors may persist. This reinforces the need for a randomized controlled trial. The data, however, do not suggest any harm, and provide solid information for further interventional randomized controlled trials. Secondly, the exact date of ACEi/ARB initiation and patient compliance after ICU discharge was not controlled in the study, and the introduction or interruption of treatment after ICU discharge was not available or controlled. Third, the dose ACEi was not recorded. Even though the cohort was large, the sample size of patients treated with ACEi/ARB at discharge may limit power for sub-groups analysis. Urine output and true baseline Screat were not available in this cohort. Acute kidney disease was also assessed at ICU discharge before 3 months of follow-up in most cases, which may lead to under-evaluation of recovery. Also, kidney and heart function was not assessed during the follow-up, and therefore the impact of ACEi-induced cardiovascular and renal disease prevention on the outcome could not be confirmed. Finally, as the causes of death after ICU are not known in FROG-ICU, it was unfortunately not possible to show a decrease in the incidence of cardiovascular or renal deaths between exposed and unexposed populations.
However, whatever the patient compliance, the dose and the effects on different organs, we report a reduction in 1-year mortality. This main result could encourage the performing of a large randomized study in ICU patients experiencing an AKI during ICU stay.
Conclusion
To conclude, the results of this study suggest that ACEi/ARB may be considered as a preventive strategy for long-term outcome for patients discharged alive from ICU, after having experienced an episode of AKI. Whether ACEi/ARB could prevent chronic organ damage and ultimately improve outcome in patients recovering from AKI needs to be properly validated in further randomized controlled clinical trials.
References
Cheng S-Y, Chou Y-H, Liao F-L et al (2016) Losartan reduces ensuing chronic kidney disease and mortality after acute kidney injury. Sci Rep 6:34265. https://doi.org/10.1038/srep34265
Efrati S, Berman S, Hamad RA et al (2012) Effect of captopril treatment on recuperation from ischemia/reperfusion-induced acute renal injury. Nephrol Dial Transplant 27:136–145. https://doi.org/10.1093/ndt/gfr256
Burrell LM, Burchill L, Dean RG et al (2012) Chronic kidney disease: cardiac and renal angiotensin-converting enzyme (ACE) 2 expression in rats after subtotal nephrectomy and the effect of ACE inhibition. Exp Physiol 97:477–485. https://doi.org/10.1113/expphysiol.2011.063156
Chou Y-H, Huang T-M, Pan S-Y et al (2017) Renin-angiotensin system inhibitor is associated with lower risk of ensuing chronic kidney disease after functional recovery from acute kidney injury. Sci Rep 7:46518. https://doi.org/10.1038/srep46518
Horkan CM, Purtle SW, Mendu ML et al (2015) The association of acute kidney injury in the critically ill and postdischarge outcomes: a cohort study. Crit Care Med 43:354–364. https://doi.org/10.1097/CCM.0000000000000706
Parr SK, Matheny ME, Abdel-Kader K et al (2017) Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int. https://doi.org/10.1016/j.kint.2017.07.007
Sawhney S, Marks A, Fluck N et al (2017) Acute kidney injury as an independent risk factor for unplanned 90-day hospital readmissions. BMC Nephrol 18:9. https://doi.org/10.1186/s12882-016-0430-4
Rana I, Velkoska E, Patel SK et al (2015) MicroRNAs mediate the cardioprotective effect of angiotensin-converting enzyme inhibition in acute kidney injury. Am J Physiol Renal Physiol 309:F943–F954. https://doi.org/10.1152/ajprenal.00183.2015
Evans M, Carrero J-J, Szummer K et al (2016) Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in myocardial infarction patients with renal dysfunction. J Am Coll Cardiol 67:1687–1697. https://doi.org/10.1016/j.jacc.2016.01.050
Burchill L, Velkoska E, Dean RG et al (2008) Acute kidney injury in the rat causes cardiac remodelling and increases angiotensin-converting enzyme 2 expression. Exp Physiol 93:622–630. https://doi.org/10.1113/expphysiol.2007.040386
Joannidis M, Druml W, Forni LG et al (2017) Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017: expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med 43:730–749. https://doi.org/10.1007/s00134-017-4832-y
Cheungpasitporn W, Thongprayoon C, Srivali N et al (2015) Preoperative renin-angiotensin system inhibitors use linked to reduced acute kidney injury: a systematic review and meta-analysis. Nephrol Dial Transplant 30:978–988. https://doi.org/10.1093/ndt/gfv023
Gayat E, Cariou A, Deye N et al (2018) Determinants of long-term outcome in ICU survivors: results from the FROG-ICU study. Crit Care Lond Engl 22:8. https://doi.org/10.1186/s13054-017-1922-8
Mebazaa A, Casadio MC, Azoulay E et al (2015) Post-ICU discharge and outcome: rationale and methods of the The French and euRopean Outcome reGistry in Intensive Care Units (FROG-ICU) observational study. BMC Anesthesiol 15:143. https://doi.org/10.1186/s12871-015-0129-2
Hoste EAJ, Bagshaw SM, Bellomo R et al (2015) Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41:1411–1423. https://doi.org/10.1007/s00134-015-3934-7
Forni LG, Darmon M, Ostermann M et al (2017) Renal recovery after acute kidney injury. Intensive Care Med 43:855–866. https://doi.org/10.1007/s00134-017-4809-x
Grambsch P, Therneau T (1994) Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81:515–526
Harrell FE, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387. https://doi.org/10.1002/(SICI)1097-0258(19960229)15:4%3c361:AID-SIM168%3e3.0.CO;2-4
Prescott HC, Angus DC (2018) Enhancing recovery from sepsis: a review. JAMA 319:62–75. https://doi.org/10.1001/jama.2017.17687
Dépret F, Prud’homme M, Legrand M (2017) A role of remote organs effect in acute kidney injury outcome. Nephron 137:273–276. https://doi.org/10.1159/000476077
Simões E, Silva AC, Teixeira MM (2016) ACE inhibition, ACE2 and angiotensin-(1-7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res 107:154–162. https://doi.org/10.1016/j.phrs.2016.03.018
Zhong J, Basu R, Guo D et al (2010) Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation 122:717–728. https://doi.org/10.1161/circulationaha.110.955369 (18 p following 728)
Whelton PK, Carey RM, Aronow WS et al (2017) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertens Dallas Tex. https://doi.org/10.1161/HYP.0000000000000065
Chawla LS, Eggers PW, Star RA, Kimmel PL (2014) Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371:58–66. https://doi.org/10.1056/NEJMra1214243
Acknowledgements
The authors are particularly grateful to CRAs and healthcare providers of all the investigating centers. We also thank the Centre de Recherche Clinique (CRC) of Lariboisière University Hospital for his support.
Funding
FROG-ICU was funded by the Programme Hospitalier de la Recherche Clinique (AON 10-216) and by a research grant from the Société Française d’Anesthésie—Réanimation.
Author information
Authors and Affiliations
Consortia
Contributions
Study concept and design: Gayat, Legrand, Mebazaa; Acquisition of data: Gayat, Mebazaa, Vieillard-Baron, Cariou, Deye, Jaber, Chousterman, Lu, Laterre, Monnet, Leone, Guidet, Lefrant, Fournier, Legrand; Analysis and interpretation of data: Gayat, Legrand, Mebazaa; Drafting of the manuscript: Legrand, Gayat, Hollinger, Mebazaa; Critical revision of the manuscript for important intellectual content: all declared authors; Statistical analysis: Gayat; Obtained funding: Gayat, Mebazaa; Administrative, technical, or material support: Gayat, Mebazaa, Fournier; Study supervision: Gayat, Legrand, Mebazaa; All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
EG received research grants from Sphingotec, and consultancy fees from Magnisense and Roche Diagnostics. AM received speaker’s honoraria from Abbott, Novartis, Orion, Roche and Servier, and fees as a member of the advisory board and/or Steering Committee from Cardiorentis, Adrenomed, MyCartis, Neurotronik and Sphyngotec. ML received research grants from Sphingotec, consultancy fees from Astellas and Lecture fees from Gilead and Fresenius. The remaining authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
134_2018_5160_MOESM1_ESM.tiff
eFigure 1. Imbalance before (black square) and after (red bullet) propensity score matching for variables included in the propensity model with patients who developed AKI during ICU stay (TIFF 61 kb)
134_2018_5160_MOESM2_ESM.tiff
eFigure 2. Survival curves according to prescription of ACEi at ICU discharge in patients without AKI (Panel A) and Imbalance before (black square) and after (red bullet) propensity score matching for variables included in the propensity model with patients who didn’t developed AKI during ICU stay(Panel B) (TIFF 220 kb)
Rights and permissions
About this article
Cite this article
Gayat, E., Hollinger, A., Cariou, A. et al. Impact of angiotensin-converting enzyme inhibitors or receptor blockers on post-ICU discharge outcome in patients with acute kidney injury. Intensive Care Med 44, 598–605 (2018). https://doi.org/10.1007/s00134-018-5160-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-018-5160-6