Abstract
Purpose
It is unclear whether tight glycemic control is warranted in all critically ill adults. We employed network meta-analysis to examine the risk of mortality and hypoglycemia associated with different glycemic control targets in critically ill adults.
Methods
Electronic databases were searched up to 2016 for randomized controlled trials comparing various insulin regimens in critically ill adults with hyperglycemia. Two reviewers independently extracted information and evaluated quality with the Cochrane risk-of-bias tool. Four glycemic control groups were compared: tight (blood glucose: 4.4 < 6.1 mmol/l), moderate (6.1 < 7.8 mmol/l), mild (7.8 < 10.0 mmol/l), and very mild (10.0 to < 12.2 mmol/l). Network meta-analysis was performed by a frequentist approach with multivariate random effects meta-analysis.
Results
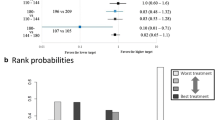
Thirty-six randomized trials (17,996 patients) were identified. Compared with very mild control, tight control did not reduce the risk of short-term mortality [relative risk (RR) 0.94 (95 % CI 0.83–1.07, p = 0.36)], and neither did mild control [RR 0.88 (0.73–1.06), p = 0.18] or moderate control [RR 1.1 (0.66–1.84), p = 0.72]. However, severe hypoglycemia (<2.2 mmol/l) was more frequent with tight control than very mild control [RR 5.49 (3.22–9.38), p < 0.001] or mild control [RR 4.47 (2.5–8.03), p < 0.001]. Stratified analyses (cause of death, ICU type, time period, or diabetes) did not find significant between-group differences. Ranking analysis revealed the following hierarchy for avoiding death (highest to lowest rank): mild control, tight control, and very mild control.
Conclusions
Network meta-analysis showed no mortality benefit of tight glycemic control in critically ill patients, but fivefold more hypoglycemia versus mild or very mild control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most observational studies have found that hyperglycemia and hypoglycemia are strongly associated with adverse outcomes in patients receiving critical care [1–3]. It is unclear whether tight glycemic control is warranted in all critically ill adults. Early randomized controlled trials (RCTs) suggested that intensive treatment of hyperglycemia improved hospital outcomes [4, 5]. However, most of the trials performed subsequently in critically ill patients have failed to show significant improvement of mortality with tight glycemic control or have even shown an increased risk of mortality [6]. Moreover, RCTs have highlighted the risk of severe hypoglycemia resulting from efforts to maintain tight glycemic control [7, 8]. Four previous meta-analyses of RCTs examined the risks and benefits of tight glycemic control compared with care in critically ill adult patients [9–12]. However, all these studies were traditional pairwise meta-analyses and only the pooled relative risk (RR) was calculated by direct comparison, although these studies had heterogeneous target blood glucose levels that limits the meaningfulness of making comparisons among them. The American Diabetes Association recommends a target glucose range of 7.8–10.0 mmol/l (140–180 mg/dl) for the majority of critically ill patients receiving insulin therapy for persistent hyperglycemia (Grade A) [13]. However, no meta-analysis has confirmed the clinical significance of these recommendations.
In the present study, our objective was to systematically evaluate the mortality rate and frequency of severe hypoglycemia associated with various target glucose levels in critically ill patients with hyperglycemia receiving insulin therapy. We investigated RCTs using a network meta-analysis approach, which enabled us to assess four glucose targets by mixed and direct comparison to determine their efficacy and safety.
Methods
Search strategy
We searched PubMed (from 1950 to March 2, 2016), the Cochrane library (to issue 3, 2016), and Web of Science (from 1970 to March 2, 2016) for original reports of RCTs that compared different glycemic control strategies using insulin in critically ill patients with hyperglycemia (Supplementary Table 1). We checked the reference lists of the original studies, review articles, and meta-analyses identified by our electronic searches to find other eligible trials. There were no language restrictions on the search.
Study selection
Eligible randomized trials fulfilled the twin criteria of (1) comparing various insulin regimens in critically ill (e.g., ICU) adult patients (≥18 years old) with hyperglycemia, and (2) reporting outcomes of interest. Studies were excluded if other aspects of care apart from the target glucose level (e.g., oral hypoglycemic agents) differed between the groups. Studies assessing children or glucose-potassium-insulin (GKI) regimens were also not eligible. Furthermore, reviews and abstracts reported at scientific sessions were excluded.
Quality assessment
Two authors (T.Y., N.S.) independently assessed the eligibility of each trial, extracted data, and evaluated trial bias risk. Disagreements were resolved by consensus. We used the trial risk of bias assessment scheme recommended by the Cochrane Collaboration for assessment of quality [14]. Trials with a high or unclear risk of bias for any components were classed as trials with a high risk of bias, while all other trials were classed as having a low risk of bias.
Data extraction and synthesis
Baseline demographic data, trial design, insulin therapy, and outcomes were extracted from each trial. The investigators of each study were contacted by e-mail to obtain missing information as needed. For this analysis, four treatment groups were identified: (1) a tight glycemic control group (target glucose level of 4.4 to <6.1 mmol/l), (2) a moderate glycemic control group (target glucose level of 6.1 to <7.8 mmol/l), (3) a mild glycemic control group (target glucose level of 7.8 to <10.0 mmol/l), and (4) a very mild glycemic control group (target glucose level of 10.0 to <12.2 mmol/l). When the glucose targets of candidate trials were not consistent with the above four categories, the trials were excluded. We chose these four target glucose ranges in accordance with the 2015 clinical practice recommendations of the American Diabetes Association [13].
Study outcomes
In accordance with a previous meta-analysis [9–12], the primary efficacy outcome was short-term mortality, which was defined as 90-day mortality or (if unavailable) hospital mortality, 28-day mortality, ICU mortality, or 6-month mortality. The secondary efficacy outcomes were (1) ICU mortality, (2) 28-day mortality, (3) hospital mortality, (4) 90-day mortality, and (5) 6-month mortality. The primary safety outcome was hypoglycemia (defined as a blood glucose level <3.3 mmol/l), while the secondary safety outcome was severe hypoglycemia (defined as a blood glucose level <2.2 mmol/l). Other definitions of hypoglycemia (e.g., <4.4 mmol/l) were not classified as hypoglycemia and were excluded from the analysis.
Statistical analyses
Comparison of treatments
Network meta-analysis was performed by a frequentist-based approach with multivariate random effects meta-analysis [15–18]. The covariance between two estimates from the same study (sharing a common treatment group) is the variance of data in the shared arm and is calculated by the multivariate meta-analysis method. Inconsistency of the network model was estimated by using inconsistency factors and their uncertainty.
In addition, ranking plots (rankograms) were constructed using the probability that a given treatment had the highest event rate for each outcome. The surface under the cumulative ranking curve (SUCRA), which is a simple transformation of the mean rank, was used to set the hierarchy of the treatments. As the SUCRA becomes larger, the treatment in question receives a lower rank [19, 20]. In addition, clustered ranking plots were constructed using SUCRA values for efficacy (mortality) and safety (hypoglycemia) outcomes to obtain information on treatments that maximized the benefit for both mortality and hypoglycemia. We performed ranking analysis of three regimens [tight control (4.4 to <6.1 mmol/l) vs. mild control (7.8 to <10.0 mmol/l) vs. very mild control (10.0 to <12.2 mmol/l) and excluded moderate control (6.1 to <7.8 mmol/l)] because fewer studies (n = 4) assessed this regimen than any other regimen, and one of the four studies [21] had a high risk of bias that might have led to misinterpretation of the results.
Direct comparison meta-analysis
In addition to network meta-analysis, direct comparison meta-analysis was performed by using data for the closed loop in the network analysis. Meta-analyses were performed in line with the recommendations of the Cochrane Collaboration, the Preferred Reporting Items for Systematic Reviews, Meta-Analyses (PRISMA) statement [14, 22], and the GRADE Working Group approach (Checklists 1, 2) [23].
Heterogeneity was assessed by using the I 2 statistic [24]. Summary effect sizes and their 95 % confidence intervals were calculated by the random effects model of DerSimonian and Laird [24]. Publication bias was estimated visually with funnel plots, and also by using Begg’s test and the weighted regression test of Egger. All analyses were performed using standard software (Stata 13.0, Stata, TX, USA) and p < 0.05 indicates statistical significance.
Sensitivity analyses
Three sensitivity analyses were performed to assess heterogeneity by (1) investigating trial quality, (2) by excluding studies in which the glycemic control regimen differed somewhat from the four categories that we selected, and (3) by stratification for the cause of death [sepsis (septic shock), or cardiovascular mortality] and the intensive care unit (ICU) setting. The ICU setting was stratified into 3 categories: (1) surgical (including general surgical, cardiothoracic, neurosurgical, and trauma ICUs); (2) medical (including general medical, cardiac, and neurologic ICUs); and (3) mixed medical–surgical ICUs. For trials that did not specify the ICU setting, we categorized it as mixed medical–surgical. Analyses stratified for the presence of diabetes were also performed, and we used the method of Altman et al. [25] to evaluate whether the pooled RRs differed between groups stratified by diabetes. Moreover, we performed a meta-regression analyses to explore the sources of heterogeneity, and we examined whether variables such as diabetes had a significant influence on the risk of mortality.
Results
Selection and characteristics of the studies
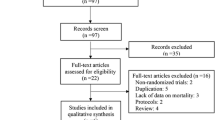
We identified 36 randomized trials that satisfied the inclusion criteria, which covered a total of 17,996 patients (n = 8956 in the intensive care group and n = 9050 in the control care groups) [4–6, 21, 26–57] (Supplementary Fig. 1). The network of treatment comparisons are displayed in Fig. 1.
The baseline characteristics of the trials are presented in Table 1 and Supplementary Table 2. Trials were conducted in diverse countries, but most often at a single center. The prevalence of hypoglycemia ranged widely among the studies. The definition of hypoglycemia was heterogeneous, and five studies were excluded from analysis of hypoglycemia because of the lack of data describing hypoglycemic events [26–29] or an unsuitable definition of hypoglycemia (<4.4 mmol/l) [30].
Risk of bias
Supplementary Fig. 2 summarizes the risk of bias for the domains included in the Cochrane tool of risk assessment. In all 36 studies, the risk of bias was high for blinding of participants and personnel because physicians were not blinded to the interventions in any study. Fourteen studies (39 %) had a high (6 %) [21, 29] or unclear (33 %) risk of bias in the domain of allocation concealment. Conversely, there was generally a low risk of bias for blinding of outcome assessment, generation of the randomization sequence, and incomplete outcome data. However, one study [21] had a high risk of bias with regard to generation of the randomization sequence because it was a randomized quasi-experimental trial. The attrition rate was reasonable in most studies with loss to follow-up of 0–2 %, but was unknown for 5 studies. All studies employed intention-to-treat (ITT) analysis.
Network meta-analysis
Efficacy outcomes
All-cause mortality
Analysis performed with mixed treatment comparison models (data from 36 randomized trials including 17,996 patients and 4684 events) using very mild glycemic control as the reference revealed no statistically significant difference of all-cause mortality among the glycemic control regimens [RR 0.88 (0.73–1.06), p = 0.18 for mild control; RR 1.1 (0.66–1.84), p = 0.72 for moderate control; and RR 0.94 (0.83–1.07), p = 0.36 for tight control] (Fig. 2). Ranking analysis revealed that the hierarchy for efficacy in avoiding death (highest to lowest rank) was mild control (SUCRA 14.0), followed by tight control (SUCRA 52.6) and then very mild control (SUCRA 81.4) (Supplementary Fig. 3a, b).
There was no evidence of statistical inconsistency between the direct and indirect estimates (p = 0.05 for the very mild–mild–tight control loop vs. p = 0.92 for the mild–moderate–tight control loop). Similarly, when very mild glycemic control was used as the reference, there was no significant difference in the rate of all-cause death compared with the other regimens (Fig. 3; Supplementary Fig. 4). There was no evidence of statistical inconsistency between the direct and indirect estimates (data not shown).
Safety outcome
Hypoglycemia
In the mixed treatment comparison models with data from 31 randomized trials including 17,502 patients and 1449 events, tight control was associated with a higher risk of hypoglycemic events when compared with very mild glycemic control and mild control [RR 5.64 (95 % confidence interval 3.69–8.61, p < 0.001) vs. very mild control and RR 4.67 (95 % confidence interval 2.75–7.94), p < 0.001 vs. mild control] (Fig. 2).
Ranking analysis revealed that the hierarchy for safety in avoiding hypoglycemia (highest to lowest rank) was very mild control (SUCRA 14.6), followed by mild control (SUCRA 35.4) and tight control (SUCRA 100.0) (Supplementary Fig. 5a, b).
There was no evidence of statistical inconsistency between the direct and indirect estimates (data not shown). There was also no evidence of a small-study effect in any of the models (data not shown).
Severe hypoglycemia
Similarly, analysis of data from 22 randomized trials including 16,120 patients and 1020 events showed that tight control was associated with a higher risk of severe hypoglycemic events compared with very mild control and mild control (RR 5.49 (95 % confidence interval 3.22–9.38), p < 0.001 vs. very mild control and RR 4.47 (95 % confidence interval 2.5–8.03), p < 0.001 vs. mild control] (Fig. 2). There was no evidence of statistical inconsistency between the direct and indirect estimates (data not shown).
Clustered ranking plot
Figure 4 showed a plot of the combined ranking for both all-cause mortality (efficacy) and hypoglycemia (safety). The ideal glycemic control regimen would decrease mortality and also reduce the occurrence of hypoglycemic events. Unfortunately, none of the reported regimens maximized the benefit for both mortality and hypoglycemia. However, the results of our analysis suggested that mild glycemic control (7.8 to <10.0 mmol/l) achieves the best outcome for all-cause mortality.
Clustered ranking plot of short-term mortality and hypoglycemia with various glycemic control strategies. As the surface under the cumulative ranking curve (SUCRA) value becomes higher, the efficacy (no short-term mortality) and safety (no hypoglycemia) both decrease. The hypothetical ideal glycemic control regimen has the best outcomes for both mortality and hypoglycemia
Sensitivity analyses
The results were largely similar for sensitivity analysis (1) based on trial quality (excluding data [21] that were considered to have a high risk of selection bias) and sensitivity analysis (2) excluding studies in which the glycemic control regimen differed somewhat from our 4 categories (e.g., the target glucose level was <12.2 mmol/l with no lower limit, which we categorized as very mild control). The sensitivity analysis employing stratification by the type of ICU revealed no significant difference of mortality among the four glycemic control targets (Fig. 5).
Analyses stratified by presence of diabetes also revealed no significant interaction for this relationship (all p values for interaction >0.05) (Supplementary Fig. 6).
Furthermore, we performed a sensitivity analysis stratified by the cause of death (Supplementary Figs. 7, 8). Similar to the findings for all-cause mortality, this analysis indicated that mortality due to sepsis or cardiovascular causes did not differ significantly between treatment groups (all p values were >0.05, as shown in Supplementary Fig. 7).
Ranking analysis suggested that the hierarchy for avoiding death due to sepsis (highest to lowest rank) was mild glycemic control (target glucose level: 7.8 to <10.0 mmol/l), followed by tight control (4.4 to <6.1 mmol/l) and then very mild control (10.0 to <12.2 mmol/l). However, the hierarchy for avoiding cardiovascular mortality was very mild glycemic control (target glucose level: 10.0 to <12.2 mmol/l), followed by mild control (7.8 to <10.0 mmol/l) and then tight control (4.4 to <6.1 mmol/l) (Supplementary Fig. 8).
Direct comparison
Regarding the efficacy outcomes, the direct comparison meta-analysis found that there was no statistically significant difference of short-term mortality between tight glycemic control and very mild control [pooled RR 0.95 (95 % CI 0.83–1.08), p = 0.46, I 2 = 0 %)] (Supplementary Fig. 9) or mild control (pooled RR 1.06 (95 % CI 0.97-1.15), p = 0.18, I 2 = 0 %)] (Supplementary Fig. 10). These results were similar to those obtained using the fixed comparison model. Funnel plots showed relatively good symmetry, arguing against the presence of publication bias (Supplementary Fig. 11). There was also no evidence of publication bias according to Begg’s test and Egger’s test (see p values in the figures).
Analysis stratified by the presence of diabetes showed similar results [with diabetes: RR 1.07 (0.9–1.28), p = 0.42, I 2 = 0 %, p for heterogeneity =0.75; without diabetes: RR 1.0 (0.91–1.1), p = 0.99, I 2 = 50 %, p for heterogeneity =0.054; p for interaction 0.52] (Supplementary Fig. 12a and b).
Finally, meta-regression analysis demonstrated that the presence of diabetes at baseline was not significantly related to the risk of mortality (p = 0.96) (Supplementary Fig. 13).
Discussion
The present network meta-analysis of randomized trials of intensive insulin therapy in critically ill patients failed to find any benefit of tight glycemic control for all-cause mortality, but tight glycemic control increased the frequency of hypoglycemia and severe hypoglycemia by about fivefold compared with mild or very mild glycemic control. All stratified analyses of mortality [stratified by the type of ICU (medical, surgical, or mixed), the time period (ICU stay, hospital stay, 28 days, 3 months, or 6 months), or the presence of diabetes] did not identify any significant differences among the glycemic control groups. Even though better glycemic control is potentially beneficial, achieving tight control for a short period could have too small an effect to influence mortality.
Our network meta-analysis did not assess whether short-term hypoglycemia actually increased mortality. Even though hypoglycemia itself has a potential adverse influence on the risk of death, transient hypoglycemia in the hospital setting over a short period (several days to weeks) might not have a significant impact. However, hypoglycemia could cause other harms that are less obvious than death, but may well be clinically significant, such as cognitive and vision-related sequelae [58, 59]. Complex trade-offs may occur between tight glycemic control and hypoglycemia in critically ill patients. Moreover, it is possible that there was no statistically significant impact due to the short follow-up period.
Ranking analysis suggested that the hierarchy for avoiding death due to sepsis (highest to lowest rank) was in the order of mild control, tight control, and then very mild control, while the hierarchy for avoiding cardiovascular mortality was very mild control, mild control, and then tight control.
Since sepsis was the chief cause of death in all of the studies used for analysis of cause-specific mortality, we considered that sepsis might have the strongest influence on all-cause mortality in relation to glycemic control. It is possible that sepsis-related mortality was lowest in patients managed with mild glycemic control (target glucose: 7.8 to <10.0 mmol/l) due to the balance between the adverse influence of hyperglycemia on immune function and the adverse influence of hypoglycemia on infection. In contrast, the finding that cardiovascular mortality was highest with tight glycemic control (target glucose: 4.4 to <6.1 mmol/l), followed by mild control (target glucose: 7.8 to <10.0 mmol/l) and then very mild control (target glucose: 10.0 to <12.2 mmol/l) suggested that hypoglycemia might have an adverse influence on cardiovascular events. These differing results indicate that the optimal glycemic target might vary depending on the cause of death.
It is also possible that these results of ranking analysis (probability analysis) represent random chance, because there were no significant differences among the different glycemic control regimens with regard to sepsis-related or cardiovascular-related death.
In contrast to our present results, most observational studies have found that hyperglycemia and hypoglycemia are strongly associated with adverse outcomes in critically ill patients [1–3], which suggests that observational studies are inevitably influenced by various biases. It is also possible that patients with better glycemic control were healthier than patients with poor control, so the difference in mortality might simply reflect a difference in the severity of their underlying diseases (reverse causality). There is no doubt that the influence of reverse causality could explain some of the discrepancies between our findings and those reported previously.
The controversy concerning glycemic control was initiated by the first report of van den Berghe et al. [4] and continued by the results of studies such as the NICE-SUGAR trial [6]. While van den Berghe et al. [4] reported that intensive glycemic control was beneficial for ICU patients, the NICE-SUGAR trial did not show any such benefit, and actually demonstrated an increase of mortality in the intensive insulin therapy group [6]. Many authors and editorialists have since argued that the differing results could be explained by differences in patient characteristics (reason for hospitalization, glycemic control before admission, dialysis, septicemia, etc.) and the setting (surgical vs. medical vs. mixed ICU). Other factors have also been suggested, such as the age, sex, comorbidities, staff-to-patient ratio, staff experience, and frequency of blood glucose measurement.
The present network meta-analysis found no increase in the risk of mortality with tight glycemic control, while previous studies have demonstrated an increase. This difference may have arisen because previous investigations employed simple pairwise meta-analysis and ignored the differing glycemic control targets of the studies analyzed. Accordingly, the present network meta-analysis may provide more precise risk estimates and better information about the hierarchy of target glucose ranges for achieving safe and effective glycemic control with insulin in critically ill patients. The clustered ranking plot (Fig. 4) suggests that mild glycemic control (7.8 to <10.0 mmol/l) achieves the best outcome in relation to all-cause mortality and hypoglycemia, which is consistent with the American Diabetes Association guideline [13] and the AACE/ADA target glucose levels [60].
Strengths and limitations
The strengths of this network meta-analysis were as follows. First, it included several large-scale randomized controlled trials based on intention-to-treat analysis of target glycemic control goals. Second, we performed sensitivity analyses of cause-specific mortality that yielded important findings.
Regarding limitations, we did not review all the available research because we did not include grey literature (conference proceedings and unpublished data) due to concern about the reliability and quality of such information and the potential for large biases. However, we consider that the sample of studies was adequate because we carefully reviewed the references of all literature identified (including reviews) and analyzed approximately 40 articles. In addition, we evaluated publication bias and confirmed that there was no significant bias. Second, there was no blinding of the patients and physicians in any of the RCTs that we analyzed. Also, there were wide variations among the studies with regard to baseline patient characteristics (e.g., age and sex), insulin protocols, target glucose levels, and nutritional supplementation, all of which might have significantly impacted the endpoints.
Third, although network meta-analysis allowed us to compare the efficacy and safety of several glycemic control strategies, we acknowledge the limitation that interpretation of a network meta-analysis relies on there being sufficient homogeneity among trials to allow indirect comparison of target outcomes through common comparators [61].
Fourth, there are practical difficulties with implementing tight glucose control both inside and outside the clinical trial setting. It can be very hard to actually achieve the target glucose level, even in a clinical trial with close supervision. In fact, among the 72 comparison groups in the 36 RCTs we reviewed, 36 groups (50 %) did not achieve a mean glucose level within the target range. Failure to achieve the target glucose level could also bias our findings in either direction. To overcome such problems, advanced glycemic control techniques using new devices (such as continuous glucose monitoring or an artificial pancreas) should be employed. As Friedrich [11] and Griesdale [9] have stated, performing individual meta-analyses could also help to obtain further insights into optimal glycemic control for critically ill patients [62].
In conclusion, tight glycemic control did not improve (or worsen) mortality in critically ill patients, but there was an approximately fivefold increase of hypoglycemia and severe hypoglycemia compared with mild or very mild glycemic control. Accordingly, we do not recommend tight control in critically ill patients, and our analyses support the selection of intermediate target glucose levels.
References
Norhammar AM, Rydén L, Malmberg K (1999) Admission plasma glucose. Independent risk factor for long-term prognosis after myocardial infarction even in nondiabetic patients. Diabetes Care 22(11):1827–1831
Kosiborod M, Rathore SS, Inzucchi SE et al (2005) Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation 111:3078–3086
Svensson AM, McGuire DK, Abrahamsson P et al (2005) Association between hyper- and hypoglycaemia and 2 year all-cause mortality risk in diabetic patients with acute coronary events. Eur Heart J 26:1255–1261
Van den Berghe G, Wouters P, Weekers F et al (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345(19):1359–1367
Van den Berghe G, Wilmer A, Hermans G et al (2006) Intensive insulin therapy in the medical ICU. N Engl J Med 354(5):449–461
NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY et al (2009) Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 360(13):1283–1297
NICE-SUGAR Study Investigators, Finfer S, Liu B, Chittock DR et al (2012) Hypoglycemia and risk of death in critically ill patients. N Engl J Med 367(12):1108–1118
Krinsley JS, Grover A (2007) Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 35(10):2262–2267
Griesdale DE, de Souza RJ, van Dam RM et al (2009) Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ 180(8):821–827
Kramer AH, Roberts DJ, Zygun DA (2012) Optimal glycemic control in neurocritical care patients: a systematic review and meta-analysis. Crit Care 16(5):R203
Friedrich JO, Chant C, Adhikari NK (2010) Does intensive insulin therapy really reduce mortality in critically ill surgical patients? A reanalysis of meta-analytic data. Crit Care 14(5):324
Wiener RS, Wiener DC, Larson RJ (2008) Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA 300(8):933–944
American Diabetes Association (2015) Standards of medical care in diabetes-2015. Diabetes Care 38(suppl 1):S80–S85
Higgins J, Green S (2011) Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Cochrane Collaboration. http://handbook.cochrane.org/. Accessed 1 Sep 2015
Bangalore S, Toklu B, Kotwal A et al (2014) Anticoagulant therapy during primary percutaneous coronary intervention for acute myocardial infarction: a meta-analysis of randomized trials in the era of stents and P2Y12 inhibitors. BMJ 11(349):g6419
Higgins JPT, Jackson D, Barrett JK et al (2012) Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 3:98–110
White IR, Barrett JK, Jackson D et al (2012) Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 3:111–125
White IR (2009) Multivariate random-effects meta-analysis. Stata J 9:40–56
Chaimani A, Higgins JP, Mavridis D et al (2013) Graphical tools for network meta-analysis in STATA. PLoS ONE 8(10):e76654
Salanti G, Ades AE, Ioannidis JP (2011) Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 64(2):163–171
Giakoumidakis K, Eltheni R, Patelarou E et al (2013) Effects of intensive glycemic control on outcomes of cardiac surgery. Heart Lung 42(2):146–151
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7):e1000100
Puhan MA, Schünemann HJ, Murad MH et al (2014) GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 24(349):g5630
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Altman DG, Bland JM (2003) Interaction revisited: the difference between two estimates. BMJ 326:219
Iapichino G, Albicini M, Umbrello M et al (2008) Tight glycemic control does not affect asymmetric-dimethylarginine in septic patients. Intensiv Care Med 34(10):1843–1850
Farah R, Samokhvalov A, Zviebel F et al (2007) Insulin therapy of hyperglycemia in intensive care. Isr Med Assoc J 9(3):140–142
Bilotta F, Spinelli A, Giovannini F et al (2007) The effect of intensive insulin therapy on infection rate, vasospasm, neurologic outcome, and mortality in neurointensive care unit after intracranial aneurysm clipping in patients with acute subarachnoid hemorrhage: a randomized prospective pilot trial. J Neurosurg Anesthesiol 19(3):156–160
Savioli M, Cugno M, Polli F et al (2009) Tight glycemic control may favor fibrinolysis in patients with sepsis. Crit Care Med 37(2):424–431
Lazar HL, McDonnell MM, Chipkin S et al (2011) Effects of aggressive versus moderate glycemic control on clinical outcomes in diabetic coronary artery bypass graft patients. Ann Surg 254(3):458–463
Mitchell I, Knight E, Gissane J, Australian and New Zealand Intensive Care Society Clinical Trials Group et al (2006) A phase II randomised controlled trial of intensive insulin therapy in general intensive care patients. Crit Care Resusc 8(4):289–293
McMullin J, Brozek J, McDonald E et al (2007) Lowering of glucose in critical care: a randomized pilot trial. J Crit Care 22(2):112–118
Brunkhorst FM, Engel C, Bloos F et al (2008) German competence network sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 358(2):125–139
De La Gdel C, Donado JH, Grupo de Investigacion en Cuidado intensivo: GICI-HPTU et al (2008) Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Crit Care 12(5):R120
Arabi YM, Dabbagh OC, Tamim HM et al (2008) Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med 36(12):3190–3197
Oksanen T, Skrifvars MB, Varpula T et al (2007) Strict versus moderate glucose control after resuscitation from ventricular fibrillation. Intensiv Care Med 33(12):2093–2100
Bruno A, Kent TA, Coull BM et al (2008) Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke 39(2):384–389
Grey NJ, Perdrizet GA (2004) Reduction of nosocomial infections in the surgical intensive-care unit by strict glycemic control. Endocr Pract 10(Suppl 2):46–52
Preiser JC, Devos P, Ruiz-Santana S et al (2009) A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensiv Care Med 35(10):1738–1748
Staszewski J, Brodacki B, Kotowicz J et al (2011) Intravenous insulin therapy in the maintenance of strict glycemic control in nondiabetic acute stroke patients with mild hyperglycemia. J Stroke Cerebrovasc Dis 20(2):150–154
Green DM, O’Phelan KH, Bassin SL et al (2010) Intensive versus conventional insulin therapy in critically ill neurologic patients. Neurocrit Care 13(3):299–306
Coester A, Neumann CR, Schmidt MI (2010) Intensive insulin therapy in severe traumatic brain injury: a randomized trial. J Trauma 68(4):904–911
Johnston KC, Hall CE, Kissela BM, GRASP Investigators et al (2009) Glucose regulation in acute stroke patients (GRASP) trial: a randomized pilot trial. Stroke 40(12):3804–3809
Yang M, Guo Q, Zhang X et al (2009) Intensive insulin therapy on infection rate, days in NICU, in-hospital mortality and neurological outcome in severe traumatic brain injury patients: a randomized controlled trial. Int J Nurs Stud 46(6):753–758
Kreisel SH, Berschin UM, Hammes HP et al (2009) Pragmatic management of hyperglycaemia in acute ischaemic stroke: safety and feasibility of intensive intravenous insulin treatment. Cerebrovasc Dis 27(2):167–175
Azevedo JR, Lima ER, Cossetti RJ et al (2007) Intensive insulin therapy versus conventional glycemic control in patients with acute neurological injury: a prospective controlled trial. Arq Neuropsiquiatr 65(3B):733–738
Cappi SB, Noritomi DT, Velasco IT et al (2012) Dyslipidemia: a prospective controlled randomized trial of intensive glycemic control in sepsis. Intensiv Care Med 38(4):634–641
COIITSS Study Investigator, Annane D, Maxime A, Cariou V (2010) Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA 303(4):341–348
Rosso C, Corvol JC, Pires C et al (2012) Intensive versus subcutaneous insulin in patients with hyperacute stroke: results from the randomized INSULINFARCT trial. Stroke 43(9):2343–2349
Gandhi GY, Nuttall GA, Abel MD et al (2007) Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med 146(4):233–243
Bilotta F, Caramia R, Paoloni FP et al (2009) Safety and efficacy of intensive insulin therapy in critical neurosurgical patients. Anesthesiology 110(3):611–619
Cao SG, Ren JA, Shen B et al (2011) Intensive versus conventional insulin therapy in type 2 diabetes patients undergoing D2 gastrectomy for gastric cancer: a randomized controlled trial. World J Surg 35(1):85–92
Arabi YM, Tamim HM, Dhar GS et al (2011) Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr 93(3):569–577
Desai SP, Henry LL, Holmes SD et al (2012) Strict versus liberal target range for perioperative glucose in patients undergoing coronary artery bypass grafting: a prospective randomized controlled trial. J Thorac Cardiovasc Surg 143(2):318–325
Chan RP, Galas FR, Hajjar LA et al (2009) Intensive perioperative glucose control does not improve outcomes of patients submitted to open-heart surgery: a randomized controlled trial. Clinics (Sao Paulo) 64(1):51–60
Kalfon P, Giraudeau B, Ichai C, CGAO-REA Study Group et al (2014) Tight computerized versus conventional glucose control in the ICU: a randomized controlled trial. Intensiv Care Med 40(2):171–181
Henderson WR, Dhingra V, Chittock D, Canadian Critical Trials Group et al (2009) The efficacy and safety of glucose control algorithms in intensive care: a pilot study of the Survival Using Glucose Algorithm Regulation (SUGAR) trial. Pol Arch Med Wewn 119(7–8):439–446
Whitmer RA, Karter AJ, Yaffe K et al (2009) Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 301(15):1565–1572
Khan MI, Barlow RB, Weinstock RS (2011) Acute hypoglycemia decreases central retinal function in the human eye. Vision Res 51(14):1623–1626
Moghissi ES, Korytkowski MT, DiNardo M, American Association of Clinical Endocrinologists et al (2009) American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 15(4):353–369
Mills EJ, Thorlund K, Ioannidis JP (2013) Demystifying trial networks and network meta-analysis. BMJ 14(346):f2914
Stewart LA, Tierney JF (2002) To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Eval Health Prof 25(1):76–97
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(6):e1000097. doi:10.1371/journal.pmed1000097
Acknowledgments
There was no acknowledgment in this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
T.Yamada was funded by Japan Diabetes Society, Banyu Foundation, KAKENHI (Grants-in-Aid for Scientific Research), Japan Foundation for Applied Enzymology, and Japan Association for Diabetes Education and Care. We declare that these funds have not influenced this research.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Conflicts of interest
No potential conflicts of interest relevant to this article were reported.
Additional information
Take-home message:
Network meta-analysis showed no mortality benefit of tight glycemic control in critically ill patients, but fivefold more hypoglycemia versus mild or very mild control.
Electronic supplementary material
Below is the link to the electronic supplementary material.
134_2016_4523_MOESM1_ESM.pptx
Supplementary Figure 1. Flowchart of study selection. Supplementary Figure 2. Summary of the risk of bias: our judgements about the risk of bias for each item in each study. Blue low risk of bias, Yellow unclear risk of bias, Red high risk of bias. Supplementary Figure 3a. Rankogram for short-term mortality. The plot presents the ranking probabilities and their uncertainty. Supplementary Figure 3b. Hierarchy of outcomes for short-term mortality according to surface under the cumulative ranking curve (SUCRA) values. As the SUCRA value becomes higher, the efficacy (no short-term mortality) decreases. Supplementary figure 4. Network plot comparing the different treatment strategies. Nodes and edges are weighted according to the number of studies with the respective interventions. Supplementary Figure 5a. Rankogram for hypoglycemia. The plot presents the ranking probabilities and their uncertainty. Supplementary Figure 5b. Hierarchy of outcomes for hypoglycemia according to surface under the cumulative ranking curve (SUCRA) values. As the SUCRA value becomes higher, the safety (no hypoglycemia) decreases. Supplementary Figure 6. Comparison of various glycemic targets by using the mixed treatment comparison model stratified by the presence of diabetes. Supplementary Figure 7. Comparison of various glycemic targets using the mixed treatment comparison model with stratification by the cause of death (sepsis mortality, cardiovascular mortality, respiratory mortality, or neurological mortality). Supplementary Figure 8. Hierarchy of mortality outcomes according to the surface under the cumulative ranking curve (SUCRA). Mortality increases as the SUCRA value becomes higher. Supplementary Figure 9. Direct comparison meta-analysis between tight control and very mild control. Supplementary Figure 10. Direct comparison meta-analysis between tight control and mild control. Supplementary Figure 11. Funnel plots and the results of Begg’s test and Egger’s test. Supplementary Figure 12a. Direct comparison meta-analysis between tight control and very mild (or mild) control in patients with diabetes. Supplementary Figure 12b. Direct comparison meta-analysis between tight control and very mild (or mild) control in patients without diabetes.Supplementary Figure13. Meta-regression analysis of the relation between diabetes and the relative risk of short-term mortality with tight glycemic control versus mild and very mild control (PPTX 4424 kb)
134_2016_4523_MOESM2_ESM.docx
Supplementary Table 1. Terms used to search the electronic databases. Supplementary Table 2. Type of ICU and cause of death in all trials included in the network meta-analysis (DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Yamada, T., Shojima, N., Noma, H. et al. Glycemic control, mortality, and hypoglycemia in critically ill patients: a systematic review and network meta-analysis of randomized controlled trials. Intensive Care Med 43, 1–15 (2017). https://doi.org/10.1007/s00134-016-4523-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4523-0