Abstract
Purpose
We compared hemodynamic and biological effects of the Cascade system, which uses very high volume hemofiltration (HVHF) (120 mL kg−1 h−1), with those of usual care in patients with septic shock.
Methods
Multicenter, prospective, randomized, open-label trial in three intensive care units (ICU). Adults with septic shock with administration of epinephrine/norepinephrine were eligible. Patients were randomized to usual care plus HVHF (Cascade group), or usual care alone (control group). Primary end point was the number of catecholamine-free days up to 28 days after randomization. Secondary end points were number of days free of mechanical ventilation, renal replacement therapy (RRT) or ICU up to 90 days, and 7-, 28-, and 90-day mortality.
Results
We included 60 patients (29 Cascade, 31 usual care). Baseline characteristics were comparable. Median number of catecholamine-free days was 22 [IQR 11–23] vs 20 [0–25] for Cascade vs control; there was no significant difference even after adjustment. There was no significant difference in number of mechanical ventilation-free days or ICU requirement. Median number of RRT-free days was 85 [46–90] vs 74 [0–90] for Cascade vs control groups, p = 0.42. By multivariate analysis, the number of RRT-free days was significantly higher in the Cascade group (up to 25 days higher after adjustment). There was no difference in mortality at 7, 28, or 90 days.
Conclusion
Very HVHF using the Cascade system can safely be used in patients presenting with septic shock, but it was not associated with a reduction in the need for catecholamines during the first 28 days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite considerable progress in our understanding of the pathophysiology of septic shock, septic shock remains associated with a high mortality rate, ranging from 30 to 50 % [1–4]. Hemofiltration is widely used to manage renal failure, but most controlled trials have not shown a clinically significant and sustained effect on either cytokine removal or overall survival, even with high volume hemofiltration (above the dose of 35 mL kg−1 h−1) [5, 6]. Very high volume hemofiltration (HVHF) (above 100 mL kg−1 h−1) is a new concept for blood purification developed to attenuate the overwhelming systemic overflow of pro- and anti-inflammatory mediators released at the early phase of sepsis [7].
Journois et al. were among the first to study very HVHF as a blood purification technique in humans [8]. Very HVHF has been shown to be effective in ischemia–reperfusion syndrome (mimicking sepsis) [5, 6] or in adults resuscitated after an out-of-hospital cardiac arrest [5, 6, 9]. Using very HVHF (150 mL kg−1 h−1) with a high flux membrane, Grootendorst et al. showed an improvement in right ventricular function and cardiac performance in a porcine model of septic shock [10]. Findings suggest that high and middle molecular weight substance removal might be beneficial. However these techniques are labor intensive, require large quantities of substitution fluid, and result in undesired removal of significant amounts of vital low molecular weight substances (nutrients, trace elements, antibiotics) [11, 12].
A new hemofiltration system called Cascade (Gambro Industries, France) has been developed to perform very HVHF (120 mL kg−1 h−1) without these drawbacks. The system is designed to achieve a high removal rate for middle and high molecular weight substances. The removal rate of low molecular weight substances is equivalent to currently used RRT doses (20 mL kg−1 h−1) and requires limited consumption of substitution fluid compared to HVHF. The basic principles of the system are described in more detail in the “Methods” section below. This technique has been shown to be feasible and safe in an experimental porcine model of septic shock [12].
In the present study, we evaluated the hemodynamic and biological effects of the Cascade system in patients with septic shock and compared them to those of usual care.
Methods
Study design
This study was a multicenter, prospective, randomized, open-label trial conducted in three intensive care units (ICUs) that compared the Cascade system to usual care in patients with septic shock.
The study protocol was approved by the Ethics Committee of the Pitié-Salpêtrière Hospital, Paris, France and Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM). The study was registered with ClinicalTrials.gov under the number NCT00922870.
Study population
All patients diagnosed with either community-acquired or nosocomial septic shock, as defined by the American College of Chest Physicians/Society of Critical Care Medicine criteria [13], were screened. Inclusion criteria focused on two major points: septic shock diagnosed by the medical staff and administration of epinephrine and/or norepinephrine (namely above 0.27 μg kg−1 min−1) after adequate fluid resuscitation for more than 120 min but less than 24 h. To be included, patients had to be aged at least 18 and at most 85 years old with a real body weight below 120 kg, without contraindication to heparin coagulation, and without thrombocytopenia (below 50 × 109/L) or neutrophils below 0.5 × 109/L. Patients meeting these criteria were eligible for inclusion, irrespective of their renal function. Informed consent was obtained from either the patient or their next of kin before inclusion in the study.
Exclusion criteria were need for catecholamines for more than 24 h and admission to the ICU more than 7 days previously. Patients who could not be treated for 48 h or those with cardiac arrest without recovery of cardiac and neurological functions were not included. Patients with limited autonomy in daily life, those included in another study (within 28 days), pregnant patients, patients under legal guardianship, those treated for cancer or hematological malignancy, those treated with immunosuppressors or steroids (excluding hydrocortisone), and patients immunocompromized by immunological disease were also considered ineligible for this study.
End points
The primary end point was the number of catecholamine-free days (epinephrine or norepinephrine at any dose, dopamine at least 5 μg kg−1 min−1, dobutamine at least 5 μg kg−1 min−1) at day 28 after randomization. Secondary end points comprised the number of patients on norepinephrine and median dose of norepinephrine during the first 72 h, mean relative variation in catecholamine dose between 0 and 72 h [defined as (catecholamine dose at 72 h—catecholamine dose at 0 h)/catecholamine dose at 0 h)], the number of days without mechanical ventilation up to 90 days, without renal replacement therapy (RRT) (for acute renal failure) up to 90 days, and without ICU requirement up to 90 days, and survival at 72 h, and at 7, 28, and 90 days.
Randomization
Randomization was performed using a randomization table kept at the sponsor’s office with sealed envelopes sent to the study investigators containing the group to which the patient was assigned. Patients were assigned to either (1) usual care plus hemofiltration using the Cascade method or (2) usual care in accordance with current guidelines for the management of septic shock [14, 15].
Hemofiltration using the Cascade method
The Cascade system has been developed to perform very HVHF (120 mL kg−1 h−1) without the drawbacks of usual HVHF. The system is based on the use of two hemofilters with different cutoffs. The first hemofilter filters blood through a conventional membrane (cutoff in the range of 30–40 kDa). This first ultrafiltrate is refiltered through a second membrane with a lower cutoff (15 kDa). This second ultrafiltrate (100 mL kg−1 h−1) is reinjected into the blood circuit upstream of the first hemofilter. High and middle molecular weight molecules are retained by the second membrane and are concentrated in a limited volume of fluid effluent (20 mL kg−1 h−1) (Fig. 1). Depletion of low molecular weight molecules is thereby limited and consumption of substitution fluids is decreased. Cascade hemofiltration was started after a brief period of optimization and then continued for 48 h with a maximum of six circuit changes.
A commercial monitor (Prismaflex, Gambro Lundia AB, Sweden) was used with an external add-on device (Gambro Industries, France) to perform treatment. A more detailed description of the system is provided in the online supplement.
Usual care
Usual care was based on the recommendations of the Surviving Sepsis Campaign for severe sepsis, with some minor adaptations in order to standardize the clinical care given by the participating centers [14, 15]. There were no specific recommendations regarding the reasons to initiate RRT. Each center had the possibility to initiate hemofiltration according to usual practice, if required [14, 15]. Target mean arterial blood pressure was 65 mmHg or over [15].
Data collection
Senior physicians and research nurses in the participating ICUs collected data daily. Data were entered into a dedicated case-report form. The age and sex of each patient were recorded. The severity of illness was evaluated on the first ICU day using the Simplified Acute Physiology Score (SAPS III) [16] and during the first 24 h after onset of septic shock with the Sequential Organ Failure Assessment (SOFA) score [17]. Treatments in ICU were recorded.
Blood samples (4.5 mL, Barrier BD Vacutainer Systems, Beckton Dickinson, Plymouth, UK) were drawn at 0, 3, 6, 12, 24, 36, 48, and 72 h, centrifugated and cooled (4 °C), and stored at −20 °C for 4 h then at −80 °C for later analysis. For the Cascade group, the start of filtration treatment was designated 0 h, whereas for the control group, 0 h was defined as the time of randomization. Blood gases, lactates, bicarbonate, and electrolyte analyses were performed. In addition, for the Cascade group, samples of the effluent were also taken at 30 min and at 6, 12, 24, 36, and 48 h in order to identify which middle weight substances were eliminated by the Cascade filtration.
Cytokine measurements
The concentrations of IL-6, IL-8, IL-10, and TNF-alpha in the plasma samples were quantified using a Milliplex MAP Human Cytokine/Chemokine Panel (# HCYTOMAG-60 K, Millipore, Billerica, MA, USA). The assays were performed according to the manufacturer’s instructions. Standards and samples were analyzed on a LuminexR® apparatus (Bio-Plex 200, BioRad, München, Germany) using the BioPlex Manager Software (Version 5, BioRad, Hercules, CA, USA). Cytokines are expressed in picograms per milliliter.
Amino acid measurements
Free amino acid concentrations in plasma and hemofiltrate were determined using an automated amino acid analyzer, namely Jeol Aminotac 500 (Tokyo, Japan). The analysis was based on ion exchange chromatographic separation followed by ninhydrin detection (see supplementary online data for details). Data acquisition and calculations were made using the Jeol Workstation software. Amino acid results are expressed in micromoles per liter.
Adverse events (AE)
AE were recorded throughout the study and then summarized according to severity and resolution status. The number and percentage of patients experiencing at least one adverse event were analyzed. In any given category, subjects were only counted once. Safety was evaluated by recording the number of hypotension episodes (below 65 mmHg) per patient in each group; electrolyte imbalance, and course of plasma potassium, sodium, chloride, lactate, bicarbonate, creatinine, and albumin levels, and amino acids loss.
Statistical analysis
We calculated that 60 patients were needed to detect an improvement of 2 days of hemodynamic stability (catecholamine-free days) in the Cascade group, with α = 0.05 and β = 0.05, and assuming a standard deviation (SD) of 2 days for the primary end point. All analyses were performed according to the intention-to-treat principle.
Continuous variables are described as median with interquartile range [IQR] or as mean ± standard deviation when normally distributed. Categorical variables are described as number (percentage). Survival probabilities were described using the Kaplan–Meier method.
The Student’s t test or the non-parametric Mann–Whitney test was used for continuous variables and the χ 2 or Fisher’s exact tests for categorical variables, as appropriate. Variables with p < 0.15 by univariate analysis were included in the multivariable analyses, which was stratified by center.
Efficacy end points
The number of catecholamine-free days in the first 28 days following randomization was compared using a Mann–Whitney test. Adjustment for imbalanced covariates was performed using linear regression, after log-transformation of the dependant variable. In case of death, patients were considered to have received catecholamines between the date of death and day 28. The number of days without mechanical ventilation, without RRT, and without need for ICU up to 90 days were analyzed in the same way. For survival analysis, baseline date corresponded to the date of randomization. Patients alive at 90 days were censored. Survival probabilities were compared using the log-rank test. A Cox proportional hazards model was used to adjust the Cascade filtration effect for imbalanced characteristics. The risk of early death was analyzed using the Weibull function and the Peto–Turnbull test in order to take into account interval censored data (i.e., when the exact date of the event is not known, but is contained between two dates, i.e., within an interval). Kinetics of cytokines were described and compared between the two groups using appropriate tests.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). A p value less than 0.05 was considered statistically significant.
Results
Patient characteristics
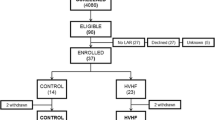
From May 2009 to November 2012, 60 patients were included in the study; 31 in the control group and 29 in the Cascade group. The baseline characteristics of the study population are shown in Table 1. The two groups were comparable in terms of age, gender, comorbidities, sepsis etiology, baseline SAPS III, and SOFA at admission (Table 1).
The median time between randomization and Cascade treatment initiation was 3.0 h [IQR 2.5–4.3]. Details of Cascade treatment administration are given in the supplementary online data appendix 1.
Primary end point
Overall, the median number of catecholamine-free days during the first 28 days was 28 days [IQR 2–25] (Table 2). There was no significant difference between the Cascade group (20 days [IQR 0–25]) and the control group (22 days [IQR 11–23]; p = 0.78) in bivariate analysis. Adjustment for SOFA, Gram-negative bacilli infection, and previous COPD did not change this result (data not shown).
Secondary outcomes
The median relative variation in catecholamine doses between 0 and 72 h did not differ significantly between treatment groups (0.85 [IQR −0.05 to 1.00] for control group and 0.83 [IQR 0–1.00] for the Cascade group, p = 0.69—supplementary file, appendices 2 and 3). This result was confirmed by multivariate analysis.
The median number of days without mechanical ventilation up to 90 days was 70 [IQR 0–85] and did not significantly differ between the control group (72 days [IQR 0–85]) and the Cascade group (68 days [IQR 16–83], p = 0.66) by multivariate analysis (Table 2).
Fourteen patients in the control group required RRT, and the median time to initiation of RRT was 3 h [IQR 3–15]. In the Cascade group, 14 patients required additional RRT after the end of treatment with the Cascade system, p < 0.0001. The median number of days without RRT up to 90 days was 84 days [IQR 1–90] and did not differ significantly between groups (74 days [IQR 0–90] vs 85 days [IQR 46–90] Control vs Cascade respectively, p = 0.42) in the bivariate model. By multivariate analysis, there was a significantly higher number of days without RRT in the Cascade group (22 days more without RRT in the Cascade group after adjustment for SOFA score, Gram-negative bacilli, and breathing disorders (22.33, 95 % CI 0.51–44.1, p = 0.045).
The number of days without need for ICU up to 90 days was 69 days [IQR 0–83] and did not differ significantly between groups (70 [IQR 0–82] vs 69 days [IQR 0–83], control vs Cascade, p = 0.92). Adjustment did not change this result.
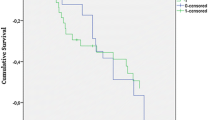
By multivariate analysis, after adjustment for SOFA score, Gram-negative bacilli, and breathing disorders, the risk of death at 72 h was significantly lower in the Cascade group compared to the control group [hazard ratio (HR) 0.22 (95 % CI 0.05–0.95), p = 0.043]. There was no significant difference in the rate of death at 7, 28, and 90 days between groups (Table 2; Fig. 2).
Tolerance and side effects
The median number of hypotensive episodes tended to be slightly higher in the Cascade group (median 2, IQR 1–4) than in control group (median 1, IQR 0–3; p = 0.05), but the difference was no longer significant by multivariable analysis. The doses of intravenous fluid therapy used did not significantly differ between groups (p = 0.60) and tended to decrease over time (p = 0.05). Among 35 adverse events, 34 (97.1 %) were not linked to treatment. There was no significant difference in the rate of events between groups (16 vs 18 for control versus Cascade group, p = 0.18). Details of the adverse events are given in the supplementary file, appendix 6.
Electrolyte depletion
Potassium, sodium, chlorides, bicarbonates, and albumin levels did not differ between the treatment groups (supplementary online data, appendix 4). Similarly, although cytokine levels (IL-6, IL-8, IL-10, TNFα) decreased significantly over time, there was no difference between groups, except for IL-8, which decreased more rapidly in the Cascade group (Fig. 3). Lastly, the relative variation of amino acids between 0 and 48 h did not significantly differ between groups, except for 3-methylhistidine (supplementary online data, appendix 5).
Discussion
In this prospective, multicenter, randomized, controlled phase 3 study, the use of very HVHF (120 mL kg−1 h−1) in patients presenting with septic shock did not reduce the length of use of catecholamines.
A recently published systematic review of the literature [18] included three randomized studies [19–21] that evaluated very HVHF (50–120 mL kg−1 h−1) in severe sepsis and septic shock patients with acute kidney failure. Two of these studies [19, 20] demonstrated results similar to ours. Conversely, Boussekey et al. [21] demonstrated a more rapid decrease in noradrenaline doses after 24 h of hemofiltration in the very HVHF group as compared to the low-volume hemofiltration group. However, the number of subjects included in the study was limited (n = 19).
Previous reports of animal models [10, 22–25] and clinical studies [8, 9, 26] showed predominantly positive results, but our study did not replicate these findings. This discrepancy may be explained by the use of different hemofiltration techniques, as well as heterogeneity between the populations included.
In this study, cytokine levels (IL-6, IL-8, IL-10, TNFα) decreased significantly over time in both groups independently of treatment group (except for IL-8). Previous studies have shown an early reduction in cytokine levels after initiation of hemofiltration, followed by a return to baseline levels within hours [27, 28]. To explain this result, Honoré’s “threshold immunomodulation hypothesis” proposes a more dynamic view, whereby cytokine removal from the blood compartment leads to removal of cytokines at the tissue level through equilibration of their concentrations between these two compartments [29]. This theory is interesting because it affects cytokines at the tissue level, which is where cytokines may mediate a harmful effect. It also explains why several studies assessing blood purification techniques have observed improved outcomes without modification of cytokine blood concentrations, since cytokines from the tissues replace those removed from the blood [30]. Other authors have put forward alternative explanations for the lack of effect on cytokine levels. For example, it has been hypothesized that tempering the cytokine peak by removing the cytokines from the blood during the early phase of sepsis could reduce inflammation, limit organ damage, and as a result decrease the incidence of multiorgan failure syndrome. This is the so-called peak concentration hypothesis advanced by Ronco and Bellomo [31].
In contrast, an older study conducted by Journois et al. in children undergoing cardiac surgery showed a reduction of cytokine plasma levels with high volume hemofiltration (100 mL kg−1 h−1) [8]. Among the secondary objectives, only the number of days of RRT (from randomization up to 90 days) was significantly reduced in a multivariate model integrating SOFA score, presence of Gram-negative bacilli infections, and respiratory disorders. During sepsis, inflammatory mediators derived from pathogens and activated immune cells alert the immune system, and this may induce the inflammation cascade [32]. Our working hypothesis was that the Cascade system could limit the effects of inflammation and its deleterious consequences at the systemic level.
The Cascade system did not influence mortality at 7, 28, or 90 days. Previous prospective, controlled, and randomized studies in severe sepsis and septic shock patients that evaluated HVHF and very HVHF failed to show a statistically significant reduction in mortality and dialysis dependence at discharge from ICU [19–21]. However, there were several differences between these studies and our study reported here. Firstly, in the previous reports, the control group underwent hemofiltration at doses of 25 and 30 mL kg−1 h−1 because all patients included presented with acute kidney injury. Secondly, the prescribed effluent rate was 55–85 mL kg−1 h−1, and two of the studies were single-center studies [19, 21]. Lastly, no specific dosages of small molecules were performed, except in the IVOIRE study, where electrolytes, antibiotics, and amino acids were dosed [20]. Nonetheless, despite being uncontrolled and thus limited in their ability to draw strong conclusions, at least six studies have reported significant reductions in mortality rates with HVHF compared to predicted mortality [33–38].
Our study showed that Cascade hemofiltration did not lead to significant loss of amino acids (among the 26 dosed) compared to the control group (except for 3-methylhistidine). Furthermore, natremia, kalemia, chloremia, and albuminemia were not significantly affected by the Cascade system. This is the only study in recent years to dose electrolytes and amino acids [19–21].
We also failed to observed a significant difference in the number of hypotension episodes or in the quantity of vascular filling used between the two groups, contrary to the findings of the VA/NIH study [39], where the intensive RRT group demonstrated significantly more hypotension and the need for more catecholamines.
This study has several strongpoints, including a homogenous population of septic shock patients, absence of serious events, and absence of electrolyte disorders and amino acid loss. However, it also has some limitations. Firstly, this study has a limited sample size (n = 60) and likely suffered from a lack of power. Indeed, the sample size for the study was calculated on the basis of an expected standard deviation of 2 days in the duration of catecholamine treatment. However, this was substantially lower than what the actual standard deviation turned out to be (9 days in the Cascade group and 11 in the control group), resulting in a loss of power. Secondly, the open-label design is associated with inherent bias, since the patients’ treatment status was known. In addition, there was no standardized protocol for catecholamine withdrawal or vasopressor titration (other than MAP greater than 65 mmHg). Furthermore, there were no standardized indications for initiation of RRT, except the usual indications for emergency situations, namely hyperkalemia greater than 6.5 mmol/L, metabolic acidosis with pH below 7.15 and acute pulmonary edema. In the control group, patients underwent RRT with the usual systems used in the participating center’s routine practice. Third, no systematic dosage of antibiotics was performed. Fourth, only a selected panel of cytokines were dosed. Lastly, acute renal failure could not be taken into account in the multivariable analysis, since hemofiltration was initiated immediately in the Cascade group.
Conclusion
Very HVHF using the Cascade system can be safely used to purify blood in patients presenting with septic shock, but it was not associated in our study with a reduction in the need for catecholamines during the first 28 days of septic shock. Further development and investigations are warranted to confirm its efficiency in reducing the inflammatory process.
References
Adrie C, Alberti C, Chaix-Couturier C, Azoulay E, De Lassence A, Cohen Y, Meshaka P, Cheval C, Thuong M, Troche G, Garrouste-Orgeas M, Timsit JF (2005) Epidemiology and economic evaluation of severe sepsis in France: age, severity, infection site, and place of acquisition (community, hospital, or intensive care unit) as determinants of workload and cost. J Crit Care 20:46–58
Annane D, Bellissant E, Cavaillon JM (2005) Septic shock. Lancet 365:63–78
Quenot JP, Binquet C, Kara F, Martinet O, Ganster F, Navellou JC, Castelain V, Barraud D, Cousson J, Louis G, Perez P, Kuteifan K, Noirot A, Badie J, Mezher C, Lessire H, Pavon A (2013) The epidemiology of septic shock in French intensive care units: the prospective multicenter cohort EPISS study. Crit Care 17:R65
Russell JA (2006) Management of sepsis. N Engl J Med 355:1699–1713
Tolwani A (2012) Continuous renal-replacement therapy for acute kidney injury. N Engl J Med 367:2505–2514
Payen D, Mateo J, Cavaillon JM, Fraisse F, Floriot C, Vicaut E (2009) Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit Care Med 37:803–810
Honore PM, Jacobs R, Boer W, Joannes-Boyau O, De Regt J, De Waele E, Van Gorp V, Collin V, Spapen HD (2012) New insights regarding rationale, therapeutic target and dose of hemofiltration and hybrid therapies in septic acute kidney injury. Blood Purif 33:44–51
Journois D, Israel-Biet D, Pouard P, Rolland B, Silvester W, Vouhe P, Safran D (1996) High-volume, zero-balanced hemofiltration to reduce delayed inflammatory response to cardiopulmonary bypass in children. Anesthesiology 85:965–976
Laurent I, Adrie C, Vinsonneau C, Cariou A, Chiche JD, Ohanessian A, Spaulding C, Carli P, Dhainaut JF, Monchi M (2005) High-volume hemofiltration after out-of-hospital cardiac arrest: a randomized study. J Am Coll Cardiol 46:432–437
Grootendorst AF, van Bommel EF, van der Hoven B, van Leengoed LA, van Osta AL (1992) High volume hemofiltration improves right ventricular function in endotoxin-induced shock in the pig. Intensive Care Med 18:235–240
Honore PM, Joannes-Boyau O, Boer W, Collin V (2009) High-volume hemofiltration in sepsis and SIRS: current concepts and future prospects. Blood Purif 28:1–11
Rimmele T, Wey PF, Bernard N, Monchi M, Semenzato N, Benatir F, Boselli E, Etienne J, Goudable J, Chassard D, Bricca G, Allaouchiche B (2009) Hemofiltration with the Cascade system in an experimental porcine model of septic shock. Ther Apher Dial 13:63–70
(1992) American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864874
Brochard L, Abroug F, Brenner M, Broccard AF, Danner RL, Ferrer M, Laghi F, Magder S, Papazian L, Pelosi P, Polderman KH (2010) An official ATS/ERS/ESICM/SCCM/SRLF statement: prevention and management of acute renal failure in the ICU patient: an international consensus conference in intensive care medicine. Am J Respir Crit Care Med 181:1128–1155
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR (2005) SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 31:1345–1355
Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26:1793–1800
Clark E, Molnar AO, Joannes-Boyau O, Honore PM, Sikora L, Bagshaw SM (2014) High-volume hemofiltration for septic acute kidney injury: a systematic review and meta-analysis. Crit Care 18:R7
Zhang P, Yang Y, Lv R, Zhang Y, Xie W, Chen J (2012) Effect of the intensity of continuous renal replacement therapy in patients with sepsis and acute kidney injury: a single-center randomized clinical trial. Nephrol Dial Transplant 27:967–973
Joannes-Boyau O, Honore PM, Perez P, Bagshaw SM, Grand H, Canivet JL, Dewitte A, Flamens C, Pujol W, Grandoulier AS, Fleureau C, Jacobs R, Broux C, Floch H, Branchard O, Franck S, Roze H, Collin V, Boer W, Calderon J, Gauche B, Spapen HD, Janvier G, Ouattara A (2013) High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): a multicentre randomized controlled trial. Intensive Care Med 39:1535–1546
Boussekey N, Chiche A, Faure K, Devos P, Guery B, d’Escrivan T, Georges H, Leroy O (2008) A pilot randomized study comparing high and low volume hemofiltration on vasopressor use in septic shock. Intensive Care Med 34:1646–1653
Yekebas EF, Eisenberger CF, Ohnesorge H, Saalmuller A, Elsner HA, Engelhardt M, Gillesen A, Meins J, The M, Strate T, Busch C, Knoefel WT, Bloechle C, Izbicki JR (2001) Attenuation of sepsis-related immunoparalysis by continuous veno-venous hemofiltration in experimental porcine pancreatitis. Crit Care Med 29:1423–1430
Bellomo R, Kellum JA, Gandhi CR, Pinsky MR, Ondulik B (2000) The effect of intensive plasma water exchange by hemofiltration on hemodynamics and soluble mediators in canine endotoxemia. Am J Respir Crit Care Med 161:1429–1436
Wang H, Zhang ZH, Yan XW, Li WQ, Ji DX, Quan ZF, Gong DH, Li N, Li JS (2005) Amelioration of hemodynamics and oxygen metabolism by continuous venovenous hemofiltration in experimental porcine pancreatitis. World J Gastroenterol 11:127–131
Rogiers P, Zhang H, Pauwels D, Vincent JL (2003) Comparison of polyacrylonitrile (AN69) and polysulphone membrane during hemofiltration in canine endotoxic shock. Crit Care Med 31:1219–1225
Cole L, Bellomo R, Hart G, Journois D, Davenport P, Tipping P, Ronco C (2002) A phase II randomized, controlled trial of continuous hemofiltration in sepsis. Crit Care Med 30:100–106
Bellomo R, Tipping P, Boyce N (1991) Tumor necrosis factor clearances during veno-venous hemodiafiltration in the critically ill. ASAIO Trans 37:M322–M323
Bellomo R, Tipping P, Boyce N (1993) Continuous veno-venous hemofiltration with dialysis removes cytokines from the circulation of septic patients. Crit Care Med 21:522–526
Honoré PM, Joannes-Boyau O, Gressens B (2007) Blood and plasma treatments: the rationale of high-volume hemofiltration. Contrib Nephrol 156:387–395
Klouche K, Cavadore P, Portales P, Clot J, Canaud B, Beraud JJ (2002) Continuous veno-venous hemofiltration improves hemodynamics in septic shock with acute renal failure without modifying TNFalpha and IL6 plasma concentrations. J Nephrol 15:150–157
Ronco C, Tetta C, Mariano F, Wratten ML, Bonello M, Bordoni V, Cardona X, Inguaggiato P, Pilotto L, d’Intini V, Bellomo R (2003) Interpreting the mechanisms of continuous renal replacement therapy in sepsis: the peak concentration hypothesis. Artif Organ 27:792–801
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150
Cornejo R, Downey P, Castro R, Romero C, Regueira T, Vega J, Castillo L, Andresen M, Dougnac A, Bugedo G, Hernandez G (2006) High-volume hemofiltration as salvage therapy in severe hyperdynamic septic shock. Intensive Care Med 32:713–722
Honore PM, Jamez J, Wauthier M, Lee PA, Dugernier T, Pirenne B, Hanique G, Matson JR (2000) Prospective evaluation of short-term, high-volume isovolemic hemofiltration on the hemodynamic course and outcome in patients with intractable circulatory failure resulting from septic shock. Crit Care Med 28:3581–3587
Joannes-Boyau O, Rapaport S, Bazin R, Fleureau C, Janvier G (2004) Impact of high volume hemofiltration on hemodynamic disturbance and outcome during septic shock. ASAIO J 50:102–109
Oudemans-van Straaten HM, Bosman RJ, van der Spoel JI, Zandstra DF (1999) Outcome of critically ill patients treated with intermittent high-volume haemofiltration: a prospective cohort analysis. Intensive Care Med 25:814–821
Piccinni P, Dan M, Barbacini S, Carraro R, Lieta E, Marafon S, Zamperetti N, Brendolan A, D’Intini V, Tetta C, Bellomo R, Ronco C (2006) Early isovolaemic haemofiltration in oliguric patients with septic shock. Intensive Care Med 32:80–86
Ratanarat R, Brendolan A, Piccinni P, Dan M, Salvatori G, Ricci Z, Ronco C (2005) Pulse high-volume haemofiltration for treatment of severe sepsis: effects on hemodynamics and survival. Crit Care 9:R294–R302
Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P (2008) Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359:7–20
Acknowledgments
We thank all the following contributors to the study: Dijon University Hospital (Elisabeth Cornot and Thérèse Devaux as well as the nursing team); Clermont-Ferrand University Hospital (Mireille Adda and the nursing team); Melun Hospital (Cécile Lormail and the nursing team); and the Gambro study team (Nicolas Semenzato, Nathalie Loughraieb and Hiram Rada). The authors also thank Fiona Ecarnot (EA3920, University Hospital Besancon, France) for translation and editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors of this paper have no conflicts of interest to report.
Funding
This study was sponsored by Gambro Industries, Meyzieu, France.
Additional information
For the Cascade study group.
Take-home message: Very high volume hemofiltration using the Cascade system can safely be used in patients presenting with septic shock, but it is not associated with a reduction in the need for catecholamines during the first 28 days.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Quenot, JP., Binquet, C., Vinsonneau, C. et al. Very high volume hemofiltration with the Cascade system in septic shock patients. Intensive Care Med 41, 2111–2120 (2015). https://doi.org/10.1007/s00134-015-4056-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-4056-y