Abstract
Importance
Acute kidney injury (AKI) is characterized by severe loss of glomerular filtration rate (GFR) and is associated with a prolonged intensive care unit (ICU) stay and increased risk of death. No interventions have yet been shown to prevent AKI or preserve GFR in critically ill patients. Evidence from mammalian physiology and small clinical trials suggests higher amino acid intake may protect the kidney from ischemic insults and thus may preserve GFR during critical illness.
Objective
To determine whether amino acid therapy, achieved through daily intravenous (IV) supplementation with standard amino acids, preserves kidney function in critically ill patients.
Design, setting, and participants
Multicenter, phase II, randomized clinical trial conducted between December 2010 and February 2013 in the ICUs of 16 community and tertiary hospitals in Australia and New Zealand. Participants were adult critically ill patients expected to remain in the study ICU for longer than 2 days.
Interventions
Random allocation to receive a daily supplement of up to 100 g of IV amino acids or standard care.
Main outcomes and measures
Duration of renal dysfunction (primary outcome); estimated GFR (eGFR) derived from creatinine; eGFR derived from cystatin C; urinary output; renal replacement therapy (RRT) use; fluid balance and other measures of renal function.
Results
474 patients were enrolled and randomized (235 to standard care, 239 to IV amino acid therapy). At time of enrollment, patients allocated to receive amino acid therapy had higher APACHE II scores (20.2 ± 6.8 vs. 21.7 ± 7.6, P = 0.02) and more patients had pre-existing renal dysfunction (29/235 vs. 44/239, P = 0.07). Duration of renal dysfunction after enrollment did not differ between groups (mean difference 0.21 AKI days per 10 patient ICU days, 95 % CI −0.27 to 1.04, P = 0.45). Amino acid therapy significantly improved eGFR (treatment group × time interaction, P = 0.004), with an early peak difference of 7.7 mL/min/1.73 m2 (95 % CI 1.0–14.5 mL/min/1.73 m2, P = 0.02) on study day 4. Daily urine output was also significantly increased (+300 mL/day, 95 % CI 145–455 mL, P = 0.0002). There was a trend towards increased RRT use in patients receiving amino acid therapy (13/235 vs. 25/239, P = 0.062); however, this trend was not present after controlling for baseline imbalance (P = 0.21).
Conclusion and relevance
Treatment with a daily IV supplement of standard amino acids did not alter our primary outcome, duration of renal dysfunction.
Trial registration
anzctr.org.au Identifier: ACTRN12609001015235.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately one-third of all critically ill patients develop acute kidney injury (AKI) during their stay in the intensive care unit (ICU) [1]. Onset of AKI is associated with a prolonged need for intensive care [2], an increased risk of developing chronic kidney disease after ICU discharge, accelerated progression to end-stage kidney disease [3], and an increased risk of short- and long-term mortality [4].
In critically ill humans, AKI is characterized by markedly decreased glomerular filtration rate (GFR) arising from ischemia due to either a global reduction in renal blood flow [5] or local microcirculatory changes within the functional units of the kidney [6]. Agents with selective effects on local blood flow within the kidney, such as dopamine and fenoldopam, have been evaluated in major clinical trials and have not been effective at preserving GFR in critical illness [6, 7].
In the healthy adult, a high protein meal is known to enhance GFR [8, 9] mediated by a global increase in renal blood flow resulting from afferent arteriolar dilation [10]. Although it is accepted that patients with chronic kidney disease (CKD) may progress to advanced stages of CKD more rapidly if they consume a high-protein diet over a prolonged period of time, animal models have demonstrated that an increase in renal blood flow in response to a short-term amino acid infusion can protect the kidney from acute ischemic insults [11]. These nephro-protective effects may be preserved in critical illness.
Published in 1973, a 53-patient clinical trial conducted in critically ill patients demonstrated that a short-term infusion of amino acids led to faster recovery from severe acute renal failure [12]. In addition, a 14-patient clinical trial published in 2007 enrolled critically ill patients with creatinine clearance below 50 mL/min and showed that patients randomized to intravenously receive a short-term higher dose of amino acids were more likely to preserve diuresis and required less furosemide to achieve negative fluid balance [13]. Furthermore, hypothesis-generating subgroup analysis of a cluster randomized controlled trial (RCT) evaluating nutrition guidelines [14] identified 242 critically ill patients at high risk of renal dysfunction at study entry, and found that patients randomized to receive higher daily protein intake were significantly less likely to require RRT [15].
Accordingly, we performed a phase II RCT to investigate the impact of providing a short-term daily intravenous supplement of standard amino acids on kidney function in critical illness, compared to standard care.
Methods
Adult patients were eligible for enrollment on day 1 or 2 of ICU stay if they were expected to remain in ICU at least 2 days after enrollment, had central venous access through which the study intervention could be delivered and were not fluid restricted to less than 1 L/day. See Online Supplement for complete eligibility criteria. Approval was obtained from each participating site’s Human Research Ethics Committee. Patient consent was obtained in accordance with local and national laws.
Allocation concealment was maintained by use of a central randomization Web server. The sequence was generated using blocks of variable size with random seeds [16] and stratified within study site by high risk of renal dysfunction and body mass index (BMI) greater than 18 kg/m2. High risk of renal dysfunction was defined using the APACHE II variable: creatinine increase over the previous 24 h by at least 20 % to over 120 µmol/L [17].
Investigators were required to attend a small-group start-up meeting and complete a formal study run-in phase to become familiar with the application of eligibility criteria and the dosing algorithm prior to recruiting their first patient [18].
Interventions
A continuous infusion of a standard mixture of 100 g/L of l-amino acids (Synthamin 17 Electrolyte Free, Baxter Healthcare, Australia) provided a maximum supplement of 100 g of amino acids per day. On the basis of the patient’s ideal body weight and protein intake from other standard nutrition sources, the infusion of amino acids was reduced such that a maximum total daily protein intake of 2.0 g/kg/day was achieved. The study intervention was continued until the patient was discharged from ICU. The study intervention was not blinded. Complete details of the dosing algorithm are provided in the Online Supplement.
Standard care was defined pragmatically. In both the study intervention and the standard care group, the attending ICU clinician selected the route, starting rate, metabolic targets and composition of nutrition to be provided to patients on the basis of current practice in their ICU, independent of the study intervention.
Outcomes
The primary outcome was duration of renal dysfunction, adjusted for time at risk (ICU stay). Renal dysfunction was defined using the validated threshold for clinically significant kidney dysfunction determined by the Brussels table (creatinine >168 μmol/L) [19].
Secondary outcomes consisted of additional measures of renal function, including use of RRT and eGFR estimated from serum creatinine using the equations developed by Levey et al. [20] and eGFR estimated from serum cystatin C using the equations developed by Stevens et al. [21]. Tertiary outcomes included vital status at study day 90; Zubrod/WHO Performance Status [22]; RAND-36 General Health Status Ver 1; and RAND-36 Physical Function scale Ver 1 [23] and other measures of in-hospital care (ICU stay, hospital stay, mechanical ventilation days, organ dysfunctions [19] etc.).
Creatinine and cystatin C assays
Creatinine was assayed at each participating site’s clinical laboratory using the standard Jaffe method. The assay has a coefficient of variation (CV) of 2.7 % at 70 μmol/L and 1.7 % at 540 μmol/L.
Serum cystatin C was assayed at a central clinical laboratory using the particle enhanced tubidometric immunoassay on the Abbot Architect chemistry analyzer. The cystatin C assay has a CV of 3.5 % at 0.34 mg/L and 3.6 % at 0.58 mg/L. To reduce sample collection, handling and processing costs, a skip pattern was used to optimize information gain: samples for cystatin C assay were obtained every second day for the first week of ICU stay and every third day thereafter, up to study day 16. This skip pattern was developed by evaluating the timing of onset of AKI in patients enrolled into a published clinical trial [14].
Interim analysis
The independent Safety and Data Monitoring Committee (SDMC) was required to conduct interim analysis using Haybittle–Peto stopping rules [24, 25] if reporting of serious adverse events generated concerns for patient safety.
Sample size, power, and statistical analysis
Sample size estimation calculated that 474 patients would be required to provide 90 % power to detect a reduction in duration of 0.54 days of renal dysfunction per 10 patient ICU days, assuming a variance of 3.31 [26]. Estimates of the potential treatment effect and variance were obtained from a previous publication demonstrating a reduction in duration of renal dysfunction attributable to improved protein intake [14].
A detailed intention to treat analysis plan was published prior to completion of recruitment [26].
Crude (unadjusted) analysis of the effect of treatment on the primary outcome, and all outcomes based on count data (e.g., length of stay, days of clinically significant organ failure etc.), was conducted using Poisson regression. If the scaled deviance exceeded 1.4 units per degree of freedom, a conservative negative-binomial model was employed. For the primary outcome an offset term (ICU length of stay) was used to account for time at risk. Other count outcomes were adjusted for time at risk where appropriate.
Measures of renal function over time were assessed using a fully factorial nested analysis of variance (ANOVA). Dichotomous outcomes were assessed using an exact Pearson chi-squared test, with unconditional exact 95 % CIs calculated around the risk difference (RD).
A prespecified algorithm was used to identify baseline characteristics for inclusion in a covariate adjusted regression model to control for confounding [26].
Two a priori defined subgroup analyses were conducted based on BMI (≤18 kg/m2) and high risk of renal dysfunction at time of enrollment. If the two-sided P value for a formal test of the subgroup × treatment interaction was less than 0.10, differential treatment effects within subgroups were declared to be present [26].
Missing data was accepted to be missing at random unless prespecified thresholds were exceeded [26].
Two-sided P value less than 0.05 was accepted to indicate statistically significant results. All analyses were conducted in SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
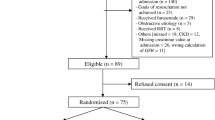
From 2 December 2010 to 26 February 2013, 474 adult critically ill patients were enrolled from 16 participating hospitals throughout Australia and New Zealand. Two patients with short stays (1 and 2 days each) did not have creatinine assays conducted, thus 472 enrolled patients were included in the analysis of the primary outcome. These two patients, who were both allocated to the study intervention group, were eligible for inclusion in analyses of all other outcomes. Figure 1 presents a CONSORT 2010 patient flow diagram.
The mean age of enrolled patients was 63.0 years (SD 16.0) and 35 % (169/474) were female. At enrollment, the mean APACHE II score was 21.0 (SD 7.3) with 82.5 % (391/474) of patients requiring mechanical ventilation. Baseline eGFR was 67.1 mL/min/1.73 m2 (SD 31.0). Table 1 presents population descriptors by study group.
Measures of study conduct
Twenty-one per cent (101/474) of patients were enrolled into the trial on their first day of ICU stay with the remaining 79 % (373/474) enrolled during their second ICU day. All 239 patients randomized to the amino acid supplementation group received the study intervention. At least 80 % of the appropriate study amino acid dose was provided on 99.4 % (2248/2262) of eligible ICU days. There were significant differences in total protein intake (sum of protein from enteral nutrition, parenteral nutrition and study amino acids) between the two study groups on each of the first 7 days of ICU stay (eFig. 1 in the Electronic Supplementary Material).
One serious adverse event (SAE) was reported. The case was reviewed by the Chair of the SDMC and was not considered to be related to the study intervention.
Baseline characteristics
There was imbalance between study groups with regards to higher APACHE II severity of illness scores (20.2 ± 6.8 vs. 21.7 ± 7.6, P = 0.02) and more patients with pre-existing renal dysfunction (29/235 vs. 44/239 patients, P = 0.07) in the study intervention group. See Table 1 for complete details.
Primary and secondary outcomes: measures of renal function
The mean duration of renal dysfunction in standard care patients was 0.99 days per 10 patient ICU days [95 % confidence interval (CI) 0.76–1.28] compared to 1.20 days per 10 patient ICU days (95 % CI 0.93–1.56) in patients receiving the study intervention. Crude (unadjusted) analysis revealed that there was no significant difference between groups: mean difference 0.21 renal dysfunction days per 10 patient ICU days, 95 % CI −0.27 to 1.04, P = 0.45.
Covariate adjusted analysis was undertaken according to a prespecified algorithm [26]. After assessing all potential confounders, there was no difference between groups with regards to duration of renal dysfunction (RR 1.07, 95 % CI 0.69–1.64, P = 0.78). The covariate adjusted model controlled for BMI, mechanical ventilation, baseline renal dysfunction, serum urea, vasoactive drugs, high risk of renal dysfunction and trauma admission.
Patients allocated to the amino acid supplement group received significantly more fluid (+380 mL/day, 95 % CI 221–539, P < 0.0001) and had significantly higher urine output (+300 mL/day, 95 % CI 145–455, P = 0.0002), resulting in no difference in daily net fluid balance (+83 mL/day, 95 % CI −92 to 259, P = 0.35).
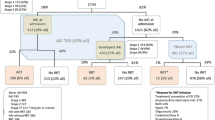
Using estimates of GFR from serum creatinine [20], fully factorial repeated measures ANOVA demonstrated a significant difference in treatment effects over time (treatment group × time interaction P = 0.004) attributable to the amino acid supplement, with a statistically significant early peak difference of 7.7 mL/min/1.73 m2 (95 % CI 1.0–14.5 mL/min/1.73 m2, P = 0.02) on study day 4 (Fig. 2). Estimation of GFR from serum cystatin C [21] confirmed the presence of this effect attributable to the amino acid supplement (treatment group × time interaction P = 0.097), with a peak difference between groups of 5.4 mL/min/1.73 m2 (95 % CI −2.7 to 13.4 mL/min/1.73 m2, P = 0.19) on study day 7.
Estimated glomerular filtration rate (CKD-EPIcreatinine) by day, post-randomization. CKD-EPIcreatinine was estimated from creatinine using the equations developed by Levey et al. [20]. ICU intensive care unit. P = 0.004 for treatment × time interaction from repeated measures ANOVA. Error bars indicate 95 % confidence intervals around differences between groups at each time point
There were no significant differences in any other a priori defined measures of renal function (Table 2); however, on univariate analysis there was a trend towards more patients allocated to the amino acid supplement group receiving RRT (13/235 standard care vs. 25/239, P = 0.062).
Tertiary outcomes
The average ICU stay was 11.2 days (SD 10.8) and the average hospital stay was 25.4 days (SD 24.0). Eighty-three per cent (394/474) of patients survived to hospital discharge. Except for a significantly shorter duration of respiratory failure in patients receiving the amino acid supplement (8.6 vs. 8.0 failure days per 10 patient ICU days, P = 0.042), there were no significant differences between groups with regards to duration of mechanical ventilation, other measures of organ dysfunction, mortality, length of ICU or hospital stay, or other tertiary outcomes. Tables 3 and 4 provide complete results.
Subgroup analyses
With regards to the primary outcome, duration of renal dysfunction, there were no differential treatment effects across a priori defined subgroups: BMI ≤ 18 kg/m2 vs. BMI > 18 kg/m2, P = 0.49; and high risk of renal dysfunction at time of enrollment vs. not high risk, P = 0.61.
Post hoc evaluation of patients receiving RRT
Controlling for baseline imbalance in severity of illness (APACHE II score) and presence of renal dysfunction at study baseline, covariate adjusted logistic regression found no evidence of a difference in RRT rates between groups (P = 0.21).
At time of initiation of RRT, serum urea was significantly higher in patients randomized to receive the amino acid supplement (21.4 ± 7.0 vs. 29.9 ± 13.5 mmol/L, P = 0.02) and urine output was also significantly greater (285 ± 107 vs. 1291 ± 830 mL/24 h, P = 0.002). There was no significant difference between groups with regards to creatinine levels at initiation of RRT (359 ± 169 vs. 278 ± 123 mmol/L, P = 0.11). eTable 1 in the Electronic Supplementary Material presents patient characteristics and biochemistry at initiation of RRT for all 38 patients who received RRT during the study.
On assessment of serum urea levels for all patients enrolled into the trial, fully factorial repeated measures ANOVA revealed a significant difference between groups (10.9 ± 6.3 vs. 15.0 ± 9.3 mmol/L, P < 0.0001) and a significant treatment group × time interaction (P < 0.0001), with higher serum urea in the group receiving the amino acid supplement. eFigure 2 in the Electronic Supplementary Material presents additional details regarding daily serum urea levels for all enrolled patients.
Post hoc hypothesis-generating subgroup analysis
Upon review, we were required to investigate the onset of new renal dysfunction in the subgroup of 401 patients who did not have renal dysfunction at time of study enrollment. There were no significant differences between randomized groups with regards to the number of patients who developed new onset renal dysfunction (31/206 vs. 25/195, P = 0.57) or the duration of new onset renal dysfunction: 0.57 days per 10 patient ICU days (95 % CI 0.40–0.82) compared to 0.53 days per 10 patient ICU days (95 % CI 0.37–0.76), P = 0.85.
Discussion
The provision of a daily intravenous supplement of up to 100 g amino acids during ICU stay did not alter our primary outcome, duration of renal dysfunction, but the study intervention did improve eGFR and increased urine output. Serum urea was significantly increased and there was a trend towards increased use of RRT in patients receiving the amino acid supplement. This suggestion of increased RRT was, in part, attributable to baseline imbalance of key prognostic factors. Furthermore, on the basis of a clinical assessment of patients’ biochemistry at time of onset of RRT, we conclude that the increase in serum urea was attributable to increased protein intake as directed by the study protocol, and not as a result of a decrease in renal function.
Effects on renal function
By itself, creatinine provides only a crude reflection of a patient’s renal function, with measured GFR accepted to be the best single index of renal function in health and disease [20]. We were not able to measure GFR in this multicenter clinical trial of critically ill patients. However, GFR was estimated from creatinine and from cystatin C using widely accepted predictive equations [20, 21], which are known to perform acceptably in critical illness [27]: estimates of GFR obtained from creatinine are accepted to be unbiased in critically ill patients with a measured GFR greater than 90 mL/min/1.73 m2; whereas estimates of GFR obtained from cystatin C are accepted to be unbiased in critically ill patients with measured GFR less than 90 mL/min/1.73 m2 [28]. On the basis of both creatinine and cystatin C derived estimates of GFR, the critically ill patients recruited into our clinical trial revealed an effect on renal functional reserve remarkably similar to healthy adults.
Although laboratory studies demonstrate that GFR may increase by 30–60 % after a large bolus dose of protein (10 g/kg) [29], studies with longer follow-up demonstrate more modest effects from increasing daily protein intake. Relative to a normal diet, the OmniHeart Trial demonstrated that healthy adults were able to significantly improve eGFR by 4 mL/min/1.73 m2 over a 6-week period in response to increasing protein in their daily diet [30]. Our critically ill patients increased eGFR by 7.7 mL/min/1.73 m2 in response to amino acids. Furthermore, the amino acid supplement led to a significant increase in urine output, to compensate for increased total fluid intake.
Previous large scale clinical trials have demonstrated that an increase in fluid intake is not associated with a compensatory increase in urine output, leading to significant positive fluid balances in general critically ill patients [31] and in critically ill patients with acute lung injury [32]. Thus, amino acid infusion appears to have preserved a unique diuretic effect, as shown in another small clinical trial conducted in 14 critically ill patients with non-oliguric renal failure [13].
We interpret the improved eGFR and the increased urine output as suggesting that the hyperfiltration response to amino acids may be preserved in general critically ill patients [8]. Although we did not measure renal blood flow in this multicenter clinical trial, the most likely mechanism of action explaining the observed effects is through maintaining or increasing renal perfusion [10].
This phase II study focused on renal physiological effects and was not powered to detect differences in patient-centered clinical outcomes. Additional studies, conducted in focused patient groups demonstrating maximum benefits, are needed to investigate whether the physiological effects observed in this study translate to improvements in patient-centered clinical outcomes.
Protein intake and RRT
Elevated urea levels in critically ill patients receiving total protein intakes of 2 g/kg ideal body weight per day or higher are expected. Previous research has reported that elevated urea levels due to higher total protein intakes are safe [33]. However, in some patients, a higher degree of uremia may trigger initiation of RRT despite the absence of other indications. This may have happened in our patients. In support of this notion, at the time of RRT start, patients randomized to amino acid therapy had significantly greater overall urinary output, lower potassium levels, less incidence of oliguria, lower serum creatinine, and no difference in pH. Moreover, it is important to consider that there was a prima facie baseline imbalance in severity of illness (APACHE II score) and baseline renal function between the two treatment groups [34]. After statistical control for these baseline imbalances, there was no suggestion of a difference in RRT rates between groups. Furthermore, if we had protocolized the initiation of RRT in both groups, we would have addressed the issue of increasing urea by recommending a reduction in protein intake, which may subsequently have reduced the use of RRT.
Strengths and limitations
This study has several strengths. To our knowledge this is the first multicenter randomized controlled trial to show a clear simultaneous physiological effect of an intervention on both eGFR and urinary output in critically ill patients. The robustness of these effects is supported by their confirmation with cystatin C levels, their biological plausibility, and their similarity with observations in healthy subjects. Internal validity is supported by the rigor of the study design and execution while external validity is supported by conduct at multiple sites with broad patient inclusion characteristics.
Duration of renal dysfunction was defined using a creatinine threshold that is widely accepted in the critical care literature [19]. Although the interpretation of relative changes in creatinine to define AKI is gaining popularity [35], pre-critical illness creatinine levels are unavailable in 45 % of ICU patients enrolled into clinical trials [36]. With a large proportion of missing information, the standard approach of imputing ‘normal’ pre-disease creatinine values [1] introduces significant uncertainty into a clinical trial [3]. Use of a creatinine threshold, combined with other measures of renal function, overcomes this potential source of bias due to excessive missing values.
As a result of costs, the study intervention was not blinded, which does increase the potential for bias. However, conduct across 16 sites and use of an objective primary outcome (creatinine levels) helps reduce the possibility of major bias.
Conclusions
We conducted a phase II multicenter clinical trial involving 474 critically ill patients to investigate the physiological effects on renal function of IV amino acid therapy. Although IV amino acid supplementation did not alter our primary outcome, duration of renal dysfunction, it did increase eGFR and urine output. These physiological effects suggest the existence of renal functional reserve in critical illness and justify further investigations of this treatment in targeted high-risk populations.
References
Bagshaw SM, George C, Dinu I, Bellomo R (2008) A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transpl 23:1203–1210
Bagshaw SM, George C, Bellomo R (2008) Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care 12:R47
Rewa O, Bagshaw SM (2014) Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10:193–207
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S et al (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818
Prowle JR, Ishikawa K, May CN, Bellomo R (2010) Renal plasma flow and glomerular filtration rate during acute kidney injury in man. Ren Fail 32:349–355
Bove T, Zangrillo A, Guarracino F (2014) Effect of fenoldopam on use of renal replacement therapy among patients with acute kidney injury after cardiac surgery: a randomized clinical trial. JAMA 312:2244–2253
Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J (2000) Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet 356:2139–2143
Woods LL (1993) Mechanisms of renal hemodynamic regulation in response to protein feeding. Kidney Int 44:659–675
Sharma A, Mucino MJ, Ronco C (2014) Renal Functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract 127:94–100
Meyer TW, Ichikawa I, Zatz R, Brenner BM (1983) The renal hemodynamic response to amino acid infusion in the rat. Trans Assoc Am Physicians 96:76–83
Roberts PR, Black KW, Zaloga GP (1997) Enteral feeding improves outcome and protects against glycerol-induced acute renal failure in the rat. Am J Respir Crit Care Med 156:1265–1269
Abel RM, Beck CH Jr, Abbott WM, Ryan JA Jr, Barnett GO, Fischer JE (1973) Improved survival from acute renal failure after treatment with intravenous essential L-amino acids and glucose. Results of a prospective, double-blind study. N Engl J Med 288:695–699
Singer P (2007) High-dose amino acid infusion preserves diuresis and improves nitrogen balance in non-oliguric acute renal failure. Wien Klin Wochenschr 119:218–222
Doig GS, Simpson F, Finfer S, Delaney A, Davies AR, Mitchell I et al (2008) Effect of evidence-based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA 300:2731–2741
Doig GS, Simpson F, Bellomo R, The ANZICSCTG (2009) Improved nutritional support is associated with reduced renal dysfunction in critical illness: a post hoc exploratory subgroup analysis. Am J Respir Crit Care Med 179:A1567
Schulz KF, Grimes DA (2002) Allocation concealment in randomised trials: defending against deciphering. Lancet 359:614–618
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Simpson F, Sweetman EA, Doig GS (2010) A systematic review of techniques and interventions for improving adherence to inclusion and exclusion criteria during enrolment into randomised controlled trials. Trials 11:17
Bernard GR, Doig GS, Hudson LD, Lemeshow S, Marshall JC, Russel J et al (1995) Quantification of organ failure for clinical trials and clinical practice. Am J Respir Crit Care Med 151:A323
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J et al (2008) Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3418 individuals with CKD. Am J Kidney Dis 51:395–406
Zubrod C, Schneiderman MA, Frei E, Brindley C, Gold GL, Shnider B et al (1960) Appraisal of methods for the study of chemotherapy of cancer in man: comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis 11:7–33
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483
Haybittle JL (1971) Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol 44:793–797
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV et al (1976) Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer 34:585–612
Doig GS, Simpson F, Sweetman EA, Heighes PT, on behalf of the Nephro-Protective Trial Management Committee (2013) Statistical analysis plan for a multi-centre randomised controlled trial: nephro-protective effects of L-amino acids in critically ill patients. EvidenceBased.net, Sydney
Delanaye P, Cavalier E, Morel J, Mehdi M, Maillard N, Claisse G et al (2014) Detection of decreased glomerular filtration rate in intensive care units: serum cystatin C versus serum creatinine. BMC Nephrol 15:9
Carlier M, Dumoulin AF, Janssen AF, Picavet S, Vanthuyne S, Vanthuyne S, Van Eynde R, Van Eynde RF et al (2015) Comparison of different equations to assess glomerular filtration in critically ill patients. Intensive Care Med 41:427–435
Woods LL, Smith BE, De Young DR (1993) Regulation of renal hemodynamics after protein feeding: effects of proximal and distal diuretics. Am J Physiol 264:R337–R344
Juraschek SP, Appel LJ, Anderson CA, Miller ER III (2013) Effect of a high-protein diet on kidney function in healthy adults: results from the OmniHeart trial. Am J Kidney Dis 61:547–554
Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R (2004) A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 350:2247–2256
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B et al (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354:2564–2575
Dickerson RN, Medling TL, Smith AC, Maish GO III, Croce MA, Minard G et al (2013) Hypocaloric, high-protein nutrition therapy in older vs. younger critically ill patients with obesity. J Parenter Enteral Nutr 37:342–351
Bagshaw SM, Uchino S, Kellum JA, Morimatsu H, Morgera S, Schetz M et al (2013) Association between renal replacement therapy in critically ill patients with severe acute kidney injury and mortality. J Crit Care 28:1011–1018
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004) Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212
Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S et al (2009) Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 361:1627–1638
Acknowledgments
Dr. Doig reported receiving academic research grants from Fresenius Kabi Deutschland GmbH and Baxter Healthcare Pty Ltd and speakers’ honoraria from Fresenius Kabi Deutschland GmbH, Baxter Healthcare Australia, Pty Ltd and Nestle Healthcare, Vevy, Switzerland. Ms. Simpson reported receiving academic research grants from Fresenius Kabi Deutschland GmbH and Baxter Healthcare Australia Pty Ltd and speakers’ honoraria from Fresenius Kabi Pty Ltd and Baxter Healthcare Australia Pty Ltd. Dr. Harrigan has no potential conflicts to declare. No other authors reported disclosures.
Funding/support
This work was supported by a peer-reviewed academic grant from the Australian National Health and Medical Research Council (NH&MRC). Baxter Healthcare Pty Ltd supplied the study amino acids.
Role of the sponsors
As a peer review funding body, the NH&MRC provided constructive comments on the study design. Baxter Healthcare Pty Ltd played no role in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. Although participating sites were compensated for the costs of conducting the trial, site investigators did not receive financial compensation for their contributions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: This multicenter clinical trial investigated whether an infusion of amino acids could preserve renal function during critical illness. Although the study intervention did not reduce the duration of renal dysfunction, estimated glomerular filtration rate and urine output were improved.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Doig, G.S., Simpson, F., Bellomo, R. et al. Intravenous amino acid therapy for kidney function in critically ill patients: a randomized controlled trial. Intensive Care Med 41, 1197–1208 (2015). https://doi.org/10.1007/s00134-015-3827-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-3827-9