Abstract
Purpose
External cooling is largely employed to induce hypothermia in comatose survivors of cardiac arrest (CA), but can fail to reach the target temperature in a reasonable time. We aimed to assess the rate of failure of external cooling after CA and to determine failure predictors.
Methods
The study was a retrospective review of a prospectively acquired database in the setting of a 24-bed ICU in a university hospital. All consecutive patients admitted for CA from May 2002 to April 2010 and treated by external cooling were considered. Patients who were already hypothermic on admission, patients dying within 24 h, patients cooled by an internal technique and patients in whom hypothermia had not been attempted were not studied. External cooling failure was defined as the inability to reach a temperature below 34 °C during the first 12 h after CA onset.
Results
Among 1,036 patients admitted to the ICU, 594 were included in the analysis and in 191 (32 %) the target temperature could not be achieved within the 12 h following CA. Independent risk factors for external cooling failure were an early coronary angiography intervention (OR 3.75, p < 0.001), a high body weight (OR 1.02 per kilogram, p = 0.007), a high temperature on ICU admission (OR 1.47 per degree, p = 0.001) and a long delay between collapse and the start of cooling (OR 1.15, p = 0.05). Conversely, early haemodialysis (OR 0.27, p < 0.001) and male gender (OR 0.47, p = 0.02) were significantly associated with cooling success.
Conclusion
External cooling failure occurred in nearly one-third of patients with CA and was associated with easily identified risk factors. This emphasizes the interest in early cooling and alternative techniques in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last decade, introduction of mild therapeutic hypothermia (MTH) has been demonstrated to improve neurological outcome and survival after out-of-hospital cardiac arrest (CA) due to ventricular fibrillation [1, 2]. On the basis of several randomized and nonrandomized clinical trials, current guidelines recommend that MTH is maintained at between 32 °C and 34 °C for 12–24 h in comatose survivors of out-of-hospital ventricular fibrillation [3, 4]. For other rhythms or in-hospital CA, guidelines suggest that such cooling may also be beneficial and should be discussed on a case by case basis. However, there is no firm recommendation on the optimal speed needed to reach MTH in these patients. In two pivotal studies [1, 2], the targeted temperature was reached in approximately 2 and 8 h, respectively. However, it seems highly likely that a shorter interval between return of spontaneous circulation (ROSC) and achievement of cooling could positively impact neurological outcome and survival [5]. In animal models of cooling after CA, a better outcome has been obtained when MTH was initiated between 0 and 4 h after ROSC, whereas the benefit was completely lost when MTH was initiated 8 h after ROSC [6]. Furthermore, some recent clinical studies have also suggested an improvement in survival and neurological outcome with early achievement of MTH [7–9]. Thus measures to speed up the initiation of cooling therapy may be warranted.
Different techniques can be used to induce and maintain cooling in patients with CA but the optimal method is not yet defined [10]. The choice of method is usually driven by different considerations such as efficacy, cost and risk-benefit ratio. Regarding induction of MTH, rapid intravenous (IV) infusion of cold saline is the most commonly recommended method for inducing hypothermia, particularly in the pre-hospital setting [11]. However, even if safe and efficient, its use is still restricted, and a large number of candidates for cooling are actually treated with other methods. Several studies have shown earlier achievement of MTH and better control of temperature with endovascular systems, but such approaches are more invasive and expensive [12, 13]. Furthermore, their use did not demonstrate any improvement in neurological outcome or survival in comparison with surface cooling techniques [13, 14]. Finally, the combination of lower cost and less invasiveness may both explain why surface cooling methods are currently widely used. However, using these methods it is sometimes difficult to achieve the target temperature in a clinically acceptable time. We hypothesized that some factors could contribute to difficulties or failure to induce and achieve hypothermia using external cooling in patients with CA. Identification of these factors could be helpful for selecting patients who might be eligible for alternative cooling methods, such as cold IV fluid loading or endovascular techniques.
Based on a large cohort of patients with CA treated with MTH, we first assessed the rate of surface cooling failure and second identified risk factors associated with difficulty in reaching the target temperature.
Materials and methods
Population
After institutional review board approval, an 8-year observational cohort study was conducted in all consecutive successfully resuscitated patients aged over 18 years admitted to our closed 24-bed medical ICU from May 2002 to April 2010 for out-of-hospital or in-hospital CA and cooled with an external device. Patients who died within the first 24 h, those who were hypothermic before ICU admission (i.e. central temperature ≤34 °C), those cooled with any other method (including cold IV fluids or an endovascular method), those in whom hypothermia had not been attempted, and those with missing data were not considered in the analysis.
We retrospectively reviewed all medical records and data from our prospectively acquired ICU database, in which the characteristics of all patients with CA are registered according to the Utstein style [15]. The following information was recorded prospectively for every patient: demographic data, clinical parameters, hypothermia management, cause of CA, CA location, “no-flow” and “low-flow” duration, initial cardiac rhythm, development of infection, hospital discharge status and ICU mortality. Postresuscitation shock was defined as a requirement for vasopressors (epinephrine or norepinephrine) lasting more than 6 h despite adequate fluid loading, or the need for ventricular assistance (intra-aortic balloon pump counterpulsation) [16].
Patient management
ICU CA management was strictly standardized. Following the publication of international guidelines [17], MTH was progressively implemented over the study period. Severe bleeding or life-threatening rhythm abnormalities were the sole contraindications. In the studied population, hypothermia was started immediately on ICU admission using external cooling with forced cold air (using a tent, fans and ice packs) during the first 24 h to obtain a target temperature between 32.0 °C and 34.0 °C. During therapeutic hypothermia, sedation was induced by adjusted doses of midazolam and morphine. Neuromuscular blocker agent infusion was systematically applied to prevent shivering. In the absence of shock or complications, sedation was interrupted at the end of the hypothermia period. Normothermia between 37.0 °C and 37.5 °C was then achieved using passive rewarming, and was maintained over the next 24 h. Central temperature was measured via a bladder probe.
Dialysis was considered necessary in the presence of severe metabolic acidosis (i.e. pH <7.20) or acute renal failure with severe hyperkalaemia, anuria or pulmonary oedema. When required, renal replacement therapy was always performed using an intermittent rather than a continuous technique. In our analysis, we only considered the potential influence of haemodialysis when performed during the first 12 h following ICU admission.
Early-onset pneumonia was defined as the presence of a new and persistent pulmonary infiltrate on chest radiography, associated with either positive quantitative culture of the endotracheal aspirates, or, in the absence of a bacteriological sample, a combination of purulent sputum and hypoxaemia (PaO2/FiO2 <200) in the first 5 days following CA.
Our practice also included a target of a mean arterial blood pressure between 65 and 75 mmHg. Since acute coronary syndrome is a frequent cause of CA in adults [18], early coronary angiography was often performed in our cardiology department before ICU admission and followed by percutaneous coronary interventions (PCI) if required. Cranial computed tomography and/or pulmonary angiotomography were also performed when the CA had a possible extracardiac cause. Extubation was carried out as soon as the neurological and respiratory examination permitted.
Data analysis
The primary endpoint was the failure of therapeutic hypothermia, defined as failure to achieve a target temperature of 34 °C or less in the first 12 h following CA. Thus two groups of patients were evaluated. In the “success” group, a target temperature of 34 °C or less was achieved in the first 12 hours following CA. In the “failure” group, patients never achieved a temperature ≤34 °C in the first 12 hours.
Statistical analysis
Categorical variables are given as counts and percentages, and continuous variables are reported with their medians and interquartile ranges (IQR). Categorical variables were compared using Pearson’s chi-squared test or Fisher’s exact test, as appropriate, and continuous variables using the nonparametric Wilcoxon test. We also performed a multivariable analysis using a logistic regression model to identify the predictive factors for failure of MTH from among all potential confounders.
All tests were two-sided and a p value <0.05 was considered significant. Analyses were performed using Stata/SE 11.0 software (Stata Corporation, College Station, TX).
Results
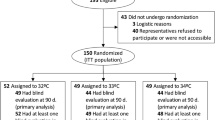
During the 8-year study period, 1,036 patients were admitted to our ICU after being resuscitated from a CA. Of these 1,036 patients, 442 were not included in the analysis due to the presence of one or more exclusion criteria (there were several duplicates between patients who died in the first 24 h and patients who were in hypothermia before admission). After application of exclusion criteria, 594 patients were finally analysed (Fig. 1). “Success” of external cooling was achieved in 403 patients (68 %) who had a temperature below 34 °C within the first 12 h, and “failure” of external cooling was seen in 191 patients (32 %) who never reached the target temperature of 34 °C within the first 12 h.
Patients were predominantly men (439 patients, 74 %) with a median age of 59 years (IQR 49–71 years). Table 1 shows the baseline characteristics, ICU management and outcome in the “success” and “failure” groups. Baseline characteristics were similar in the two groups except for weight that was significantly higher in the “failure” group than the “success” group (81 kg, IQR 71.6–93 kg, and 74 kg, IQR 65–84 kg; p < 0.001). CA occurred most frequently at home (249 patients, 42 %) with a cardiac cause in the majority (334 patients, 60 %). Among the whole population, 400 patients (68 %) underwent early coronary angiography on hospital admission, just before ICU admission. Coronary angiography was more frequently performed in the “success” group than in the “failure” group (77 vs. 63 %; p = 0.001) and time from collapse to start of external cooling was longer in the “failure” group (3 vs. 2.5 h, p = 0.002). Early-onset pneumonia was more frequent in the “failure” group (74 % vs. 63 %, p = 0.009) and haemodialysis was significantly less frequently performed in the “failure” group (18 % vs. 34 %; p < 0.001).
Regarding hospital outcome, there were no significant differences between the two groups concerning both postresuscitation shock (38.2 vs. 43.9 %, p = 0.19) and death (56.5 vs. 62.3 %, p = 0.18).
Detailed temperature data are presented in Table 2. Temperature on ICU admission was significantly higher in the “failure” group than in the “success” group (36 °C, IQR 35.7–37 °C and 36 °C, IQR 35–36 °C; p < 0.001) and the lowest temperature remained higher in the “failure” group during the first 2 days following CA (p > 0.001). In the whole population, the median time to reach the target temperature was 10 h (IQR 7–14 h), but this delay was significantly longer in the “failure” group (17 h, IQR 14–20 h and 8 h, IQR 6–10 h; p < 0.001).
The risk factors for failure of external cooling identified in the multivariable analysis are presented in Table 3 and Fig. 2. Coronary angiography intervention (OR 3.75, 95 % CI 1.79–7.82; p < 0.001), elevated body weight (OR 1.02 per kg, 95 % CI 1.01–1.03; p = 0.007), high temperature on ICU admission (OR 1.47 per degree, 95 % CI 1.17–1.83; p = 0.001) and a longer delay between collapse and the start of cooling (OR 1.15, 95 % CI 1.00–1.31; p = 0.05) were all predictors of external cooling failure. On the other hand, early haemodialysis (OR 0.27, 95 % CI 0.14–0.53; p < 0.001) and male gender (OR 0.47, 95 % CI 0.25–0.90; p = 0.02) were significantly associated with success of cooling. Figures 3 and 4 show the distributions of “failure” and “success” patients according to, respectively, body weight and the temperature on ICU admission.
Discussion
In a large proportion of patients who were eligible for MTH from among a large cohort with CA admitted to a tertiary centre the target temperature could not be achieved by external surface cooling. Factors independently associated with external cooling failure were coronary angiography intervention, a high body weight, and a high body temperature on admission. By contrast, both early haemodialysis (performed in the first 12 h) and male gender were significantly associated with cooling success.
To our knowledge, this is the first clinical study evaluating the incidence and risk factors for failure to reach the target temperature by external cooling after CA. One of the most important results of the study was that in almost one-third of the patients with CA a target temperature of 34 °C or less could not be achieved by conventional external cooling in the first 12 h following CA. This result is important since MTH is currently the only treatment that has been demonstrated to improve neurological outcome after CA [1, 2].
Conventional external cooling may not be sufficient in one-third of patients, so its use in these patients may decrease the benefit of MTH and the use of alternative cooling methods may be warranted in the presence of a risk factor for failure. Conventional external cooling techniques, which are less invasive and less expensive, are currently the most employed in clinical practice [19, 20]. However, surface cooling techniques are also likely to be less efficient in achieving the target temperature. Indeed, several studies have compared different techniques or methods, and have shown a faster achievement of cooling with the endovascular method than with conventional external cooling, despite a lack of outcome improvement [13, 14]. However, although the target temperature may be achieved more quickly using alternative methods (endovascular and various techniques such as water-circulating blankets, gel pads or convective immersion cooling), they are too costly to be employed in all patients, and should be used in selected patients [13, 20–22]. A simple way to reduce the risk of failure should be to start cooling in the period before ICU admission. This could be done using the transnasal evaporating cooling system [23] or administration of cold saline [11]. After completion of the present study, we modified our local procedure by emphasizing the systematic use of a rapid infusion of large-volume ice-cold IV fluid in patients with CA.
The highest risk factor for cooling failure was coronary angiography prior to ICU admission, which was associated with a threefold increase in the failure risk. The most likely explanation for this result is that coronary angiography delayed the arrival of the patient in the ICU and therefore delayed the initiation of cooling. Indeed, the time between collapse and the start of cooling was significantly longer among patients with failure of external cooling. Coronary angiography by itself could also be associated with external cooling failure, with a possible role of inflammation as some previous trials have shown an increase in C-reactive protein in the serum after emergency PCI [24, 25]. Finally, it is also possible that patients who had early coronary angiography had a cardiac dysfunction with a low cardiac output, which could have contributed to the failure of external cooling. In our opinion, these results do not call into question the importance of early coronary angiography after CA when a coronary cause is suspected. Indeed, the benefit of coronary angiography after CA has been widely demonstrated in several recent studies showing an association between successful immediate coronary angioplasty and improved hospital survival in patients with or without ST segment elevation after out-of-hospital CA [18]. Moreover the combination of early PCI and MTH has recently been demonstrated to be safe in patients with CA [26, 27]. Therefore recent guidelines recommend considering immediate coronary angiography in all patients with CA in whom acute coronary syndrome is suspected [28]. Thus, even if coronary angiography intervention should continue to be widely discussed after CA, our results underline that these patients would also benefit from efficient and early cooling, such as by cold IV fluid loading.
The second risk factor associated with failure of external cooling was a high body weight. Hence in our experience, the failure rate of external cooling was only 20 % in patients under 70 kg but reached nearly 50 % in patients over 90 kg. This underlines the challenge of cooling in obese patients. It might be postulated that endovascular techniques would be more efficient in obese patients. However, complications of vascular access could be a limitation of invasive methods. Another factor independently associated with external cooling failure was a more elevated temperature on ICU admission. It would be interesting to further evaluate the influence of climatic conditions on body temperature on ICU admission and on the risk of cooling failure.
Finally, early haemodialysis and male gender were associated with success of external cooling. The effect of haemodialysis is easy to understand since it is well known that using an extracorporeal blood circuit without a warming system results in rapid cooling. Furthermore, haemodialysis may also remove cytokines and decreases the SIRS response, thus resulting in an additional effect on temperature control [29]. It could be postulated that patients with CA needing renal replacement therapy can be cooled adequately by the combination of haemodialysis and surface cooling. It is more difficult to understand the effect of male sex on the success of external cooling since several confounding factors may have been unidentified.
As shown in several recent studies, early-onset pneumonia is a common complication after CA [30, 31]. We examined this factor because we thought that because of inflammatory syndrome and fever, early-onset pneumonia could be associated with difficulties in cooling. Surprisingly, although significantly more frequent in the “failure” group, early-onset pneumonia was not a significant risk factor in the multivariable analysis. One explanation is that in the present study, early-onset pneumonia was defined as pneumonia occurring in the first 5 days following CA. Thus it is possible that the delay between CA and development of infection was longer than the delay between CA and achievement of MTH. It may be interesting to look for an association between the occurrence of earlier pneumonia (i.e. pneumonia occurring in the first 24 h following CA) and hypothermia failure.
Since MTH has widely proven benefit in this setting, we may speculate that failure of MTH could have been associated with a worse outcome among patients with CA. However, our results did not show significant differences in survival or neurological outcome between the two groups. First, this may be because the study was not designed in this way, and may be have been underpowered to evaluate the impact of cooling failure on prognosis. When comparing the characteristics of the two groups, the severity in the “success” group appeared more important, as reflected by the rate of early haemodialysis requirement. This could reflect the fact that patients in the “success” group more often experienced shock or acute renal failure. Moreover, body temperature was lower on admission to the ICU in these patients. In two recent studies, spontaneous hypothermia (i.e. <35 °C) on ICU admission was a predictor of unfavourable neurological outcome [32, 33]. Second, this finding could be because, although patients in the “failure” group did not reach the target temperature of 34 °C, the median temperature on day 1 was 35.3 °C. It is possible that the neuroprotective effects of hypothermia begin at a temperature above 34 °C. Finally our study cannot answer the question of whether the failure of surface cooling is or is not associated with a worse prognosis. It would be of particular interest to investigate this outcome in further studies.
The present study suffered from several limitations. First, this is a retrospective review of a single-institution cohort. However, although data were retrospectively studied, our databases and medical records are prospectively maintained. Second, our definition of “failure” of external cooling is debatable. We chose a delay of 12 h after CA to reach the cut-off temperature of 34 °C, but there are currently no data concerning the time needed to reach this temperature. In some clinical and experimental trials, prognosis was worse when MTH was obtained 8 h or more after ROSC [2, 6]. We decided to extend this time to 12 h because it can be consensually accepted a “failure” if cooling is not achieved by this time. Third, the median time between CA and the target temperature (34 °C) was 10 h (IQR 7–14 h), which is longer than in some previous studies evaluating external cooling. Bernard et al. were able to reach the target temperature of 33.5 °C within 120 min after ROSC. Nevertheless, in their study, cooling was started in the ambulance, resulting in a lower temperature on admission than found in our study (35 vs. 36 °C) [1]. Furthermore only 4 % of patients underwent coronary angiography, whereas nearly two-thirds of our patients had coronary angiography. Moreover, our results are consistent with those of some other trials [12, 21] with target temperature obtained at 8 h (IQR 4–16 h) in the Hypothermia after Cardiac Arrest Study [2]. Fourth, our study focused on the issues involved in reaching the target temperature, whereas the duration of MTH was not studied. Finally, we could not assess the influence of body mass index on the risk of “failure” because the height of the patients was not systematically recorded in our database.
Conclusion
This study shows that failure of an external cooling procedure to achieve the target temperature in an acceptable time is a frequent event in patients with CA in whom MTH is undertaken on ICU admission. Considering the potential benefit of MTH, this should be anticipated since a substantial proportion of these patients are at higher risk of failure and might benefit from the use of more efficient techniques for cooling induction.
References
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K (2002) Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346:557–563
Hypothermia after Cardiac Arrest Study Group (2002) Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 346:549–556
Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, Hoek TLV, Kronick SL (2010) Part 9: post cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 122:S768–S786
Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, Perkins GD (2010) European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support. Resuscitation 81:1305–1352
Chenoune M, Lidouren F, Adam C, Pons S, Darbera L, Bruneval P, Ghaleh B, Zini R, Dubois-Randé J-L, Carli P, Vivien B, Ricard J-D, Berdeaux A, Tissier R (2011) Ultrafast and whole-body cooling with total liquid ventilation induces favorable neurological and cardiac outcomes after cardiac arrest in rabbits. Circulation 124:901–911
Che D, Li L, Kopil CM, Liu Z, Guo W, Neumar RW (2011) Impact of therapeutic hypothermia onset and duration on survival, neurologic function, and neurodegeneration after cardiac arrest. Crit Care Med 39:1423–1430
Wolff B, Machill K, Schumacher D, Schulzki I, Werner D (2009) Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol 133:223–228
Bernard SA, Smith K, Cameron P, Masci K, Taylor DM, Cooper DJ, Kelly AM, Silvester W; Rapid Infusion of Cold Hartmanns Investigators (2012) Induction of prehospital therapeutic hypothermia after resuscitation from nonventricular fibrillation cardiac arrest. Crit Care Med 40:747–753
Škulec R, Truhlář A, Šeblová J, Dostál P, Černý V (2010) Pre-hospital cooling of patients following cardiac arrest is effective using even low volumes of cold saline. Crit Care 14:R231
Merchant RM, Soar J, Skrifvars MB, Silfvast T, Edelson DP, Ahmad F, Huang K-N, Khan M, Vanden Hoek TL, Becker LB, Abella BS (2006) Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med 34:1935–1940
Bernard SA, Smith K, Cameron P, Masci K, Taylor DM, Cooper DJ, Kelly A-M, Silvester W (2010) Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest : a randomized controlled trial. Circulation 122:737–742
Flemming K, Simonis G, Ziegs E, Diewok C, Gildemeister R, Wunderlich C, Strasser RH (2006) Comparison of external and intravascular cooling to induce hypothermia in patients after CPR. Ger Med Sci 4:Doc04
Gillies MA, Pratt R, Whiteley C, Borg J, Beale RJ, Tibby SM (2010) Therapeutic hypothermia after cardiac arrest: a retrospective comparison of surface and endovascular cooling techniques. Resuscitation 81:1117–1122
Deye N, Group TIS (2010) Interest of endovascular cooling after cardiac arrest. Resuscitation 81:S3
Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, Cassan P, Coovadia A, D’Este K, Finn J, Halperin H, Handley A, Herlitz J, Hickey R, Idris A, Kloeck W, Larkin GL, Mancini ME, Mason P, Mears G, Monsieurs K, Montgomery W, Morley P, Nichol G, Nolan J, Okada K, Perlman J, Shuster M, Steen PA, Sterz F, Tibballs J, Timerman S, Truitt T, Zideman D; International Liaison Committee on Resuscitation (2004) Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa). Resuscitation 63:233–249
Mongardon N, Dumas F, Ricome S, Grimaldi D, Hissem T, Pene F, Cariou A (2011) Postcardiac arrest syndrome: from immediate resuscitation to long-term outcome. Ann Intensive Care 1:45
Nolan JP, Morley PT, Vanden Hoek TL, Hickey RW, Kloeck WGJ, Billi J, Böttiger BW, Morley PT, Nolan JP, Okada K, Reyes C, Shuster M, Steen PA, Weil MH, Wenzel V, Hickey RW, Carli P, Vanden Hoek TL, Atkins D (2003) Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation 108:118–121
Dumas F, Cariou A, Manzo-Silberman S, Grimaldi D, Vivien B, Rosencher J, Empana J-P, Carli P, Mira J-P, Jouven X, Spaulding C (2010) Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv 3:200–207
Holzer M (2008) Devices for rapid induction of hypothermia. Eur J Anaesthesiol Suppl 42:31–38
Merchant RM, Becker LB, Abella BS, Asch DA, Groeneveld PW (2009) Cost-effectiveness of therapeutic hypothermia after cardiac arrest. Circ Cardiovasc Qual Outcomes 2:421–428
Hoedemaekers CW, Ezzahti M, Gerritsen A, van der Hoeven JG (2007) Comparison of cooling methods to induce and maintain normo- and hypothermia in intensive care unit patients: a prospective intervention study. Crit Care 11:R91
Howes D, Ohley W, Dorian P, Klock C, Freedman R, Schock R, Krizanac D, Holzer M (2010) Rapid induction of therapeutic hypothermia using convective-immersion surface cooling: safety, efficacy and outcomes. Resuscitation 81:388–392
Castrén M, Nordberg P, Svensson L, Taccone F, Vincent J-L, Desruelles D, Eichwede F, Mols P, Schwab T, Vergnion M, Storm C, Pesenti A, Pachl J, Guérisse F, Elste T, Roessler M, Fritz H, Durnez P, Busch H-J, Inderbitzen B, Barbut D (2010) Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 122:729–736
Aggarwal A, Blum A, Schneider DJ, Sobel BE, Dauerman HL (2004) Soluble CD40 ligand is an early initiator of inflammation after coronary intervention. Coron Artery Dis 15:471–475
Almagor M, Keren A, Banai S (2003) Increased C-reactive protein level after coronary stent implantation in patients with stable coronary artery disease. Am Heart J 145:248–253
Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V (2008) Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med 36:1780–1786
Batista LM, Lima FO, Januzzi JL Jr, Donahue V, Snydeman C, Greer DM (2010) Feasibility and safety of combined percutaneous coronary intervention and therapeutic hypothermia following cardiac arrest. Resuscitation 81:398–403
Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RSB, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T (2008) Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 118:2452–2483
Laurent I, Adrie C, Cariou A, Chiche J-D, Ohanessian A, Spaulding C, Carli P, Dhainaut J-F, Monchi M (2005) High-volume hemofiltration after out-of-hospital cardiac arrest a randomized study. J Am Coll Cardiol 46:432–437
Mongardon N, Perbet S, Lemiale V, Dumas F, Poupet H, Charpentier J, Péne F, Chiche J-D, Mira J-P, Cariou A (2011) Infectious complications in out-of-hospital cardiac arrest patients in the therapeutic hypothermia era. Crit Care Med 39:1359–1364
Perbet S, Mongardon N, Dumas F, Bruel C, Lemiale V, Mourvillier B, Carli P, Varenne O, Mira J-P, Wolff M, Cariou A (2011) Early onset pneumonia after cardiac arrest: characteristics, risk factors and influence on prognosis. Am J Respir Crit Care Med 184:1048–1054
den Hartog AW, de Pont A-CJ, Robillard LB, Binnekade JM, Schultz MJ, Horn J (2010) Spontaneous hypothermia on intensive care unit admission is a predictor of unfavorable neurological outcome in patients after resuscitation: an observational cohort study. Crit Care 14:R121
Lyon RM, Richardson SE, Hay AW, Andrews PJD, Robertson CE, Clegg GR (2010) Esophageal temperature after out-of-hospital cardiac arrest: an observational study. Resuscitation 81:867–871
Conflicts of interest
The authors have not disclosed any potential conflicts of interest. Financial support was provided solely from departmental resources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ricome, S., Dumas, F., Mongardon, N. et al. Predictors of external cooling failure after cardiac arrest. Intensive Care Med 39, 620–628 (2013). https://doi.org/10.1007/s00134-012-2794-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2794-7