Abstract
Purpose
Non-invasive positive pressure ventilation (NIV) is being increasingly used in paediatric critical care, although its use in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) is still debated. No definite data are available for the prediction of NIV outcome in such selected populations. We aimed to identify which factors might affect NIV failure in paediatric ALI/ARDS patients.
Methods
A retrospective cohort study using comprehensive predictivity analysis was performed. All children admitted to our paediatric intensive care unit over a 4-year period for ALI/ARDS were reviewed. Basic, clinical, physiological parameters and their change after 1 h of NIV were considered and subjected to univariate analysis. Candidate prognostic variables were then subjected to multicollinearity scrutiny and logistic regression. Finally, variables significant in the logistic regression were subjected to predictivity analysis.
Results
The number of organ failures at admission (NOF) is a strong predictor of NIV failure (odds ratio 5.26; p = 0.004). Having only one organ failure provides a probability of NIV success of 85.7% (sensitivity 87%; specificity 49%). One NIV failure will be predicted and avoided for every four cases in which the presence of other organ failures is incorporated into the clinical decision.
Conclusions
NOF significantly predicts the NIV failure. Children with no organ failures other than ALI/ARDS may safely be treated with NIV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-invasive positive pressure ventilation (NIV) is increasingly used in paediatric intensive care units (PICU) both for the early stage of respiratory failure and for the post-extubation phase, in patients with different diseases [1–7]. Nonetheless, NIV indications in children are not well defined unlike in adults, for which NIV is already regarded as a first-line intervention in several clinical conditions [8–10]. NIV efficacy and clinical usefulness seem to be influenced by the underlying disease and by the degree of clinical severity at the respiratory failure onset [1, 11, 12].
Actually, in children affected by acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) NIV efficacy is still debated, because both high [1] and low failure rates [13, 14] have been reported in uncontrolled retrospective or prospective studies. In these critical patients, prediction of NIV failure is particularly worth studying, because it may allow a more targeted intervention. On the other hand, prediction of NIV success might avoid several complications related to endotracheal intubation in babies who will not need such aggressive respiratory support. Furthermore, in adult patients with acute respiratory failure, NIV success or failure has been independently associated with survival or death, respectively [15].
To date, only four studies analysed the prognostic factors possibly associated with the NIV failure in unselected children with acute respiratory failure [1, 11–13] and two of these studies also included patients undergoing continuous positive airway pressure (CPAP) [11, 12].
No study has been conducted to predict NIV success in homogeneous and selected populations of ALI/ARDS children, using comprehensive predictivity analysis. Since a NIV program has been implemented in the last decade as an alternative to immediate intubation in our PICU, we aimed to identify which factors might have affected NIV failure in paediatric ALI/ARDS patients.
Methods
Patients and sample size
We performed a retrospective cohort study taking data from our unit NIV register. A sample size calculation was performed in order to choose how many admissions we would have to review. Outcome was the detection of an area under the curve (AUC) at least of 0.7 for a given prognostic variable to predict NIV failure, given an α error of 0.05 and a power of 80% [16]. This gave a sample size of 60 and we reviewed all children with ALI or ARDS where NIV had been started as first-line treatment, since the PICU admission during a 4-year period (2007–2010). ALI and ARDS were defined according to the American-European Consensus Conference criteria [17]. Children were excluded if they met one of the following criteria: (1) need for cardiopulmonary resuscitation; (2) Glasgow coma score (GCS) less than 8; (3) absent cough or gag reflex; (4) haemodynamic instability (defined as systolic blood pressure below the fifth centile for age), despite appropriate volume administration and dopamine or dobutamine infusion over 5 μg/kg/min; (5) ECG with evidence of ischaemia or arrhythmias; (6) active bleeding; (7) recurrent apnoeas; (8) infants in the first year of age. Patients treated with CPAP only and those who received NIV after a period of invasive mechanical ventilation were not considered for the study. This study was approved by our institutional review board. No change to the routine assistance was required for the study aims.
NIV protocol
NIV was applied according to a well-established protocol that we had already validated for adult patients [18] and then adapted to children [14]. Patients were ventilated using a Maquet-Servo-I (Siemens-Elema, Solna, Sweden): pressure support mode (target tidal volume 6 mL/kg body weight) or pressure-controlled, time-cycled ventilation was adopted. NIV was administered via a face mask (Respironics inc., Murraysville, PA, USA or Koo Europe, Torino, Italy) or a helmet (Castar, Starmed inc., Mirandola, Italy), depending on patient’s age and collaboration. Mask and helmet size were chosen to provide best fit and comfort to the patients. NIV-related discomfort was managed as previously described [14]. All children received the standard routine care for ALI/ARDS and their basic disease, according to our internal PICU protocols.
Outcome of interest: NIV failure
NIV failure was defined as the need for endotracheal intubation and mechanical ventilation. Our predetermined criteria for intubation were (1) the inability to maintain a PaO2/FiO2 ratio of 150 during NIV administration; (2) the onset of seizures or deterioration of mental status (GCS < 8); (3) intolerance of the technique or the inability to manage copious secretions; (4) occurrence of a “de novo” haemodynamic instability after starting NIV administration (systolic blood pressure <5th centile for age), despite appropriate fluid administration and the infusion of dopamine or dobutamine above 5 μg/kg/min; (5) ECG signs of ischaemia or arrhythmias; (6) occurrence of bradypnoea or hypoventilation reducing pH to less than 7.35.
Candidate prognostic data
The following variables were chosen among those which are collected in real time in our electronic PICU database, in order to predict NIV failure: demographics, underlying diseases, GCS and number of organ failures (NOF) at PICU admission [19]. These data were collected at the initial evaluation, in the time window from the PICU admission to the 6th hour. Ventilation data, type of interface, duration of NIV support, and PRISM-III24 were then also considered.
Cardiovascular, neurologic, haematologic, renal and hepatic failure were defined according to organ dysfunction criteria of the International Pediatric Sepsis Consensus [20].
Measurements of arterial PaO2, PaCO2 and pH, respiratory rate (RR) and heart rate (HR) were performed at admission (adm) and after 1 h of NIV (1h). PaO2/FiO2 ratio was also calculated at the same time-points. Changes in such physiological parameters (∆ in %), calculated as [(value1h − valueadm)/valueadm], were also considered. These variables were chosen because they are considered clinically relevant according to our previous feasibility study [14] and other available literature data [1, 11, 12]. NIV duration, intolerance to the technique or complications and mortality were also recorded.
Statistics
Statistical analysis was subdivided in three consecutive steps:
-
1.
Univariate analysis. Data were tested for normality using the Kolmogorov–Smirnov test and then were summarized as mean ± standard deviation (SD) or median (interquartile range, IQR). Population was divided according to NIV failure or success and univariate analysis was performed using χ 2 or Fisher’s exact test for proportions and Student t or Mann–Whitney U test for continuous variables, as appropriate.
-
2.
Multivariate analysis. Multiple logistic regression with a backward stepwise method was then performed with NIV failure as the outcome variable. We initially screened as covariates all variables which differed with a p < 0.2 in the univariate analysis. PaO2/FiO2 at admission and its change over 1 h (∆PaO2/FiO2) were highly correlated (Pearson’s r = 0.54; p < 0.001); the same applies (Pearson’s r = 0.4; p = 0.002) for PaCO2 and its change over 1 h (∆PaCO2). PRISM-III24 score and NOF at admission were also significantly correlated (Pearson’s r = 0.73; p < 0.001). Among these variables PaO2/FiO2, PaCO2 and PRISM-III24 showed the highest proportion of variance associated with the highest condition index of eigenvalues (20.7) and therefore were not included in the model [21]. Consequently, covariates inserted in the model were only female sex, number of organ failures at admission, weight, ∆PaCO2 and ∆PaO2/FiO2 at admission and thus, in this model, the variance inflation factor was always less than 2.5, confirming the absence of significant multicollinearity [22]. Some alternative models inserting PRISM-III24 instead of NOF, or PaO2/FiO2 at 1 h of NIV instead of ∆PaO2/FiO2 were also studied, because of the theoretical usefulness of such variables according to the available literature [1]. The Hosmer–Lemeshow test was used to assess the model’s goodness-of-fit.
-
3.
Predictivity. Receiver operator characteristics (ROC) analysis was performed on prognostic variables which were significant in the multivariate analysis and on their combined score based on the logistic regression model equation. AUCs (± their standard error) were compared with the DeLong’s technique [23]. Predictive values were calculated only for the variable with the best performance in the ROC analysis. Test accuracy [(number of true positives + number of true negatives)/total number of cases × 100] was also calculated. Data were analysed using SPSS for Windows 15.0 (SPSS Inc., Chicago, IL, USA) or MedCalc 9 demo (MedCalc ltd., Mariakerke, Belgium) and p values less than 0.05 were considered to be significant.
Results
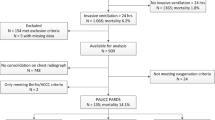
During the study period 1,670 patients were admitted to our PICU; 121 were affected by ALI/ARDS. Sixty babies were not considered eligible because they met some exclusion criteria and required invasive ventilation or were treated with CPAP only, and 61 patients received NIV as first-line treatment and were considered for the analysis. Table 1 reports the baseline data of our study population: NIV was successful in 62% of patients. The underlying diseases were distributed as follows: 7 (11.5%) solid tumours, 21 (34.4%) blood malignancies, 22 (36.1%) infections (sepsis or pneumonia), 5 (8.2%) congenital syndromes, and 6 (9.8%) other diagnoses (2 traumatic brain injuries, 1 sickle cell disease, 1 Cooley’s anaemia and 2 aplastic anaemias). In three cases, skin lesions developed at the nasal bridge as a result of the mask but were successfully relieved with ordinary care. No significant pain, anxiety or other major complication related to NIV were detected and an interruption of the technique was never needed. Causes of NIV failure were seizures or neurological deterioration (6); “de novo” haemodynamic instability (6); arrhythmias (2); inability to maintain a PaO2/FiO2 ratio of 150 (5); bradypnoea (1); and inability to manage copious secretions (4).
NIV failed at a mean of 43 ± 46 h from the beginning of the therapy. No patients died in the success group, whereas 14 out of 24 (58%) died in the failure group. Reasons for death were septic shock (5), multi-organ failure (4), intracranial haemorrhage (1), heart failure (2) and respiratory failure (2). All surviving children were discharged from the PICU and had a normal follow-up.
Table 2 shows the univariate analysis of all candidate variables for prediction of NIV failure. Factors significantly associated with NIV failure were higher PRISM-III24 and NOF along with PaCO2. Five other variables were close to the significance threshold and all eight variables were checked for multicollinearity (as described in the “Methods”).
Multivariate analysis results are shown in Table 3. Independent variables entered in the multivariate model were sex, weight, NOF, ∆PaCO2 and ∆PaO2/FiO2 at admission. According to the Hosmer–Lemeshow test, the model optimally fitted the data: NOF at admission was a strong risk factor for NIV failure, whereas female sex was slightly but significantly associated with NIV success. Two alternative multivariate models are presented in the electronic supplementary material: these significantly fit the data but gave identical results i.e. NOF and sex were the only variables associated with NIV failure.
Figure 1 shows the results of the ROC analysis for NOF at admission, sex and their combined score. AUC for NOF was 0.732 (standard error 0.06; p = 0.003), AUC for sex was 0.629 (p = 0.096), whereas AUC for the score was 0.66 (standard error 0.07; p = 0.04). Because NOF at admission was the most predictive factor for NIV failure, comprehensive results of its ROC analysis are reported in Table 4. The NOF value giving the highest accuracy was 2, which provides an accuracy of 64%. Actually, having two or more failed organs correctly identified 87% of NIV failure, increasing the risk of NIV failure from 39.3 to 51.3%. On the other hand, having only one organ failure (in other words being admitted to the PICU with only ALI/ARDS) increased the probability of NIV success from 61.7 to 85.7%. Assuming a failure risk of 39.3% (Table 1), its absolute reduction, given a NOF < 2, was calculated and this gave a number needed to prevent of 4.1 children.
ROC curves based on the multivariate analysis results. Curves represent the predictivity of the sex (grey line), NOF at admission (black line) and their combined score (dotted–dashed line), according to the equation of the multivariate model described. The considered equation was log10(NIV failure) = [5.26x (NOF)] − [0.12x (sex)] + 0.58]. Dashed line represents the prediction by chance (AUC = 0.5). More details in the text
Discussion
Our findings demonstrate that NIV may be successful in children affected by ALI/ARDS and that having organ dysfunctions other than respiratory seems a strong predictor of NIV failure, representing a useful tool for the clinical management of such patients.
In our previous study in immunocompromised children with ARDS, we found a NIV failure rate of ca. 43% [14], comparable with the present findings (ca. 40%). Such a failure rate is related to the severity of ARDS: other studies showed variable and partially similar figures (20–60%) due to the different characteristics of their populations [1, 2, 13]. In our findings, the pre-test probability is increased slightly over 50% if the baby has another organ failure, beyond ALI/ARDS. As the prediction of NIV failure in such severe cases would be useful to avoid further deterioration, it is important to identify at the bedside which patients might be safely treated with NIV. Our data allow this, providing a negative predictive value of ca. 86%: as a “screening test”, this ensures a fair confidence for applying NIV in an ALI/ARDS child, if the patient’s general conditions are stable and no other organ dysfunction is evident. The application of this simple criterion seems able to reduce the failure risk from ca. 40 to 14% and this means that one NIV failure might be predicted and avoided for every four cases in which the presence of other organ failures is incorporated into the clinical decision.
Interesting differences seem to exist with the other studies in the field. There are only four studies aimed at predicting NIV failure in PICU, but none of these focused on a selected population of ALI/ARDS [1, 11–13]. In general, these cohorts were not homogeneous; in one study, newborn infants were also enrolled [11] and in two others, the patients were treated with both CPAP and NIV [11, 12]. A strikingly different success rate was reported by Essouri et al. [1], in which ca. 80% of ARDS children failed NIV. That study was based on an unselected population of various diseases and ARDS constituted only a small group. That study also attributed a prognostic value to the general severity of illness (as estimated by the PRISM and PELOD scores) [24] and this is in agreement with our data. We used PRISM for the same purpose and the clinical severity played a role in our dataset: in fact, it is described by the NOF, which is significantly correlated with PRISM, because some of its variables describe the organ failures [24]. Consistently, another recent study confirmed the prognostic value of PRISM [12]. Various clinical scores are actually available and it is difficult to provide considerations about their use and comparison [24].
All four available studies pointed out that several physiological parameters measured after 1–2 h of NIV have a degree of prognostic value [1, 11–13]. FiO2, PaCO2, mean airway pressure, HR, RR and ∆RR have been variously indicated as candidate predictors in the unselected populations studied [1, 11–13]. We did not find a similar predictivity for such variables and this may be related to the homogeneity of our population: we only studied severely ill patients, as confirmed by the PRISM score which is higher than in the other studies by 1.4/2.2-fold [1, 12]. Moreover, the oxygenation index or PaO2/FiO2 values were not always reported [11, 13]: not using a tool estimating oxygenation impairment could have biased the interpretation of oxygen requirement.
Actually, the reported improvement of such respiratory and physiologic parameters during the early phase of NIV could be regarded as an early response, but the disease may progress and other factors may subsequently condition NIV outcome. Our study provides reasonable findings which seem to be unaffected by the disease course during the therapy, because NOF is not a physiological measurement but describes the patient’s general status, before NIV application. Our findings are consistent with those of Nichols et al. [25] who reported a higher risk of respiratory failure after bone marrow transplantation in children who developed other organ dysfunctions. However, ours is a different population, including also non-oncological patients, and has been studied when ARDS was already established.
To date, only one prospective randomized controlled trial has been published in the PICU setting, enrolling children affected by acute respiratory failure mainly of infectious origin [2]. Yanez et al. substantially confirmed the usefulness of NIV and described an improvement of HR, RR and PaO2/FiO2 over time, in NIV-treated patients. Yanez et al. called for specific trials to be designed in specific conditions, such as ALI/ARDS, probably one of the worst scenarios for a non-invasive ventilatory approach. Because immunocompromised patients are at higher risk of life-threatening complications related to invasive mechanical ventilation, we performed a NIV feasibility study in such a context and, interestingly, we found results very similar to those of Yanez et al. [2]. HR and RR were reduced along with the NIV therapy and an early and sustained PaO2/FiO2 improvement was noticed in 82 and 74% of cases, respectively [14].
Our study has several limitations: (1) It was performed in a single centre with large clinical experience in paediatric NIV. (2) A relatively small population—albeit well selected, homogeneous and comparable in size with the previously published [1, 11–13] cohorts—was evaluated. (3) Our population has a wide age range. (4) The exclusion of children less than 1 year of age could have theoretically influenced the results, but yielded a more homogeneous population. NIV application in the smallest children carries peculiar problems and might deserve specific studies; on the other hand, our population is quite representative of bigger children and adolescent. Therefore our findings could not be totally applicable to the early infancy. (5) A retrospective design was followed and a new prospective study on a distinct population is needed to validate the reliability of NOF in predicting NIV failure. (6) The lack of randomisation and control group allows no conclusion to be drawn about the success rate of NIV in ARDS patients and the effect of NIV on major outcomes. This will require a specific randomised multicentre study focused only on paediatric ARDS.
Nevertheless, our study was not targeted at studying the clinical efficacy of NIV, but only to provide a comprehensive predictivity analysis to help clinicians decide about NIV use. A Cochrane Collaboration review recently concluded that there is a lack of well-designed, controlled trials of NIV for acute hypoxemic respiratory failure in children [26]. While waiting for such trials, which are difficult to design [27], NOF could represent a practical, suitable and reliable tool to help with decisions about ventilatory strategy in paediatric ALI/ARDS.
References
Essouri S, Chevret L, Durand P, Haas V, Faroux B, Devictor D (2006) Noninvasive positive pressure ventilation: five years of experience in a pediatric intensive care unit. Pediatr Crit Care Med 7:329–334
Yanez LJ, Yunge M, Emilfork M, Lapadula M, Alcantara A, Fernandez C, Lozano J, Contreras M, Conto L, Arevalo C, Gayan A, Hernandez RN, Pedraza M, Feddersen M, Bejares M, Morales M, Mallea F, Glasinovic M, Cavada G (2008) A prospective, randomized, controlled trial of noninvasive ventilation in pediatric acute respiratory failure. Pediatr Crit Care Med 9:484–489
Mayordomo-Colunga J, Medina A, Rey C, Concha A, Menendez S, Los Arcos M, Garcia I (2010) Noninvasive ventilation after extubation in paediatric patients: a preliminary study. BMC Pediatrics 10:29
Piastra M, Antonelli M, Caresta E, Chiaretti A, Polidori G, Conti G (2006) Noninvasive ventilation in childhood acute neuromuscular respiratory failure: a pilot study. Respiration 73:791–798
Thill PJ, McGuire JK, Baden HP, Green TP, Checchia PA (2004) Noninvasive positive-pressure ventilation in children with lower airway obstruction. Pediatr Crit Care Med 5:337–342
Piastra M, Antonelli M, Chiaretti A, Polidori G, Polidori L, Conti G (2004) Treatment of acute respiratory failure by helmet-delivered noninvasive pressure support ventilation in children with acute leukemia: a pilot study. Intensive Care Med 30:472–476
Chin K, Takahashi K, Ohmori K, Toru I, Matsumoto H, Niimi A, Doi H, Ikeda T, Nakahata T, Komeda M, Mishima M (2007) Noninvasive ventilation for pediatric patients under 1 year of age after cardiac surgery. J Thorac Cardiovasc Surg 134:260–261
Antonelli M, Conti G, Bufi M, Costa MG, Lappa A, Rocco M, Gasparetto A, Meduri GU (2000) Noninvasive ventilation for the treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA 283:235–241
Antonelli M, Conti G, Rocco M, Bufi M, De Blasi RA, Vivino G, Gasparetto A, Meduri GU (1998) A comparison of noninvasive positive pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med 339:429–435
Nava S, Hill N (2009) Noninvasive ventilation in acute respiratory failure. Lancet 374:250–259
Bernet V, Hug MI, Frey B (2005) Predictive factors for the success of noninvasive mask ventilation in infants and children with acute respiratory failure. Pediatr Crit Care Med 6:660–664
Mayordomo-Colunga J, Medina A, Rey C, Diaz JJ, Concha A, Los Arcos M, Menendez S (2009) Predictive factors of noninvasive ventilation failure in critically ill children: a prospective epidemiological study. Intensive Care Med 35:527–536
Munoz-Bonet JI, Flor-Macian EM, Brines J, Rossellò-Millet PM, Llopis MC, Lòpez-Pratz JL, Castillo S (2010) Predictive factors for the outcome of noninvasive ventilation in pediatric acute respiratory failure. Pediatr Crit Care Med 11:675–680
Piastra M, De Luca D, Pietrini D, Pulitanò S, D’Arrigo S, Mancino A, Conti G (2009) Noninvasive pressure-support ventilation in immunocompromised children with ARDS: a feasibility study. Intensive Care Med 35:1420–1427
Demoule A, Girou E, Richard JC, Taille S, Brochard L (2006) Benefits and risks of success or failure of non invasive ventilation. Intensive Care Med 32:1756–1765
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R (1994) The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Rocco M, Dell’Utri D, Morelli A, Spadetta G, Conti G, Antonelli M, Pietropaoli P (2004) Noninvasive ventilation by helmet or face mask in immunocompromised patients: a case–control study. Chest 126:1508–1515
Pollack MM, Patel KM, Ruttimann UE (1996) PRISM-III: an updated pediatric risk of mortality score. Crit Care Med 24:743–752
Goldstein B, Giroir B, Randolph A, The members of the international consensus conference on pediatric sepsis (2005) International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6:2–8
Norusis M (2004) SPSS 130 advanced statistical procedures companion. Prentice Hall, Upper Saddle River
Allison PD (1999) Logistic regression using the SAS system. SAS Institute, Cary
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Lacroix J, Cotting J, Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network (2005) Severity of illness and organ dysfunction scoring in children. Pediatr Crit Care Med 6:S126–S134
Nichols DG, Walker LK, Wingard JR, Bender KS, Bezman M, Zahurak ML, Piantadosi S, Frey-Simon M, Rogers MC (1994) Predictors of acute respiratory failure after bone marrow transplantation in children. Crit Care Med 22:1485–1491
Shah PS, Ohlsson A, Shah JP (2008) Continuous negative extrathoracic pressure or continuous positive airway pressure for acute hypoxemic respiratory failure in children. Cochrane Database Syst Rev 1:CD003699
Randolph AG (2009) Management of acute lung injury and acute respiratory distress syndrome in children. Crit Care Med 37:2448–2454
Conflict of interest
Authors have neither funding nor conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Piastra, M., De Luca, D., Marzano, L. et al. The number of failing organs predicts non-invasive ventilation failure in children with ALI/ARDS. Intensive Care Med 37, 1510–1516 (2011). https://doi.org/10.1007/s00134-011-2308-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2308-z