Abstract
Objective
To prospectively assess the cost of patients in an adult intensive care unit (ICU) using bottom-up costing methodology and evaluate the usefulness of “severity of illness” scores in estimating ICU cost.
Methods and design
A prospective study costing 64 consecutive admissions over a 2-month period in a mixed medical/surgical ICU.
Results
The median daily ICU cost (interquartile range, IQR) was €2,205 (€1,932–€3,073), and the median total ICU cost (IQR) was €10,916 (€4,294–€24,091). ICU survivors had a lower median daily ICU cost at €2,164 per day, compared with €3,496 per day for ICU non-survivors (P = 0.08). The requirements for continuous haemodiafiltration, blood products and anti-fungal agents were associated with higher daily and overall ICU costs (P = 0.002). Each point increase in SAPS3 was associated with a €305 (95% CI €31–€579) increase in total ICU cost (P = 0.029). However, SAPS3 accounted for a small proportion of the variance in this model (R 2 = 0.08), limiting its usefulness as a stand-alone predictor of cost in clinical practice. A model including haemodiafiltration, blood products and anti-fungal agents explained 54% of the variance in total ICU cost.
Conclusion
This bottom-up costing study highlighted the considerable individual variation in costs between ICU patients and identified the major factors contributing to cost. As the requirement for expensive interventions was the main driver for ICU cost, “severity of illness” scores may not be useful as stand-alone predictors of cost in the ICU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intensive care is a high-cost speciality. Critically ill patients require therapies that vary widely in type, duration and cost. Reliable data from individual patients will allow a comprehensive understanding of cost drivers. Many studies evaluating patient cost and cost–effectiveness in the ICU focus on costs averaged across all patients and do not address individual patient-specific cost [1–9].
In order to prospectively identify individual patient costs associated with an expensive ICU stay, we performed a prospective bottom-up or “micro-costing” study, in which the costs were determined on an individual patient basis. Furthermore, the relationship between ICU cost and “severity of illness” scores on admission was examined.
Methods
Sixty-four consecutive patients were admitted to our nine-bed medical/surgical University Teaching Hospital adult ICU during a 2-month study period. In 2008, the mean day 1 SOFA score was 7.5 and APACHEII was 19, SMR (ICU) 0.6 and occupancy 104%. Demographic, clinical and outcome data were prospectively collected on all studied patients.
Patients’ resource utilization was identified prospectively by a combination of a daily review of medical notes and prescriptions and also bedside checklists that were contemporaneously completed by the bedside nurse and verified by the authors.
Fixed costs included capital costs—the purchase and maintenance of equipment and buildings—and non-clinical support services such as hospital administration and catering. Fixed costs were apportioned proportionate to the ICU floor area within the hospital. Nursing staff consisted of a unit manager, four associate managers, a patient care coordinator, three clinical instructors and bedside nurses. Nurse:patient dependency was 1:1. Medical staff included an ICU attending physician with two residents (day-time) or an off-site ICU attending physician with one resident (on-call). Additional staff included a ward clerk, secretary, pharmacist, dietician and physiotherapists. Semi-fixed costs included staffing costs obtained from the human resources department. Marginal costs were the costs of the patient’s treatment. Laboratory investigation and diagnostic imaging costs were calculated on a per procedure basis, taking labour, reagents, contrast and capital equipment into account. Radiology costs per procedure are apportioned into work units [10]. The hospital pharmacy supplied drug costs. The cost of ICU equipment was calculated using the Hospital Equipment Control System (HECS) from the European Care Research Institute (ECRI).
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad, San Diego, CA) and SPSS 11.0 (SPSS, Chicago, IL). Non-parametric data were compared using Mann–Whitney U or Kruskal–Wallis tests as appropriate and are presented as medians (IQR). The relative contribution of individual variables to ICU cost was explored using univariate analysis, linear or logistic regression, as appropriate. Our binomial regression model compared the most expensive 1/3 with the least expensive 2/3 of the population, allowing a clinically meaningful comparison while maintaining sufficient numbers in each group for regression analysis. Only one ICU score (SAPS3) was used in this model, due to high multicollinearity among SAPS3, APACHEII and SOFA. P < 0.05 was taken to represent statistical significance.
Results
Table 1 shows the baseline characteristics of the studied population. Sixty-four consecutive patients were admitted for 492 bed-days in total. Forty-one (63%) were male. The median age (IQR) was 58.5 (46.5–69) years. The median ICU length of stay (LOS) in the ICU was 2.9 days (1.2–9.0). ICU occupancy in the study period was 97%.
The median daily and total ICU costs were €2,205 (€1,932–€3,073) and €10,916 (€4,294–€24,091), respectively. Mean daily and total costs (±SD) were €2,659 (±€1,817) and €20,487 (±€24,187), respectively. The daily and total costs for the study period are shown in Table 2.
The requirement for continuous haemodiafiltration, blood products and antifungals was associated with higher ICU costs, shown in Table 3. On multivariate analysis, continuous haemodiafiltration [P < 0.0001, odds ratio (OR) 37, 95% confidence interval (CI) 5.5–247] and the requirement for blood products (P = 0.01; OR 9.2; 95% CI 1.7–51) were independent risk factors for more expensive daily ICU costs. The mean daily cost of continuous haemodiafiltration was €570 and antifungal therapy €700.
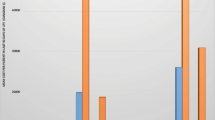
Figure 1 compares the median daily costs of survivors and non-survivors. The median LOS of survivors was 3.3 days (1.2–10.8) and non-survivors 1.9 (1.1–5.7). Non-survivors tended to be more expensive than survivors on a daily basis (P = 0.08). Non-survivor care also became more expensive as time went on, although small numbers limit the interpretation of these data.
Linear regression was used to determine if a linear relationship existed between disease severity scores and ICU cost. On first analysis, APACHEII (P = 0.03) and day 1 SOFA (P = 0.005) had statistically significant linear relationships with daily ICU cost. Day 1 SOFA (P = 0.02) and SAPS3 (P = 0.03) had statistically significant linear relationships with total cost. However, APACHEII and SOFA were strongly affected by one outlier in daily cost and two outliers in total cost. Daily SOFA scores did not have a consistent relationship with daily cost and were also affected by outliers. The linear association between SAPS3 and total cost persisted when outliers were removed (P = 0.03): total ICU cost increased by €305 (95% CI €31–€579) for each point increase in SAPS 3, as illustrated in Fig. 2a.
a Linear regression of total ICU cost on SAPS 3 scores. Regression line and 95% confidence band of the regression line are shown; P = 0.03; R2 = 0.08. b Total ICU cost stratified according to the number of “expensive” ICU interventions required per patient—dialysis, blood products and antifungal treatment; P < 0.0001. b Total ICU cost stratified according to the number of “expensive” ICU interventions required per patient—dialysis, blood products and antifungal treatment; Kruskal–Wallis test P < 0.0001. **P = 0.001; ***P < 0.0001; aP = 0.05; bP = 0.08
Patient cost is driven by the requirement for expensive interventions, especially haemodiafiltration, blood products and antifungals. When patients were stratified according to requirements for these three interventions, total cost increased significantly with the need for additional ICU interventions (P < 0.0001), which are shown in Fig. 2b. A regression model including these three markers of ICU expense accounted for 54% of the variance in total cost. The addition of LOS into this model accounted for 92% of the variance in total cost.
Discussion
This study shows the median daily and total ICU cost established by micro-costing. Even though non-survivors cost more on a daily basis, survivors and non-survivors were comparable in terms of overall resource utilisation due to survivors’ longer LOS.
Fixed costs including salaries account for 51% of ICU cost. The main driver of patient marginal cost in this study is the requirement for “expensive” interventions, specifically dialysis, blood products and antifungal treatment. Even though a statistically significant relationship between total ICU cost and SAPS3 was seen, this failed to explain the majority of variance in a regression model (R 2 = 0.08). The requirement for expensive treatments better explains the variance in total cost than SAPS3 alone (R 2 of 0.54). Moreover, adding LOS to this model improves its fit considerably (R 2 = 0.92). Although staffing costs are perceived as the main element of “global” ICU cost, our method identifies specific technological interventions as key contributors to the cost of caring for the sickest patient.
A recent top-down study by Sznajder of seven ICUs in Paris found that the mean total cost of an ICU stay was US$14,130 [11]. Chaix found a mean total cost per ICU stay of £6,279 [12]. Heyland computed a mean ICU cost per day of Can$1,565 [13]. Recently, the International Programme For Resource Use in Critical Care (IPOC), which utilised the top-down approach, estimated mean cost per patient day in the UK to be $1,512, and $934 in France [14]. Increased semi-fixed and marginal costs have been associated with University ICUs due to the specialised technology and funding for tutors and research staff [15].
Cost assessment becomes more useful with increased ICU survival. Stakeholders need to know what value they are getting for ICU expenditure; therefore, indices such as quality-adjusted life year (QALY) are becoming more important. The UK National Institute for Clinical Excellence (NICE) has adopted a cost effectiveness threshold range of £20,000 (€29,500; $40,000) to £30,000 per QALY gained. While the NICE does not accept or reject health-care technologies on cost-effectiveness grounds alone [16–18], it is undoubtedly a major deciding factor [19]. It is important to compare the cost utility of ICUs with other therapies, e.g., the unit cost per QALY of hip replacements is £1,520, coronary artery by-pass grafting £2,700, breast cancer treatment £7,460 and continuous ambulatory peritoneal dialysis £25,630 [20]. In the study by Sznajder the incremental cost-utility ratio was US$4,100 per quality-adjusted life-year saved in ICU. These data suggest that ICU costs lie within the range currently considered acceptable.
This study has a number of limitations. While we adopted a strict microcosting approach to determine marginal costs, supporting services were apportioned. Because the focus of interest of this study was the itemised, precise cost associated with more complex care, the study did not address costs once patients left ICU. Due to the labour-intensive nature of microcosting, the sample size was necessarily small. In addition the relative importance of the interventions described above may vary with case mix and other local factors.
In conclusion, our study demonstrates a method for estimating the current median and itemised total costs of intensive care in Ireland using micro-costing. As expected, ICU costs are driven both by (1) the cost of standard care common to all and (2) several costly interventions delivered to patient subgroups. Expensive cost-driving interventions, such as, haemodiafiltration, antifungals and blood products, may be more useful for identifying “expensive” patients than severity of illness scores. Our findings suggest that estimating cost effectiveness using a global approach may not have general applicability.
Abbreviations
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation Score II
- CI:
-
Confidence interval
- CT:
-
Computed tomography scan
- CXR:
-
Chest X-ray
- FFP:
-
Fresh frozen plasma
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- MRI:
-
Magnetic resonance imaging
- NICE:
-
National Institute for Clinical Excellence
- NIV:
-
Non-invasive ventilation
- SAPS 3:
-
Simplified Acute Physiology Score 3
- SD:
-
Standard deviation
- SOFA:
-
Sequential Organ Failure Assessment Score
- U/S:
-
Ultrasound scan
References
Chalfin DB, Cohen IL, Lambrinos J (1995) The economics and cost–effectiveness of critical care medicine. Intensive Care Med 21:952–961
Bams JL, Miranda DR (1985) Outcome and costs of intensive care. A follow-up study on 238 ICU-patients. Intensive Care Med 11:234–241
Bekes C, Fleming S, Scott E (1988) Reimbursement for intensive care services under diagnosis-related groups. Crit Care Med 16:478–481
Chassin MR (1982) Costs and outcomes of medical intensive care. Med Care 20:165–179
Coulton CJ, McClish D, Doremus H, Powell S, Smookler S, Jackson DL (1985) Implications of DRG payments for medical intensive care. Med Care 23:977–985
Ridley S, Biggam M, Stone P (1991) Cost of intensive therapy. A description of methodology and initial results. Anaesthesia 46:523–530
Ridley S, Biggam M, Stone P (1993) A cost–benefit of intensive therapy. Anaesthesia 48:14–19
Singer M, Myers G, Hall G, Cohen SL, Armstrong RF (1994) The cost of intensive care. A comparison on one unit between 1988 and 1991. Intensive Care Med 20:542–549
Loes O, Smith-Erichsen N, Lind B (1987) Intensive care: cost and benefit. Acta Anaesthesiol Scand 31:3–19
Health Division of Statistics Canada (1982) Canadian workload measurement system-diagnostic radiology. A schedule of unit values for diagnostic radiology, 1982–1983. Workload Measurement, Institutional Statistics Section, Health Division, Statistics Canada, Ottawa, Canada
Sznajder M, Aegerter P, Launois R, Merliere Y, Guidet CubRea B (2001) A cost–effectiveness analysis of stays in intensive care units. Intensive Care Med 27:146–153
Chaix C, Durand-Zaleski I, Alberti C, Brun-Buisson C (1999) A model to compute the medical cost of patients in intensive care. Pharmacoeconomics 15:573–582
Heyland DK, Konopad E, Noseworthy TW, Johnston R, Gafni A (1998) Is it ‘worthwhile’ to continue treating patients with a prolonged stay (>14 days) in the ICU? An economic evaluation. Chest 114:192–198
Negrini D, Sheppard L, Mills GH, Jacobs P, Rapoport J, Bourne RS, Guidet B, Csomos A, Prien T, Anderson G, Edbrooke DL (2006) International Programme for Resource Use in Critical Care (IPOC)—a methodology and initial results of cost and provision in four European countries. Acta Anaesthesiol Scand 50:72–79
Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE (2007) Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med 35:1470–1476
Rawlins M, Culyer AJ (2004) National Institute for Clinical Excellence and its value judgments. BMJ 329:224–227
Devlin N, Parkin D (2004) Does NICE have a cost effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ 13:437–452
Dakin H, Devlin N, Odeyemi I (2006) Yes, no or “yes, but”? A multinomial model of NICE decision-making. Health Policy 77:352–367
Appleby J, Devlin N, Parkin D (2007) NICE’s cost effectiveness threshold. BMJ 335:358–359
Mason J, Drummond M, Torrance G (1993) Some guidelines on the use of cost effectiveness league tables. BMJ 306:570–572
Acknowledgments
The authors would like to thank Dr. J. Boylan for his valuable advice in preparing this manuscript. The authors acknowledge financial support from Dr. Canavan from the UK Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Conflict of interest statement
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McLaughlin, A.M., Hardt, J., Canavan, J.B. et al. Determining the economic cost of ICU treatment: a prospective “micro-costing” study. Intensive Care Med 35, 2135–2140 (2009). https://doi.org/10.1007/s00134-009-1622-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1622-1