Abstract
Objective
To test the usefulness of dead space for determining open-lung PEEP, the lowest PEEP that prevents lung collapse after a lung recruitment maneuver.
Design
Prospective animal study.
Setting
Department of Clinical Physiology, University of Uppsala, Sweden.
Subjects
Eight lung-lavaged pigs.
Interventions
Animals were ventilated using constant flow mode with VT of 6 ml/kg, respiratory rate of 30 bpm, inspiratory-to-expiratory ratio of 1 : 2, and FiO2 of 1. Baseline measurements were performed at 6 cmH2O of PEEP. PEEP was increased in steps of 6 cmH2O from 6 to 24 cmH2O. Recruitment maneuver was achieved within 2 min at pressure levels of 60/30 cmH2O for Peak/PEEP. PEEP was decreased from 24 to 6 cmH2O in steps of 2 cmH2O and then to 0 cmH2O. Each PEEP step was maintained for 10 min.
Measurements and results
Alveolar dead space (VDalv), the ratio of alveolar dead space to alveolar tidal volume (VDalv/VTalv), and the arterial to end-tidal PCO2 difference (Pa-etCO2) showed a good correlation with PaO2, normally aerated areas, and non-aerated CT areas in all animals (minimum–maximum r2 = 0.83–0.99; p < 0.01). Lung collapse (non-aerated tissue > 5%) started at 12 cmH2O PEEP; hence, open-lung PEEP was established at 14 cmH2O. The receiver operating characteristics curve demonstrated a high specificity and sensitivity of VDalv (0.89 and 0.90), VDalv/VTalv (0.82 and 1.00), and Pa − etCO2 (0.93 and 0.95) for detecting lung collapse.
Conclusions
Monitoring of dead space was useful for detecting lung collapse and for establishing open-lung PEEP after a recruitment maneuver.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute lung injury (ALI) is characterized by varying degrees of atelectasis and edema [1, 2]. Ventilatory treatment with low airway pressures, low tidal volume, high inspired oxygen fraction, or tracheal suctioning may favor alveolar collapse [3, 4, 5]. Such collapse may also be cyclic with repeated opening and closing of unstable pulmonary units over the respiratory cycle, promoting lung injury and impeding gas exchange [6, 7, 8].
Lung recruitment is a process that refers to the re-aeration of previously collapsed lung areas and can be performed by so-called recruitment maneuvers (RM) [9, 10, 11, 12]. An adequate positive end expiratory pressure (PEEP) remains the cornerstone of any ventilatory approach, since it should prevent end expiratory lung de-recruitment; however, the lowest level of PEEP that avoids de-recruitment and at the same time does not overdistend the lung, thus, “open-lung” PEEP (OL-PEEP), is difficult to determine at bedside [13, 14, 15, 16, 17, 18].
Dead space can be viewed as “wasted ventilation” and its calculation is useful to study the efficiency of ventilation [19, 20, 21]. Dead space variables can reflect changes in lung condition such as collapse and recruitment. Lung overdistension can also be characterized by an increase in VDalv and by a decrease in the exhaled volume of CO2 per breath [22]. A successful RM decreases the arterial to end-tidal PCO2 difference (Pa-etCO2) [21], which in turn reduces the calculated dead space by decreasing the shunt [23, 24]. Some publications support the value of monitoring the relationship between ventilation inefficiency and efficiency during positive pressure ventilation. Suter et al. described how the “best” PEEP closely correlated with the lowest dead space as well as with the highest compliance and oxygen transport in ALI patients [25]. McMahon et al. showed that patients with respiratory failure who responded to PEEP therapy with an increase in oxygenation also showed a decrease in dead space [26]; however, other authors failed to find similar effect on dead space during PEEP titration in this kind of patients [27, 28].
We hypothesized that dead-space variables have a close relationship with collapse-recruitment phenomena within the lungs, and thus, could be useful for monitoring the process of OL-PEEP titration.
The aim of this study was to test this hypothesis in an animal model of surfactant depletion, evaluating dead-space variables on a breath-by-breath basis and comparing these values with continuous arterial oxygenation and computed tomography (CT) images of the lungs.
Methods
The study was approved by the Animal Research Committee of Uppsala University (Sweden). Eight Swedish mixed country breed pigs (weight 29.8 ± 2.1 kg) were premedicated with intramuscular zolazepam-tiletamine (6 mg/kg) and were anesthetized with fentanyl (2.5 μg kg–1 h) followed by an infusion of ketamine 25–50 mg kg–1 h, midazolam 90–180 μg kg–1 h, fentanyl 3–6 μg kg–1 h, and pancuronium 0.25–0.50 mg kg–1 h.
Animals were tracheotomized and mechanically ventilated in supine position through a 7-mm ID endotracheal tube (Mallinckrodt, Atholone, Ireland) using a constant flow volume controlled mode (Servoi, Siemens Elema AB, Solna, Sweden) with a tidal volume (VT) of 6 ml/kg, respiratory rate of 30 breaths/min, PEEP level of 6 cmH2O, an inspiratory: expiratory ratio of 1 : 2, and a FiO2 of 1.
Systemic arterial pressure was monitored invasively via a catheter in the femoral artery. Pulmonary artery pressure and cardiac output were continuously monitored by a 7.5-F pulmonary artery catheter CCOmbo (Edwards Lifesciences LLC, Irvine, Calif.). Arterial blood gases were monitored on-line using the multi-parameter intra-arterial sensor Trendcare (Diametrics Medical Ltd, High Newcombe, UK) inserted into the right carotid artery. In vitro and in vivo calibrations of devices were performed before start of the protocol. Hemodynamic and on-line blood gas parameters were stored in a data acquisition system programmed in LabView (National instruments, Austin, Texas). Independent arterial and mixed venous blood gas samples were analyzed on each PEEP level using an ABL 300 and an OSM 3 (Radiometer, Copenhagen, Denmark). Shunt was calculated using the standard formula for oxygen content in blood [29].
CO2 equipment
Dead space was analyzed by the single breath test of CO2 (SBT − CO2), computed by integrating expiratory flow and CO2 signals [21, 22]. The SBT-CO2 and standard respiratory mechanics were monitored on a breath-by-breath basis with the CO2SMOplus (Novametrix, Wallinford, Conn.) and recorded using the software Aplus. The CO2 measurement was performed by a main-stream sensor using non-dispersive infrared technique (accuracy ± 2 mmHg and resolution 1 mmHg). Airway flow was measured by a fixed orifice, differential pressure flow sensor (range 2–180 l/min and accuracy > 3%). Instrumental dead space of 10 ml was included in the airway dead-space calculations.
VDaw is the airway dead space determined by Fowler's method [20]. Physiological dead space (VDphys) was calculated using Enghoff's modification of Bohr's formula [19] as:
VDphys = (PaCO2 − PaeCO2)/PaCO2 * VT,
where PaeCO2 is the mean expired alveolar partial pressure [21, 30]. Then, the ratio of physiological dead space to tidal volume (VD/VT) was calculated as:
VD/VT = VDphys/VT
VDalv was computed subtracting VDaw from VDphys. The alveolar dead space to alveolar tidal volume ratio (VDalv/VTalv) was then obtained dividing VDalv by alveolar tidal volume (VTalv, i. e., the difference between VT and VDaw). Pa-etCO2 was defined as the difference between arterial and end-tidal partial pressure of CO2.
To study lung aeration at the end of each PEEP step, computed tomography (CT) of the chest was used (Somatom Sensation 16, Siemens, Forchheim, Germany). Prior to obtaining a transverse CT slice 2 cm cranial of the right diaphragmatic dome during an end-expiratory hold maneuvers of > 4 s, a scout view was obtained on each PEEP level. Exposure time was 0.75 s at 120 mA and 100 kV. Images were reconstructed with 6-mm slice thickness using a standard body reconstruction filter (Siemens notation: B40s). Attenuations of the pulmonary parenchyma were analyzed using the CT image analysis software Maluna (Mannheim Lung Analyzing Tool, version 2.02, Mannheim, Germany). Regions of interest of lungs were manually delineated taking the inner rib cage and the mediastinal structures as the lung boundaries. We used standard definitions of lung aeration according to the attenuation values in Hounsfield units (HU) [31]. Differently aerated lung regions were classified as non aerated (+100 to –100 HU), poorly aerated (–100 to –500 HU), normally aerated (–500 to –900 HU) and hyperinflated (–900 and –1000 HU), respectively. The percentage of voxels per CT slice showing these predefined four ranges of HU was calculated. To avoid inter-observer variations, the CT analysis was performed by the same investigator who was blinded as to the level of PEEP used.
Protocol
The model was induced by surfactant depletion after lung lavage with 35 ml/kg of warm (37 °C) isotonic saline [32]. Lavages were repeated every 5 min until PaO2 decreased to values below 100 mmHg (FiO2 of 1). A final observation period of 1 h was added to assure the stability of the model.
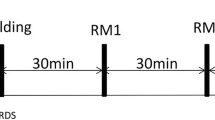
A schematic representation of the protocol is shown in Fig. 1. We studied the following ventilation sequence:
-
1.
Baseline measurement at 6 cmH2O of PEEP. Then, PEEP level was increased in 6 cmH2O steps up to 24 cmH2O.
Fig. 1 Protocol design. Baseline measurement was performed at 6 cmH2O of positive end-expiratory pressure (PEEP). Then, PEEP was increased in steps of 6 cmH2O of PEEP, from 6 to 24 cmH2O in a volume control mode of ventilation (VCV). After PEEP reached 24 cmH2O a recruitment maneuver (RM) was performed for 2 min (60 breaths) in a pressure control mode of ventilation (PCV) with 60/30 cmH2O of peak inspiratory pressure (PIP) and PEEP, respectively. After RM, ventilation was returned to VCV and PEEP was decreased by 2 cmH2O every 10 min starting at 24 cmH2O and going down to 6 cmH2O and finally to ZEEP. Computer tomography images of the chest (CT; asterisks) were taken at the eighth minute of each PEEP step. Arterial partial pressure of oxygen (PaO2) and the single-breath test of CO2 (SBT-CO2) were recorded continuously
-
2.
A cycling lung recruitment maneuver, performed in pressure control ventilation reaching maximum pressures of 60/30 cmH2O for PIP/PEEP for 2 min, was performed [32]. Ventilation was then returned to the previous ventilatory setting but with a PEEP of 24 cmH2O. We assumed that this high level of PEEP would surely exceed the OL-PEEP to be determined by the titration method in any of the animals.
-
3.
A descending PEEP titration, where PEEP level was decreased by 2 cmH2O, from 24 to 6 cmH2O and then to 0 cmH2O. Each PEEP step was maintained for 10 min. In pilot measurements a time lapse of 10 min was enough to reach steady-state conditions in real-time CO2 and O2 signals. A complete CO2 data set was obtained for every breath cycle. Average data from the 30 breaths during the eighth minute of every PEEP step just prior to taking the CT scans and recording the hemodynamic variables represented each PEEP level.
Definition of lung recruitment and collapse
The PaO2 values > 400 mmHg [32], an amount of non-aerated zones < 5%, and zones of overly aerated regions > 85% of the total lung area were considered markers of total lung recruitment and were used to define the “open-lung” condition, i. e., a lung without collapse. During the PEEP trial, we assumed that an increase in non-aerated areas > 5% defined the start of lung collapse after the RM; therefore, OL-PEEP was defined as the minimum PEEP level that maintained the lungs recruited. Per this definition, OL-PEEP was 2 cmH2O above the PEEP level at which first signs of collapse occur.
Statistical analysis was performed using MatLab (MatLab, The MathWorks, Inc., Natick, Mass.). Variables were analyzed by ANOVA with Dunnett's post test adjustment taking baseline (6 cmH2O of PEEP prior to RM) or 24 cmH2O of PEEP after RM as reference values. Pearson's correlation coefficient (r2) between dead-space variables and oxygenation and CT data was calculated. Data are presented as mean and SD or minimum–maximum values. Analysis of the receiver operating characteristics curve (ROC) was used to determine the sensitivity and specificity of each dead space variable in predicting lung derecruitment as pre-defined by a cut-off level > 5% of non-aerated lung on CT.
Results
Measurements were performed in eight pigs. None of the animals presented barotrauma or died during the protocol. All variables of the protocol are shown in Table 1.
Results revealed a good correlation between PaO2 and normally aerated (r2 minimum–maximum: 0.92–0.99; p < 0.001) and an inverse correlation between PaO2 and non-aerated regions (r2 minimum–maximum: 0.88–0.99; p < 0.001), confirming that, in all animals, arterial oxygenation—and thus, the amount of lung tissue available for gas exchange—matched closely with the amount of normally aerated areas. Dead-space variables were closely related to the recruitment-collapse phenomena as observed by CT and PaO2. During the PEEP titration phase, VDalv, VDalv/VTalv, and Pa-etCO2 were the dead-space variables that correlated best with the amount of non-aerated tissue (Table 2).
After successful lung recruitment, the “open lung” condition had been reached at 24 cmH2O of PEEP with corresponding mean plateau pressure (Pplat) of 41 ± 3.5 cmH2O. Lung collapse during the PEEP trial occurred at a mean PEEP value of 12 cmH2O (Pplat of 21 ± 1.6 cmH2O). Dead-space data showed also a significant change at 12 cmH2O of PEEP (Table 1); thus, the “open-lung” condition was maintained during decremental PEEP until a mean PEEP value of 14 cmH2O (Pplat of 23 ± 1.2 cmH2O), defining the OL-PEEP in these pigs.
The ROC analysis demonstrated a high specificity and sensitivity of VDalv, VDalv/VTalv, and Pa-etCO2 for detecting lung collapse, whereas VDaw and VD/VT did not show such good estimations (Fig. 2; Table 3).
Analysis of the receiver operating characteristic curve (ROC) for dead-space variables and lung collapse as defined by the level of PEEP at which the percentage of non aerated tissue exceeded 5% of the total CT slices. p-value < 0.05 for alveolar dead space (VD alv ), alveolar dead space to alveolar tidal volume ratio (VD alv /VT alv ), the arterial to end-tidal PCO2 difference (Pa-et CO 2), and physiological dead space (VD phys ). VD aw airway dead space, VD/VT ratio of physiological dead space to tidal volume
Fig. 3 shows the parallel behavior of shunt and VDalv. The changes of VDaw along the protocol are presented as the ratio of VDaw/VT and its relationship with VD/VT and VDalv/VTalv (Fig. 4).
Ratio of airway dead space to tidal volume (VD aw /VT) during PEEP titration. The effect of airway distension on the ratio of physiological dead space to tidal volume (VD/VT), but not on the ratio of alveolar dead space to alveolar tidal volume (VD alv /VT alv ), is clearly visible. Arrows indicate the moment of lung collapse
Ventilatory data are shown in Table 4. At levels greater than 20 cmH2O of PEEP plateau pressures presented values above those recommended [33]; however, according to CT definition less than 0.7% of lung tissue on the CT slices was hyperinflated even at PEEP > 20 cmH2O. For this reason, hyperinflated areas are not included in the results. In addition, dead-space data did not show a functional alveolar overdistension at 24 cmH2O of PEEP and corresponding Pplat of 41 ± 3.5 cmH2O, despite known increases in the volume of the conducting airways (Fig. 3; Table 1).
Highest dynamic compliance and lowest dynamic expiratory airway resistance were observed at the OL-PEEP. The opposite findings were observed at lowest and highest PEEP levels.
At the highest PEEP level, the lowest but still acceptable mean arterial pressures (61 ± 7 mmHg) and cardiac indeces (3.2 ± 0.9 l min–1/m2) were observed, whereas mean pulmonary arterial pressure remained almost constant along the study. We did not apply any specific hemodynamic treatment during the protocol other than fixed i.v. fluid of 5 ml kg–1 and dopamine infusion of 3–4 μ kg–1.
Discussion
In this surfactant-depleted model, a decremental PEEP trial was performed after a cycling recruitment maneuver. Dead-space variables were compared with two well-established methods for monitoring the lung's volumetric state and gas exchange. We found that VDalv, VDalv/VTalv, and Pa–etCO2 were the parameters most closely correlated with atelectatic lung areas on CT scan as well as with arterial oxygenation. These variables also showed a high sensitivity and specificity for detecting early lung collapse during a PEEP titration trial following lung recruitment.
Our results, if confirmed in patients with ALI, suggest that dead-space variables, in the context of recruitment and a PEEP titration procedure, might become a clinically useful bedside tool for implementing a lung protective ventilation strategy based on OL-PEEP.
As lung collapse induces changes in both, ventilation distribution and gas exchange, VD should be a better tool for assessing overall respiratory function than oxygenation, because the latter one does not allow inferences on “ventilation.” Despite the fact that VD suffers from the same limitation as PaO2 (i. e., they both require arterial blood gases), VD may become a practical bedside tool, thus replacing CT images for OL-PEEP determination.
At lowest PEEP levels the pigs showed a similar behavior between VDalv and shunt, supporting the notion that alveolar dead space is “fictitious” in origin, meaning that shunt creates a dead-space effect when VDalv is calculated according to Enghoff's modification of Bohr's formula [19, 21]. This is a known effect of an increased Pa-etCO2 difference and was called shunt-related dead space (Fig. 3). An increase in “real” VDalv, i. e., the true presence of areas with a high V/Q, did not occur even at 24 cmH2O of PEEP, suggesting that the more homogeneous distribution of ventilation prevented the occurrence of a significant alveolar overdistension even at these high pressures. This phenomenon is clearly shown in Fig. 3 and Table 1, where VDalv remains at low values paralleled by minimal amounts of non-aerated lung and shunt during the open-lung condition.
Pa-etCO2, a clinical index commonly used to estimate dead space, showed a similar behavior as VDalv during the protocol (Table 1). As soon as PEEP decreased below OL-PEEP, Pa-etCO2 started to increase again, marking the beginning of lung collapse.
Our data are in agreement with those of Gattinoni et al. [34] in patient with ARDS. In those patients who “responded” to prone positioning, PaCO2 decreased. This decrement in PaCO2 was most likely due to a recruitment of some additional tissue for gas exchange which was finally associated with a better outcome. Our results indicated the same recruitment effect: After the recruitment, the lung's capacity for CO2 elimination is increased. This, in turn, corresponds with a decrement in arterial PCO2 (Tables 1, 4).
VDaw presented a close positive correlation with PaO2 and normally aerated areas. A similar change in airway caliber related to transmural pressures has been reported earlier [35]; therefore, it seems that gas exchanging peripheral units are kept open at the expense of an increase in airway dead space. The increase in airway diameter was also responsible for the differences observed between VD/VT and VDalv/VTalv, as the former more global ratio is “contaminated” by the effect of PEEP on airway dead space, whereas the latter is not (Figs. 3, 4). This fact suggests that the dead space portion of the alveolar gas provides more meaningful information than the classical VD/VT ratio when monitoring of the lung collapse-recruitment phenomena is of the essence.
These changes in dead space agree with those reported by Wenzel et al. in an animal model of ALI [36]. In their study, the administration of exogenous surfactant decreased dead space due to a partial recruitment of atelectatic lung areas. Furthermore, the results of the current study agree well with the findings of our previous studies in anesthetized patients [23, 24].
On the contrary, neither Blanch et al. [27] nor Beydon et al. [28] showed the same positive effect on dead space when using levels of PEEP between 0 and 15 cmH2O in acutely injured lungs. These opposing results are most likely due to the absence of any genuine lung-recruitment effect in their patients. Putting these thoughts together, differences in the results of these studies may in fact be the demonstration of the significant physiological changes in dead space, which are induced by lung-recruitment maneuvers as compared with the isolated use of PEEP without them.
Limitations of the study
The saline lavage model behaves differently from clinical ALI and fails to reproduce the vascular and inflammatory pathology of early acute respiratory failure [37]. This model is generally more “recruitable,” i. e., it responds more to increases in airway pressures than other models of lung injury but shows less hemodynamic compromise [37]. Therefore, changes in dead-space variables could have been overestimated in the experimental model used as compared with real patients. Rosenthal et al. demonstrated that there is no such thing as a perfect animal model that could imitate the complex nature of ALI or ARDS [38]; thus, data from other experimental models of ALI, but more importantly from patients, would be needed to confirm our results.
The use of two-dimensional CT scans for assessing three-dimensional volumes of lung aeration has limitations. The main limitation of our approach was to take only one juxta-diaphragmatic CT slice for analysis, a technique that need not be representative for the entire lung [31]. Also, analysis of hyperinflated areas was difficult due to inherent limitations of CT technology as a tool for assessing hyperinflation, especially when a single-slice technique is used [1, 2, 14, 31, 39].
Conclusion
Monitoring of dead space during PEEP titration was useful for detecting early signs of lung collapse in an experimental model of surfactant depletion. After lung recuitment, the parameters alveolar dead space, ratio of alveolar dead space to alveolar tidal volume, and Pa-etCO2 showed high specificity and sensitivity for establishing OL-PEEP. VDalv/VTalv, as opposed to VD/VT, agreed well with the gold standard defined by CT. VDalv/VTalv should replace the classical ratio whenever alveolar aeration is to be optimized.
References
Gattinoni L, Pelosi A, Crott S, Valenza F (1995) Effects of positive end-expiratory pressure on regional distribution of tidal volume and recruitment in adult respiratory distress syndrome. Am J Respir Crit Care Med 151:1807–1814
Puybasset L, Cluzel P, Chao N, Slutsky AS, Coriat P, Rouby JJ (1998) A computed tomography scans assessment of regional lung volume in acute lung injury. Am J Respir Crit Care Med 158:1644–1655
The Acute Respiratory Distress Syndrome Network (2004) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Reber A, Endberg G, Wegenius G, Hedenstierna G (1996) Lung aeration: the effect of pre-oxygenation and hyperoxygenation during total intravenous anesthesia. Anesthesia 51:733–777
Reissmann H, Böhm SH, Suarez-Sipmann F, Tusman G, Buschmann C, Maisch S, Pesch T, Tham O, Plumers C, Schulte am Esch J, Hedenstierna G (2005) Suctioning through a double-lumen endotracheal tube helps to prevent alveolar collapse and to preserve ventilation. Intensive Care Med 31:431–440
Muscedere JG, Mullen JBM, Gan K, Bryan AC, Slutsky AS (1994) Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149:1327–1334
Abraham E, Andrews P, Antonelli M, Brochard L, Brun-Buisson C, Dobb G, Fagon J-Y, Groeneveld J, Mancebo J, Metnitz P, Nava S, Pinsky M, Radermacher P, Ranieri M, Richard C, Tasker R, Vallet B (2004) Year in review in Intensive Care Medicine, 2003. I. Respiratory failure, infection and sepsis. Intensive Care Med 30:1017–1031
Andrews P, Azoulay E, Antonelli M, Brochard L, Brun-Buisson C, de Backer D, Dobb G, Fagon J-Y, Gerlach H, Groeneveld J, Mancebo J, Metnitz P, Nava S, Pugin J, Pinsky M, Radermacher P, Richard C, Tasker R (2006) Year in review in Intensive Care Medicine, 2005. I. Acute respiratory failure and acute lung injury, ventilation, hemodynamics, education, renal failure. Intensive Care Med 32:207–216
Lachmann B (1992) Open up the lung and keep the lung open. Intensive Care Med 118:319–321
Rothen HU, Sporre B, Wegenius G, Hedenstierna G (1993) Reexpansion of atelectasis during general anaesthesia: a computed tomography study. Br J Anaesth 71:788–795
Amato MBP, Barbas CSV, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354
Tusman G, Böhm SH, Vazquez de Anda GF, do Campo JL, Lachmann B (1999) “Alveolar Recruitment Strategy” improved arterial oxygenation during general anaesthesia. Br J Anaesth 82:8–13
Richard JC, Maggiore SM, Mercat A (2004) Clinical review: bedside assessment of alveolar recruitment. Crit Care Med 8:163–169
Malbouisson LM, Muller JC, Constantin JM, Lu Q, Puybasset L, Rouby JJ (2001) Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 163:1444–1450
Hickling KG (2001) Best compliance during a decremental, but not incremental, positive end-expiratory pressure trial is related to open-lung positive end-expiratory pressure. A mathematical model of acute respiratory distress syndrome lungs. Am J Respir Crit Care Med 163:69–78
Rouby JJ, Lu Q, Goldstein I (2002) Selecting the right level of positive end-expiratory pressure in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 165:1186–1186
Ranieri M, Zhang H, Mascia L, Aubin M, Lin C, Mullen B, Grasso S, Binnie M, Volgyesi GA, Slutsky AS (2000) Pressure-time curve predicts minimally injurious ventilatory strategy in an isolated rat lung model. Anesthesiology 93:1320–1328
Sipmann FS, Böhm SH, Tusman G, Reissmann H, Pesch T, Thamm O, Hedenstierna G (2004) Selecting Open Lung PEEP in an experimental model of ARDS: comparison of oxygenation and compliance with CT scan. Am J Resp Crit Care Med 169:A721
Enghoff H (1938) Volume inefficax. Bemerkungen zur Frage des schädlichen Raumes. Upsala Läkaref Förh 44:191–218
Fowler WS (1948) Lung function studies. II. The respiratory dead space. Am J Physiol 154:405–416
Fletcher R, Jonson B (1981) The concept of deadspace with special reference to the single breath test for carbon dioxide. Br J Anaesth 53:77–88
Breen PH, Mazumdar B (1996) How does positive end-expiratory pressure decrease CO2 elimination from the lung? Respir Physiol 103:233–242
Tusman G, Böhm SH, Suárez Sipmann F, Turchetto E (2004) Alveolar recruitment improves ventilatory efficiency of the lungs during anesthesia. Can J Anesth 51:723–727
Tusman G, Böhm SH, Suárez Sipmann F, Maisch S (2004) Lung recruitment improves the efficiency of ventilation and gas exchange during one-lung ventilation anesthesia. Anesth Analg 98:1604–1609
Suter PM, Fairley B, Isenberg MD (1975) Optimum end-expiratory pressure in patients with acute pulmonary failure. N Engl J Med 292:284–289
McMahon SM, Halprin GM, Sieker HO (1973) Positive end-expiratory airway pressure in severe arterial hypoxemia. Am Rev Respir Dis 108:526–535
Blanch LL, Lucangelo U, Lopez-Aguilar J, Fernandez R, Romero PV (1999) Volumetric capnography in patients with acute lung injury: effect of positive end-expiratory pressure. Eur Respir J 13:1048–1054
Beydon L, Uttman L, Rawal R, Jonson B (2002) Effects of positive end-expiratory pressure on dead space and its partitions in acute lung injury. Intensive Care Med 28:1239–1245
Berggren SM (1942) The oxygen deficit of arterial blood caused by non-ventilated parts of the lung. Acta Physiol Scand (Suppl 4):4–92
Breen PH, Mazumdar B, Skinner SC (1996) Comparison of end-tidal PCO2 and average alveolar expired PCO2 during positive end-expiratory pressure. Anesth Analg 82:368–373
Gattinoni L, Caironi P, Pelosi P, Goodman LR (2001) What has computed tomography taught us about the acute respiratory distress syndrome. Am J Respir Crit Care Med 164:1701–1711
Lachmann B, Jonson B, Lindroth M, Robertson B (1982) Modes of artificial ventilation in severe respiratory distress syndrome. Lung function and morphology in rabbits after wash-out of alveolar surfactant. Crit Care Med 10:724–732
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R (1994) The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Gattinoni L, Vagginelli F, Carlesso E, Taccone P, Conte V, Chiumello D, Valenza F, Caironi P, Pesenti A (2003) Decrease in PaCO2 with prone position is predictive of improved outcome in acute distress syndrome. Crit Care Med 31:2727–2733
Hedenstierna G, Lundberg S (1975) Airway compliance during artificial ventilation. Br J Anaesth 47:1227–1232
Wenzel U, Rüdiger M, Wagner MH, Wauer RR (1999) Utility of deadspace and capnometry measurements in determination of surfactant efficacy in surfactant-depleted lungs. Crit Care Med 27:946–952
Van der Kloot TE, Blanch L, Youngblood AM, Weinert C, Adams AB, Marini JJ, Shapiro RS, Nahum A (2000) Recruitment maneuvers in three experimental models of acute lung injury: Effects on lung volume and gas exchange. Am J Respir Crit Care Med 161:1485–1494
Rosenthal C, Caronia C, Quinn C, Lugo N, Sagy M (1998) A comparison among animal models of acute lung injury. Crit Care Med 26:912–916
Vieira SRR, Puybasset L, Richecoeur J, Lu Q, Cluzel P, Gusman PB, Coriat P, Rouby JJ (1998) A lung computed tomographic assessment of positive end-expiratory pressure-induced lung overdistension. Am J Respir Crit Care Med 158:1571–1577
Acknowledgements
We thank A. Roneus, E.-M. Hedin, K. Fagerbrink (laboratory assistants at the Department of Medical Sciences, Clinical Physiology, University Hospital, Uppsala Sweden), and O. Thamm (medical student at Department of Anesthesiology, University Hospital, Hamburg-Eppendorf, Hamburg, Germany) for their invaluable assistance, and I. Passoni (Department of Bioengineering, University of Mar del Plata, Argentina) for her expert help with data analysis. This work was performed in the Department of Medical Sciences, Clinical Physiology, University Hospital, Uppsala Sweden. Support was provided by the Swedish Medical Research Council (5315), the Swedish Heart-Lung Fund and Maquet Critical Care.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: http://dx.doi.org/10.1007/s00134-006-0372-6
Rights and permissions
About this article
Cite this article
Tusman, G., Suarez-Sipmann, F., Böhm, S.H. et al. Monitoring dead space during recruitment and PEEP titration in an experimental model. Intensive Care Med 32, 1863–1871 (2006). https://doi.org/10.1007/s00134-006-0371-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0371-7