Abstract
Objective
To investigate the potential beneficial and adverse effects of early post-pyloric feeding compared with gastric feeding in critically ill adult patients with no evidence of impaired gastric emptying.
Design
Randomised controlled studies comparing gastric and post-pyloric feeding in critically ill adult patients from Cochrane Controlled Trial Register (2005 issue 3), EMBASE and MEDLINE databases (1966 to 1 October 2005) without any language restriction were included. Two reviewers reviewed the quality of the studies and performed data extraction independently.
Measurements and results
Eleven randomised controlled studies with a total of 637 critically ill adult patients were considered. The mortality (relative risk [RR] 1.01, 95% CI 0.76–1.36, p = 0.93; I 2 = 0%) and risk of aspiration or pneumonia (RR 1.28, 95% CI 0.91–1.80, p = 0.15; I 2 = 0%) were not significantly different between patients treated with gastric or post-pyloric feeding. The effect of post-pyloric feeding on the risk of pneumonia or aspiration was similar when studies were stratified intothose with and those without the use of concurrent gastric decompression (RR ratio 0.95, 95% CI 0.48–1.91, p = 0.89). The risk of diarrhoea and the length of intensive care unit stay (weighted mean difference in days –1.46, 95% CI –3.74 to 0.82,p = 0.21; I 2 = 24.6%) were not statistically different. The gastric feeding group had a much lower risk of experiencing feeding tube placement difficulties or blockage (0 vs 9.6%, RR 0.13, 95% CI 0.04–0.44, p = 0.001; I 2 = 0%).
Conclusions
Early use of post-pyloric feeding instead of gastric feeding in critically ill adult patients with no evidence of impaired gastric emptying was not associated with significant clinical benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nutrition support has significant effects on morbidity and mortality in critically ill patients [1], and early aggressive enteral nutrition has been demonstrated to reduce infective complications and improve recovery of head-injured patients [2]. However, intolerance to gastric feeding due to impaired gastric emptying is common, and large gastric residual volumes may contribute to bacterial colonisation of the stomach and aspiration pneumonia in critically ill patients [3, 4]. Feeding via a fine-bore feeding tube that is placed beyond the pylorus can bypass the problem of impaired gastric emptying and may improve the amount of enteral nutrition delivered to the patients [1]. However, two meta-analyses evaluating the benefits of post-pyloric feeding come to different conclusions [1, 5]. The meta-analysis demonstrating the beneficial effect of post-pyloric feeding on pneumonia depended critically on the results of one study [2]. This study was not a direct comparison of gastric and post-pyloric feeding. Rather, it compared early aggressive enteral feeding with standard enteral feeding, and as part of the feeding protocol patients randomised to aggressive enteral feeding group could require a post-pyloric tube to achieve the feeding protocol targets. The different and more aggressive feeding protocol is potentially a significant confounding factor. Also, only 34% of the patients in the aggressive feeding group had a post-pyloric tube inserted, but all patients in the group were included in the ‘post-pyloric’ group for meta-analysis.
Evidence from neonatal studies suggests post-pyloric feeding is associated with an increase in mortality and gastrointestinal disturbance [6]. Placement of a feeding tube beyond the pylorus can be difficult, and serious complications due to malpositioning have been reported when endoscopic or fluoroscopic guidance was not used [7]. In addition, feeding through a post-pyloric tube without concurrent gastric decompression can result in significant undrained gastric residual volumes and may potentially increase the risk of aspiration [8]. The previous meta-analyses did not evaluate the potential adverse effects of post-pyloric feeding. We conducted a meta-analysis to re-evaluate both the potential beneficial and adverse effects of early post-pyloric feeding in critically ill adult patients with no evidence of impaired gastric emptying. We also assessed the potential confounding effects of concurrent gastric decompression, prokinetic agents, and the targeted location of the post-pyloric tube on the beneficial effect of post-pyloric feeding on pneumonia or aspiration.
Materials and methods
The literature search was performed on the Cochrane Controlled Trials Register (2005 issue 3), EMBASE and MEDLINE databases (1966 to 1 October 2005). Only randomised control clinical trials comparing early gastric feeding with post-pyloric feeding in critically ill adult patients were included. Studies comparing pyloric feeding with only intravenous hydration or parenteral nutrition were excluded. Studies comparing different feeding protocols in which some patients may require post-pyloric feeding were excluded. During the electronic database search, the following exploded MeSH terms were used: ‘post-pyloric’, ‘trans-pyloric’, ‘duoden*’, ‘jejun*’, ‘nasojejun*’, ‘nasoduoden*’, or ‘small bowel’ with ‘feed*’ or ‘nutrition’, and with ‘critically ill’, ‘intensive care’, ‘trauma’, ‘pancreatitis’, ‘peritonitis’ or ‘burns’. The reference lists of related reviews and original articles identified were searched for relevant trials. Finally, the web sites of the International Network of Agencies of Health Technology Assessment and the International Society of Technology Assessment in Health Care were searched to ensure all suitable studies were included. The authors of two studies were contacted for additional information and unpublished data that were important in the analysis, and one of them responded to our request. No studies published in languages other than English were found in the literature search. Two independent reviewers examined the titles and the abstracts of all identified trials to confirm they fulfilled the inclusion criteria. They examined and recorded the trial characteristics and outcomes independently, using a pre-designed data abstraction form. This abstraction form was used to record information regarding the quality of the trial such as allocation concealment, randomisation method, blinding of treatment, and inclusion and exclusion criteria. The quality of the study was scored according to the Jadad scale (range from 0 to 5, with a higher score indicating better study quality) [9], but the individual component that constitutes the quality of the study was also described. The grading of allocation concealment was based on the Cochrane approach, i.e. adequate or uncertain or clearly inadequate. Any disagreements between the two independent reviewers were resolved by consensus. Any duplicated publications were combined to represent one single trial. Data were checked and entered into the Review Manager (version 4.2.6 for Windows; Oxford, UK: The Cochrane Collaboration, 2003) database for further analyses.
The hospital mortality and the proportion of patients with aspiration or pneumonia were chosen as main outcomes of this meta-analysis because they are the most relevant clinical outcomes of post-pyloric feeding. The definitions of pneumonia varied among different studies, but the common definition involved new and persistent radiological changes with at least two other criteria. These criteria included the presence of purulent sputum or isolation of pathogenic bacteria in the sputum culture, peripheral leucocytosis, and significant fever (38.5 °C). Aspiration was defined as the presence of radioisotope-labelled feeds in the lungs or in the sputum. The use of dye-labelled feeds without clinical or radiological criteria to detect aspiration is insensitive [10], and therefore these ‘aspirations’ were not included in this meta-analysis. There were no missing data for these two main outcomes in the studies included. The other outcomes assessed in this meta-analysis included the proportion of patients who developed diarrhoea and complications related to the insertion of the feeding tube, including pneumothorax or major cardiorespiratory complications such as cardiopulmonary arrest. The proportion of patients who had to change over to the other mode of enteral feeding because of tube placement difficulties or blockage, and the difference in the length of intensive care unit (ICU) stay, were analysed. The percentage of daily nutritional targets attained and the time to achieve full feeding targets were also reported.
Statistical analyses
The differences in categorical outcomes between the treatment and placebo groups were reported as relative risk (RR) with 95% confidence intervals (CI), using a random effect model. The effect on the frequency of pneumonia from post-pyloric feeding was further stratified into studies with or without the use of concurrent gastric decompression and the interaction was tested by RR ratio [11]. The differences in total length of ICU length of stay and the nutritional outcomes between the post-pyloric and gastric feeding group were reported as weighted mean differences (WMD), using a random effect model. The presence of heterogeneity between trials was assessed by the chi-square statistics, and the extent of inconsistency was assessed by the I 2 statistics [12]. Sensitivity analyses after excluding patients who had aspiration but no pneumonia, studies with the use of gastric prokinetic agents, and studies that placed the post-pyloric tube in the duodenum instead of the jejunum were conducted. Publication bias was assessed by funnel plot using pneumonia or aspiration as an endpoint.
Results
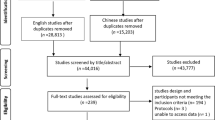
We identified 28 potentially eligible investigations, of which 11 studies [3, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22] fulfilled the inclusion criteria and were subject to meta-analysis (Fig. 1). Five studies used a blind or tactile technique to insert the post-pyloric feeding tube; in the event of failure, repeated attempts were performed with the assistance of fluoroscopy or endoscopy [3, 16, 17, 18, 19]. Three studies used endoscopy [14, 20, 21], one study used fluoroscopy [15] and one study used electromagnetic guidance for all patients [12]. One study used different techniques for different patients without specifying the criteria for the preference [22]. Five studies aimed at placing the post-pyloric tube in the duodenum [3, 13, 15, 16, 17] and four studies aimed at placing the post-pyloric tube in the jejunum [14, 20, 21, 22]. The exact targeted location of the post-pyloric tube was not specified in two studies [18, 19]. The size of the post-pyloric tube used ranged from 7 to 12 Fr (mean = 10.2). In seven studies prokinetic agents were not used in all patients [13, 15, 16, 17, 19, 20, 21]. Three studies performed concurrent gastric decompression, using either a nasogastric tube or the proximal port of the post-pyloric tube, in all patients in the post-pyloric feeding group [3, 14, 22].
All studies included patients early in the course of their ICU stay and before impaired gastric emptying was diagnosed. Eight studies recruited patients in general ICUs, one study recruited trauma patients [15], one study recruited patients from a neurological ICU [17] and one study recruited patients with acute pancreatitis [20]. The mean Acute Physiology and Chronic Health Evaluation (APACHE) II and III scores ranged from 10 to 23 and from 45 to 52, respectively. The Jadad score of the studies ranged from 2 to 4 (mean 3.1). Allocation concealment was adequate in 10 studies but only 2 studies used double blinding. The radiologists who interpreted the chest radiography of the patients were blinded to the clinical data and treatment allocation in one study [16], and the investigators interpreting the sputum results were blinded to the treatment allocation in another study [21]. The proportion of patients who were randomised but not able to complete the study was less than 5% in 10 studies. The study details are summarised in Table 1.
There was good overall consistency in most of the results without significant heterogeneity. The use of gastric feeding instead of post-pyloric feeding did not increase hospital mortality (RR 1.01, 95% CI 0.76–1.36, p = 0.93; I 2 = 0%) (Fig. 2) or the risk of pneumonia or aspiration (RR 1.28, 95% CI 0.91–1.80, p = 0.15; I 2 = 0%) (Fig. 3). The effect of post-pyloric feeding on pneumonia or aspiration was not different with and without the use of concurrent gastric decompression (RR ratio = 0.95, 95% CI 0.48–1.91, p = 0.89). The proportion of patients requiring an alternative mode of enteral feeding because of difficulties in tube placement or blockage was much lower in the gastric feeding group (0% vs. 9.6%, RR 0.13, 95% CI 0.04–0.44, p = 0.001; I 2 = 0%) (Fig. 4). The proportion of patients with diarrhoea (RR 0.85, 95% CI 0.57–1.27, p = 0.42; I 2 = 0%) was not significantly different. Complications related to the insertion of the feeding tube were reported in five studies. With the exception of one study [22], complications related to the insertion of the gastric or post-pyloric feeding tube were very rare (0.8%) and were not significantly different between the two groups (RR 0.37, 95% CI 0.06–2.15, p = 0.27; I 2 = 22.5%). The total length of ICU stay was also not significantly different (WMD –1.46 days, 95% CI –3.74 to 0.82, p = 0.21; I 2 = 24.6%) (Fig. 5). There were no significant differences in the percentage of daily nutritional targets delivered (WMD –9.1%, 95% CI–19.4 to 1.2, p = 0.08; I 2 = 38.4%) (Fig. 6) and the time required to achieve full feeding targets (WMD = −0.4 h, 95% CI –7.8 to 6.9, p = 0.91; I 2 = 79.3%) (Fig. 7) by either gastric or post-pyloric feeding.
Excluding the two studies that assessed aspiration instead of pneumonia, the four studies using prokinetic agents and the five studies targeting the post-pyloric tube in the duodenum instead of jejunum did not change the effects of post-pyloric feeding on mortality and pneumonia. One study reported cost analysis [17], but none included a formal cost-effectiveness analysis.
Discussion
Significance of our findings
This meta-analysis shows that the early use of post-pyloric feeding instead of gastric feeding was not associated with any significant clinical benefits or adverse effects. It did not change the mortality, the proportion of patients with pneumonia or aspiration, the length of ICU stay, the proportion of patients with diarrhoea, the amount of nutrition delivered or the time to achieve the feeding targets. However, difficulties in post-pyloric feeding tube placement and blockage were not uncommon.
Our results showed that the early use of post-pyloric feeding instead of gastric feeding did not reduce mortality or length of ICU stay when the same feeding protocol was used. These results are consistent with the results of previous systematic reviews [1, 5]. However, nutritional guidelines have recommended the routine use of small-bowel feeding in ICUs, when small-bowel access is feasible, because of its potential beneficial effects on two surrogate clinical outcomes, a reduced risk of pneumonia and an improvement in nutritional intake [1]. After excluding the study that assessed different feeding protocols and was not a direct comparison of gastric feeding with post-pyloric feeding [2], we could not confirm the beneficial effects of early post-pyloric feeding on these two surrogate clinical outcomes. Using post-pyloric feeding without concurrent gastric decompression can potentially leave a large volume of gastric residuals undrained and increase the risk of aspiration [8]. This may counteract the beneficial effect of post-pyloric feeding on the risk of pneumonia or aspiration. It is also possible that using prokinetic agents in the gastric feeding group or placing the post-pyloric feeding tube in the duodenum instead of the jejunum may make the beneficial effect of post-pyloric feeding on pneumonia or aspiration less apparent. However, we could not find any significant benefit on the risk of pneumonia or aspiration even when we considered the ‘best-case scenarios’ in the use of post-pyloric feeding. These scenarios included using concurrent gastric decompression with the post-pyloric feeding, omitting prokinetic agents in the gastric feeding group, and placing the post-pyloric tube in the jejunum instead of the duodenum.
Our results showed that post-pyloric feeding was not associated with significant adverse events such as diarrhoea, and the procedure of inserting the tube was very safe. However, difficulties in post-pyloric tube placement and blockage of the tube were not uncommon; therefore, about 10% of the patients in the pooled studies ‘crossed over’ to gastric feeding. This failure rate included the patients in which the passage of the tube beyond the pylorus proved to be impossible or clinically not justifiable because of repeated blockage. The actual proportion of patients with a blocked post-pyloric tube requiring tube replacement was greater than 10%. The tube placement difficulties or blockage can delay or interrupt the delivery of enteral nutrition, and therefore may reduce the amount of nutrition delivered in patients without impaired gastric emptying. Nevertheless, a new self-propelling post-pyloric tube (Tiger Tube™, Cook Group Inc.) may potentially ameliorate the tube placement problem [39], and prophylactic pancreatic enzymes may reduce the risk of blockage of the post-pyloric tube [40].
Cost analysis was reported in one study, and small bowel feeding was about 10% more expensive than gastric feeding [17]. However, placement of the post-pyloric tube was successful by a blind technique in all patients in this study. The cost of using post-pyloric feeding would probably have been much higher than gastric feeding if endoscopy or fluoroscopy had been required. In the absence of demonstrated beneficial effects on mortality and length of ICU stay, the routine early use of post-pyloric feeding without trying gastric feeding cannot be cost-effective. In patients who have high gastric volumes despite repeated attempts at gastric feeding, whether post-pyloric feeding is more preferable to parenteral nutrition remains uncertain and warrants further evaluation in large randomised control studies.
Limitations of the study
Meta-analyses are prone to bias. The quality of trials can affect the direction and magnitude of treatment effect in meta-analyses. Although most of the included studies had adequate allocation concealment, near-complete follow-up, and a Jadad score of over 3, double blinding was used in only two studies. The radiologists interpreting the chest radiography or the investigators interpreting the sputum culture were blinded to the clinical data and treatment allocation in only two studies [16, 21]. These findings confirm that the quality of many nutritional support studies was not satisfactory [41], and this might have created bias. Future nutritional support studies should consider the use of double blinding in outcome assessment. Furthermore, the number of patients included in this meta-analysis may not be enough to exclude significant clinical benefits. With the sample size of this meta-analysis (n = 500), a positive protective effect of early post-pyloric feeding on the risk of pneumonia can be demonstrated only if the associated RR reduction exceeds 40%. If post-pyloric feeding can reduce the RR of pneumonia by only 20%, a sample size of over 2,600 patients will be required to demonstrate such an effect if the baseline risk of pneumonia is 22% in the gastric feeding group. Second, publication bias can affect the direction and magnitude of the results of a meta-analysis. The funnel plot showed that publication bias was unlikely to be a significant cause of the negative results of this meta-analysis (Fig. 8). Finally, although the results of this meta-analysis were consistent across the studies included, there were significant differences in the technique used for placing the post-pyloric feeding tube and the means of confirming whether the post-pyloric tube remained in the targeted location. Evidence suggests the risk of aspiration or regurgitation may be lower if the post-pyloric tube is in the distal part of the small bowel [3]. However, the position of the post-pyloric feeding tube was not confirmed daily in some studies [14, 17, 19, 20]; therefore, some post-pyloric feeding tubes might have been displaced into the proximal duodenum or stomach, so reducing the potential benefits of post-pyloric feeding.
Conclusions
The early use of post-pyloric feeding in critically ill adult patients with no evidence of impaired gastric emptying was not associated with significant clinical benefits. Difficulties in post-pyloric tube placement or blockage were not uncommon. Routine early use of post-pyloric feeding without trying gastric feeding in critically ill adult patients is not recommended.
References
Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P, Canadian Critical Care Clinical Practice Guidelines Committee (2003) Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr 27:355–373
Taylor SJ, Fettes SB, Jewkes C, Nelson RJ (1999) Propsective, randomized, controlled trial to determine the effect of early enhanced enteral nutrition on clinical outcome in mechanically ventilated patients suffering head injury. Crit Care Med 27:2525–2531
Heyland DK, Drover JW, MacDonald S, Novak F, Lam M (2001) Effect of postpyloric feeding on gastroesophageal regurgitation and pulmonary microaspiration: results of a randomized controlled trial. Crit Care Med 29:1495–1501
Heyland DK, Tougas G, King D, Cook DJ (1996) Impaired gastric emptying in mechanically ventilated, critically ill patients.Intensive Care Med 22:1339–1344
Marik PE, Zaloga GP (2003) Gastric versus post-pyloric feeding: a systematic review. Crit Care 7:R46–51
McGuire W, McEwan P (2004) Systematic review of transpyloric versus gastric tube feeding for preterm infants. Arch Dis Child Fetal Neonatal Ed 89:F245–248
Kawati R, Rubertsson S (2005) Malpositioning of fine bore feeding tube: a serious complication. Acta Anaesthesiol Scand 49:58–61
Metheny NA, Stewart J, Nuetzel G, Oliver D, Clouse RE (2005) Effect of feeding-tube properties on residual volume measurements in tube-fed patients. JPEN J Parenter Enteral Nutr 29:192–197
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
McClave SA, Lukan JK, Stefater JA, Lowen CC, Looney SW, Matheson PJ, Gleeson K, Spain DA (2005) Poor validity of residual volumes as a marker for risk of aspiration in critically ill patients. Crit Care Med 33(2):324–330
Altman DG, Bland JM (2003) Interaction revisited: the difference between two estimates. BMJ 326:219
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Esparza J, Boivin MA, Hartshorne MF, Levy H (2001) Equal aspiration rates in gastrically and transpylorically fed critically ill patients. Intensive Care Med 2001 27:660–664
Davies AR, Froomes PR, French CJ, Bellomo R, Gutteridge GA, Nyulasi I, Walker R, Sewell RB (2002) Randomized comparison of nasojejunal and nasogastric feeding in critically ill patients. Crit Care Med 30:586–590
Kortbeek JB, Haigh PI, Doig C (1999) Duodenal versus gastric feeding in ventilated blunt trauma patients: a randomized controlled trial. J Trauma 46:992–996
Kearns PJ, Chin D, Mueller L, Wallace K, Jensen WA, Kirsch CM (2000) The incidence of ventilator-associated pneumonia and success in nutrient delivery with gastric versus small intestinal feeding: a randomized clinical trial. Crit Care Med 28:1742–1746
Day L, Stotts NA, Frankfurt A, Stralovich-Romani A, Volz M, Muwaswes M, Fukuoka Y, O'Leary-Kelley C (2001) Gastric versus duodenal feeding in patients with neurological disease: a pilot study. J Neurosci Nurs 33:148–149, 155–159
Neumann DA, DeLegge MH (2002) Gastric versus small-bowel tube feeding in the intensive care unit: a prospective comparison of efficacy. Crit Care Med 30:1436–1438
Boivin MA, Levy H (2001) Gastric feeding with erythromycin is equivalent to transpyloric feeding in the critically ill. Crit Care Med 29:1916–1919
Eatock FC, Chong P, Menezes N, Murray L, McKay CJ, Carter CR, Imrie CW (2005) A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis. Am J Gastroenterol 100:432–439
Montecalvo MA, Steger KA, Farber HW, Smith BF, Dennis RC, Fitzpatrick GF, Pollack SD, Korsberg TZ, Birkett DH, Hirsch EF, Craven DE (1992) Nutritional outcome and pneumonia in critical care patients randomized to gastric versus jejunal tube feedings. The Critical Care Research Team. Crit Care Med 20:1377–1387
Montejo JC, Grau T, Acosta J, Ruiz-Santana S, Planas M, Garcia-De-Lorenzo A, Mesejo A, Cervera M, Sanchez-Alvarez C, Nunez-Ruiz R, Lopez-Martinez J, Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units (2002) Multicenter, prospective, randomized, single-blind study comparing the efficacy and gastrointestinal complications of early jejunal feeding with early gastric feeding in critically ill patients. Crit Care Med 30:796–800
Pupelis G, Selga G, Austrums E, Kaminski A (2001) Jejunal feeding, even when instituted late, improves outcomes in patients with severe pancreatitis and peritonitis. Nutrition 17:91–94
Singh G, Ram RP, Khanna SK (1998) Early postoperative enteral feeding in patients with nontraumatic intestinal perforation and peritonitis. J Am Coll Surg 187:142–146
Hoover HC Jr, Ryan JA, Anderson EJ, Fischer JE (1980) Nutritional benefits of immediate postoperative jejunal feeding of an elemental diet. Am J Surg 139:153–159
Smith RC, Hartemink RJ, Hollinshead JW, Gillett DJ (1985) Fine bore jejunostomy feeding following major abdominal surgery: a controlled randomized clinical trial. Br J Surg 72:458–461
Hwang TL, Huang SL, Chen MF (1991) Early nasoduodenal feeding for the post-biliary surgical patient. J Formos Med Assoc 90:993–997
Malhotra A, Mathur AK, Gupta S (2004) Early enteral nutrition after surgical treatment of gut perforations: a prospective randomised study. J Postgrad Med 50:102–106
Moore EE, Jones TN (1986) Benefits of immediate jejunostomy feeding after major abdominal trauma–a prospective, randomized study. J Trauma 26:874–881
Kaur N, Gupta MK, Minocha VR (2005) Early enteral feeding by nasoenteric tubes in patients with perforation peritonitis. World J Surg 29:1023–1027
Zapas JL, Karakozis S, Kirkpatrick JR (1998) Prophylactic jejunostomy: a reappraisal. Surgery 124:715–719
Adams GF, Guest DP, Ciraulo DL, Lewis PL, Hill RC, Barker DE (2000) Maximizing tolerance of enteral nutrition in severely injured trauma patients: a comparison of enteral feedings by means of percutaneous endoscopic gastrostomy versus percutaneous endoscopic gastrojejunostomy. J Trauma 48:459–464
Spain DA, DeWeese RC, Reynolds MA, Richardson JD (1995) Transpyloric passage of feeding tubes in patients with head injuries does not decrease complications. J Trauma 39:1100–1102
Minard G, Kudsk KA, Melton S, Patton JH, Tolley EA (2000) Early versus delayed feeding with an immune-enhancing diet in patients with severe head injuries. JPEN J Parenter Enteral Nutr 24:145–149
Pandey SK, Ahuja V, Joshi YK, Sharma MP (2004) A randomized trial of oral refeeding compared with jejunal tube refeeding in acute pancreatitis. Indian J Gastroenterol 23:53–55
Levy B, Perrigault PF, Gawalkiewicz P, Sebire F, Escriva M, Colson P, Wahl D, Frederic M, Bollaert PE, Larcan A (1998) Gastric versus duodenal feeding and gastric tonometric measurements. Crit Care Med 26:1991–1994
Monteferrante E, Mancini G, Pedrazzoli C, Rozzi F, Ciampaglia F, Manso L, Listorto G (1999) The nasojejunal tube in early postoperative nutrition. Minerva Chir 54:551–555
Strong RM, Condon SC, Solinger MR, Namihas BN, Ito-Wong LA, Leuty JE (1992) Equal aspiration rates from postpylorus and intragastric-placed small-bore nasoenteric feeding tubes: a randomized, prospective study. JPEN J Parenter Enteral Nutr 16:59–63
Davies AR, Bellomo R (2004) Establishment of enteral nutrition: prokinetic agents and small bowel feeding tubes. Curr Opin Crit Care 10:156–161
Bourgault AM, Heyland DK, Drover JW, Keefe L, Newman P, Day AG (2003) Prophylactic pancreatic enzymes to reduce feeding tube occlusions. Nutr Clin Pract 18:398–401
Doig GS, Simpson F, Delaney A (2005) A review of the true methodological quality of nutritional support trials conducted in the critically ill: time for improvement. Anesth Analg 100:527–533
Acknowledgements
We would like to thank Dr. Navneet Kaur for providing information from his study.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was solely funded by the Department of Intensive Care, Royal Perth Hospital. No financial support was received for this study from pharmaceutical companies or other private companies in the form of grants and awards.
Rights and permissions
About this article
Cite this article
Ho, K.M., Dobb, G.J. & Webb, S.A.R. A comparison of early gastric and post-pyloric feeding in critically ill patients: a meta-analysis. Intensive Care Med 32, 639–649 (2006). https://doi.org/10.1007/s00134-006-0128-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0128-3