Abstract
Objective
To evaluate the prevalence of pulmonary hypertension associated with severe acute respiratory distress syndrome (ARDS) and to asses the value of pulmonary artery trunk diameter (PAT) to predict pulmonary hypertension.
Design
Prospective study
Setting
University teaching hospital and ARDS referral center.
Patients
103 patients with ARDS, who received both right heart catheterization and chest computed tomography.
Interventions
None.
Measurements and results
95 patients (92.2%) with ARDS had pulmonary artery hypertension, 16 of them (16.8%) mild, 72 (75.8%) moderate, and 7 (7.4%) severe, as assessed by right heart catheterization. Of the patients with moderate and severe pulmonary hypertension, 43 had a pulmonary artery trunk diameter ≥ 29 mm yielding a sensitivity of 0.54 and a specificity of 0.63. Pulmonary artery trunk diameter correlated significantly but weakly with mean pulmonary artery pressure (r = 0.34, p = 0.0004). The positive predictive value was 0.83, and the negative predictive value was 0.28. The diagnosis of pulmonary hypertension by PAT diameter measurements was incorrect in 43.7% of patients with ARDS.
Conclusions
Pulmonary artery hypertension has a high prevalence in patients with severe ARDS. Measurement of PAT diameter on admission CT scan is an unreliable tool for identification of ARDS patients with pulmonary hypertension.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary hypertension (PH) is a feature of various advanced chronic diseases of the pulmonary vasculature, lung parenchyma, or airways. Detecting PH provides important prognostic information [1] and can define the need for inhalational vasodilator therapy [2] or other medications such as bosentan [3] or sildenafil [4]. In patients with primary pulmonary hypertension and chronic heart or pulmonary disease, attempts have been made to estimate pulmonary artery pressure (PAP) by less-invasive techniques such as echocardiography [5, 6], computed tomography (CT) [7], or magnetic resonance imaging [8]. While echocardiography has been shown to be inaccurate in as much as 50% of patients with advanced lung disease when compared with right heart catheterization [9], a pulmonary artery trunk (PAT) diameter of 29 mm measured on CT scans had an excellent diagnostic yield in detecting PH in patients with advanced lung disease, with a sensitivity of 87% and a specificity of 89% [7].
In patients with acute respiratory distress syndrome (ARDS), PH is a frequent observation [10, 11], although little is known about the prevalence and magnitude of PH in patients with severe ARDS. That PH might be of relevance in patients suffering from ARDS is suggested by Squara et al., who observed that patients dying from ARDS had a significantly higher PAP than survivors and concluded that lowering PAP may by itself be an important therapeutic strategy [11]. Obviously, recognizing increased PAP and its decrease implies correct identification of patients with increased PAP.
Direct measurement of PAP by pulmonary artery catheterization (PAC), the gold standard for measuring pulmonary artery pressure (PAP), has been shown to be safe and not associated with increased morbidity and mortality in severely ill patients with sepsis and ARDS [12]. Nevertheless, alternative and less invasive methods would be welcome in many patients. In this respect, CT scanning is of particular interest, since CT scanning in ARDS patients is often helpful in defining pathology and treatment strategy, and in chronic cardiopulmonary disease can predict PH fairly reliably [7]. However, ARDS is an acute illness and, therefore, the extent of pulmonary artery distension or its adaptation to increased pressure is unknown, as is the transferability of results obtained in chronic illness to severe ill ARDS patients.
Therefore, we performed this study to assess (1) the prevalence of PH in patients with severe ARDS, and (2) if an increased pulmonary artery trunk diameter (≥ 29 mm) measured by CT scan on admission is reliable for predicting PH in patients with ARDS.
Material and methods
One hundred three (103) patients referred with the diagnosis of ARDS and receiving both chest CT and a pulmonary artery catheter (PAC) on admission as a routine work-up were analyzed. All patients had been referred to our unit for advanced ARDS treatment from other hospitals and were mechanically ventilated in the pressure-controlled mode with pure oxygen, with a respiratory rate of 20 breaths per minute, an inspiratory-to-expiratory ratio of unity, a positive end-expiratory pressure (PEEP) of 10 cmH2O or more, and a peak inspiratory pressure to achieve a tidal volume of approximately 6 ml/kg, as recommended elsewhere [13]. ARDS was defined as described elsewhere [14] and by a lung injury score (LIS) greater than 2.5 [15].
Measurements
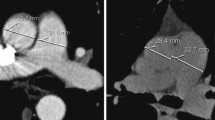
Following patients' arrival by helicopter or ambulance equipped for transport of critically ill patients, PAT diameter was measured by CT scan. All patients were examined using a standardized protocol. CT images were obtained after central venous injection (100 ml) of non-ionic iodinated contrast agent (iobitridol, Guerbet, Sulzbach, Germany) at a flow of 3 ml/s, using an automated injector (Liebel, Flarsheim, Germany). Contrast-enhanced CT scans were acquired 35 s after the start of injection to examine the thorax from the upper-abdomen to the neck. In all patients, scanning was performed using an expiratory breath hold technique with a single breath hold at adjusted PEEP, a voltage of 120 kV, and a current of 50–150 mA. Contiguous reconstructed sections were obtained with 5-mm collimation in the mediastinum and lung kernel and stored on optical disk in a digital picture archiving and communication system (PACS, Centricity, GE Medical, Dornstadt, Germany) for further review.
The pulmonary artery trunk (PAT) diameter was measured independently by two investigators (M.B., H.K.) within 3 cm below the pulmonary artery bifurcation at its widest portion, as described elsewhere [7, 16], and the mean value was used for further analysis.
Following CT scanning, the patients were transported to our ICU, where mean pulmonary artery pressure, referenced to the mid axillary line, was measured at end expiration within 120 min of the CT scan, using a flow-directed catheter (Edwards Lifesciences, Unterschleissheim, Germany). During this period, the patterns of mechanical ventilation, including PEEP, were kept constant. All vascular pressures were recorded at end expiration as displayed on the oscilloscopes of the respirator (Evita 4, Dräger Medical, Lübeck, Germany) and hemodynamic monitor (Siemens 1281, Erlangen, Germany). For cardiac output measurements by thermodilution, 10 ml of cold saline was injected in triplicate, and mean values were used for further calculations such as pulmonary vascular resistance and right ventricular stroke work index. Simplified acute physiologic score [17] and sepsis-related organ failure assessment [18] were calculated from lowest values obtained within the first 24 h after admission.
Definitions
To evaluate the prevalence of PH in patients with ARDS, we defined PH as presence of a mean pulmonary artery pressure of at least 25 mmHg, following the US National Institutes of Health (NIH) and the World Health Organization (WHO) [19]. For further correlations a mean PAP of 30 mmHg (moderate PH) and more was used. Pulmonary artery trunk (PAT) diameter was considered increased with a diameter of ≥ 29 mm, in accordance with others [7, 20].
Plot of mean pulmonary artery pressure (PAP) obtained by right heart catheterization and pulmonary artery trunk diameter measured on computed tomography scans of patients with acute respiratory distress syndrome. Threshold for pulmonary artery hypertension with computed tomography (pulmonary artery trunk diameter ≥ 29 mm, vertical bar), and with right heart catheterization (mean pulmonary artery pressure ≥ 30 mmHg, horizontal bar)
Right ventricular failure was defined as an enlarged right atrium (> 4 cm) and right ventricle (right ventricular end-diastolic area greater than left ventricular end-diastolic area) during trans-esophageal echocardiography in the presence of normal left ventricular dimension and contractility and the demand for inotropic support in the presence of pulmonary artery hypertension.
Statistical analysis
All metric data are presented as means ( ± standard deviation, SD). Correlations were performed using the Pearson correlation analysis. A p value of less than 5% was considered statistically significant. The software SAS 8.02 (SAS Institute, Cary, NC, USA) was used for statistical analysis. For comparison of groups, the Wilcoxon rank test was used, if variables were continuous. Fisher's exact test was used for dichotomous variables. Otherwise, the chi-square test was used.
To assess independent predictors of death, logistic regression modeling, combined with a backward variable elimination, was employed. The required level of significance for a variable to remain in the model was set to 0.05. Logistic regression was also applied to establish relations between high values of PAP (PAP ≥ 25 mmHg) and PAT (and compliance). As a measure of association, we report the area under the receiver-operating-characteristics (ROC) curve. For assessment of interobserver variability in measurements of PAT, we calculated the concordance correlation of Lin [21].
Results
Ninety-five (95) patients (92.2%) had a pulmonary hypertension associated with ARDS, 16 of them (16.8%) graded mild, 72 (75.8%) graded moderate, and seven (7.4%) graded severe. The etiology for the ARDS was sepsis in 32 (31.1%), trauma in seven (6.7%), aspiration in eight (7.8%), and pneumonia in 56 patients (54.4%), respectively.
Mean pulmonary artery trunk diameter in ARDS patients averaged 29.2 ± 4.1 mm at a mean pulmonary artery pressure (PAP) of 35.4 ± 8.8 mmHg (Table 1). The interobserver concordance in respect to the measurement of the PAT diameter was high, with a concordance correlation coefficient of 0.9. There was a significant correlation between PAT diameter and PAP (r = 0.34, p < 0.001). PaCO2 correlated with lung injury score (LIS, r = 0.4, p = 0.003), PaO2/FiO2 (r = −0.41, p < 0.001), compliance (−0.42, p < 0.001), but only weakly with PAP (r = 0.21, p = 0.03) and PAT (r = 0.24, p = 0.16).
Thirty-seven (37) patients (35.9%) died, 29.7% of them due to right heart failure (Table 3). Patients who died had a significantly lower compliance (24.6 ± 12.1 ml mbar−1 vs. 30.6 ± 12.1 ml mbar−1, p = 0.033), were older (51 ± 13 years vs. 37 ± 13 years, p < 0.0001), and were on mechanical ventilation for a longer time prior to admission to our ICU (9 ± 9 days vs. 5 ± 6 days, p = 0.013). Truncus diameter was not different between survivors and non-survivors (28.9 ± 4.3 mm vs. 30.6 ± 4.3 mm). A logistic regression analysis revealed older age (odds ratio (OR): 1.083, 95% confidence interval (CI): 1.03–1.13; p = 0.0001), PaCO2 (OR: 1.02, CI: 1.01–1.04; p = 0.007), and sequential organ failure assessment score on admission (SOFA, OR: 1.2, CI: 1.03–1.4; p = 0.017) as independent predictors of death.
Seventy-nine (79) patients with PH (83.2%) had moderate or severe pulmonary hypertension associated with ARDS. Of these, 43 had a PAT diameter of 29 mm or greater, yielding a sensitivity of 0.54. Specificity averaged 0.63. The positive predictive value was 0.83 (43/52), whereas the negative predictive value was 0.28 (15/51). The optimal discrimination point was determined from ROC analysis as PAT = 29 mm.
In patients with moderate or severe pulmonary hypertension, correlations were found between PaCO2 and LIS (r = 0.36, p = 0.006), PaO2/FiO2 (r = −0.37, p < 0.001), compliance (r = −0.4, p < 0.001), and PAT diameter (r = 0.33, p = 0.003), but not with PAP (r = 0.18, p = 0.11). Furthermore, PaO2/FiO2 was correlated with compliance (r = 0.38, p < 0.001).
Logistic regression of PAP ≥ 25 mmHg vs. PAP < 25 mmHg on PAT did not reveal a significant predictive value for PAT (OR = 1.07, CI: 0.86–1.33, p = 0.53). Accordingly, the area under the ROC curve was at 0.55, only marginally above the value 0.5 of the straight line. Adding compliance to the logistic model did not advance the predictive power.
Discussion
Our data show that pulmonary hypertension has a high prevalence in patients with severe ARDS. Of patients referred to our ARDS center, 92.2% met the definition for PH. Even when using the threshold for moderate PH (≥ 30 mmHg), 79 patients (76.7%) still presented with PH. Most important, PAT diameter on the CT scan performed immediately after admission was unreliable in predicting PH in patients with severe ARDS, with a negative predictive value of only 0.28. Furthermore, the prediction was incorrect in as much as 44% of patients. Even a threshold of PAT diameter greater than 29 mm resulted in an incorrect assumption of PH in 17.3% of patients, whereas 70.6% of patients with a PAT diameter of less than 29 mm did have PH.
With reported specificities and sensitivities of 89% and 87%, respectively, a PAT diameter of 29 mm predicts PH fairly well in patients with chronic pulmonary disease [7]. In patients with severe ARDS and hence rapid onset pulmonary arterial hypertension, we showed that, while a PAT diameter of 29 mm is the optimum cutoff point for PH detection, it has much less specificity and sensitivity than in chronic conditions.
Possibly, pulmonary vascular changes in chronic pulmonary arterial hypertension lead to remodeling of the pulmonary artery, resulting in increased diameter. Nevertheless, due to its incorrect prediction in almost half of the patients, PAT diameter measurements are inappropriate for clinical decision-making in ARDS.
PH in ARDS has received revived attention, as permissive hypercarbia can cause acute cor pulmonale [22], and even short-lasting elevations of PAP can lead to impairment of right ventricular function [23]. The interpretation of these results remains speculative, as the outcome of patients with ARDS and PH has not yet been determined. An indication of the relevance of PH arises from the observation that patients who died from ARDS had a significantly higher PAP over a period of 3 weeks than patients who survived [11]. The importance of looking at PAP is underlined by right heart failure being the second most common cause of death in our patients. Whether therapeutic strategies in lowering PAP [2–4] are of value in patients with ARDS is unclear. In any case, before treatment, a reliable but less invasive measurement of PAP than right heart catheterization would be welcome.
In patients with chronic cardiopulmonary disease, measurements of pulmonary artery diameter have been reported to detect PH [7, 20, 24]. However, serial measurements by CT scan have never been used to monitor either treatment or the course of illness. In contrast, echocardiography has been appraised as an excellent tool for this purpose due to its minimal invasiveness [25]. In a large prospective study on patients evaluated for lung or heart/lung transplantation, however, echocardiography was recently shown to have limited value in diagnosing PH when compared with right heart catheterization. Comparable with our study, PH prediction was incorrect in over 50% of patients [9]. By the same token, an incorrect prediction of PH by echocardiography was found in pregnant women when compared with right heart catheterization [26]. Therefore, a prediction of pulmonary artery pressure by radiological techniques is of questionable value when the pulmonary artery pressure has been estimated by echocardiography [8, 20]. Furthermore, it remains to be shown whether these results are transferable to critically ill, mechanically ventilated patients.
It has been shown that right heart catheterization in patients with acute respiratory distress syndrome changed the therapy in most cases [27], although the benefit of these changes has not been demonstrated. According to our results, PAC is the only reliable tool to identify ARDS patients with PH, and the only method to monitor PAP-lowering therapies.
Our study has limitations. First, all previous radiologic studies on PAT diameter were performed in patients breathing spontaneously at atmospheric pressure. This is an important objection, as mechanical ventilation by increasing alveolar pressure and lung volume may alter intra-mediastinal pressure, decrease transmural pulmonary artery pressure, and hence end-expiratory PAT diameter. However, since variation of positive end-expiratory pressure among patients was small and, due to the low lung compliance in ARDS, increased alveolar pressure is transmitted to only a small extent to the mediastinum, it is very unlikely that mechanical ventilation resulted in much bias. In fact, any bias decreasing PAT diameter would underestimate the presence of PH, i.e., prediction would be even worse. Second, because all patients were referred from other hospitals in severe distress by helicopter or ambulance, they received the CT scanning even before entering the ICU, where a pulmonary artery catheter was subsequently placed. Therefore, we cannot rule out that values of PAP during CT scan may have been somewhat different than after ICU admission. However, any error is likely to be random and of small magnitude, as PAP in patients with ARDS is known to change little over much longer time periods [11].
We report a high incidence of PH in severe ARDS. Furthermore, the magnitude of PH is higher than documented in large randomized trials [13] and observational studies [11], and right ventricular failure as cause of death has not been emphasized. Our patients are different from those included in studies and can be regarded as treatment failures by their referring hospitals referred as emergency admissions to our institution. This raises the question of whether investigations in ARDS patients in general correctly reflect pulmonary hemodynamics in severe ARDS. Our data confirm observations of Hemmila et al. [28], who found PH of similar magnitude in patients with severe ARDS referred to extracorporeal lung support. Interestingly, they recorded PH in the presence of normocarbia, suggesting that structural pulmonary changes rather than hypercarbia are largely responsible for PH in severe ARDS [28]. This assumption is also supported by the weak correlation between PaCO2 and PAP in our patients and no influence of PaCO2 in patients with moderate and severe PH. Therefore, it remains speculative to which extent an increased PaCO2 influences the PAP in patients with already-increased pulmonary vascular resistance due to ARDS or other parenchymal changes. PAP has been reported to be significantly higher in patients dying from ARDS than in surviving patients [11], but, in this latter series, PH was still approximately 7 torr lower than in our patients. Accordingly, the high incidence of right heart failure in our patients with severe ARDS is the consequence of PH. PaCO2, next to older age and SOFA, was an independent predictor of death.
In summary, 92% of patients with severe ARDS admitted to an ARDS treatment center suffer from PH. However, prediction of pulmonary arterial hypertension by PAT diameter measurements is of limited value, as the prediction is incorrect in almost half of the patients. Its sensitivity of 0.54 and specificity of 0.63 make CT scanning an unreliable clinical tool in predicting PH in ARDS patients.
References
Oswald-Mammosser M, Weitzenblum E, Quoix E, Moser G, Chaouat A, Charpentier C, Kessler R (1995) Prognostic factors in COPD patients receiving long-term oxygen therapy: importance of pulmonary artery pressure. Chest 107:1193–1198
Olschewski H, Simonneau G, Galiè N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Speich R, Hoeper MM, Behr J, Winkler J, Sitbon O, Popov W, Ghofrani HA, Manes A, Kiely DG, Ewert R, Meyer A, Corris PA, Delcroix M, Gomez-Sanchez M, Siedentop H, Seeger W, the Aerosolized Iloprost Randomized Study Group (2002) Inhaled Iloprost for severe pulmonary hypertension. N Engl J Med 347:322–329
Rubin LJ, Badesch DB, Barst RJ, Galiè N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G, the Bosentan Randomized Trial of Endothelin Antagonist Therapy Study Group (2002) Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 346:896–903
Sastry BK, Narasimhan C, Reddy NK, Raju BS (2004) Clinical efficacy of sildenafil in primary pulmonary hypertension. J Am Coll Cardiol 43:1149–1153
Berger M, Haimowitz A, Van Tosh A, Berdorff RL, Goldberg E (1985) Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol 6:359–365
Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, Reeder GS, Nishimura RA, Tajik AJ (1985) Continuous wave Doppler estimation of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol 6:750–756
Tan RT, Kuzo R, Goodman LR, Siegel R, Haasler GB, Presberg KW (1998) Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Chest 113:1250–1256
Krüger S, Haage P, Hoffmann R, Breuer C, Bücker A, Hanrath P, Günther RW (2001) Diagnosis of pulmonary arterial hypertension and pulmonary embolism with magnetic resonance angiography. Chest 120:1556–1561
Arcasoy SM, Christie JD, Ferrari VA, Sutton MSJ, Zisman DA, Blumenthal NP, Pochettino A, Kotloff RM (2003) Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease Am J Respir Crit Care Med 167:735–740
Zapol WM, Snider MT (1977) Pulmonary hypertension in severe acute respiratory failure. N Engl J Med 296:476–480
Squara P, Dhainaut JFA, Artigas A, Carlet J, the European Collaborative ARDS working group (1998) Hemodynamic profile in severe ARDS: result of the European Collaborative ARDS study. Intensive Care Med 24:1018–1028
Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnoud D, Boulain T, Lefort Y, Fartoukh M, Baud F, Boyer A, Brochard L, Teboul JL, for the French Pulmonary Artery Catheter Study Group (2003) Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome. A randomized controlled trial. JAMA 290:2713–2720
The Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R, The American–European Consensus Conference on ARDS (1994) Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Murray JF, Matthay MA, Luce JM, Flick MR (1988) An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 138:720–723
Edwards PE, Bull RK, Coulden R (1998) CT measurement of main pulmonary artery diameter. Br J Radiol 71:1018–1020
Le Gall JR, Lemeshow S, Saulnier F (1993) A new simplified acute physiology score (SAPS II) based on a European / North American multicenter study. JAMA 270:2957–2963
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med 22:707–710
Rich S, Dantzker DR, Ayres S, Bergofsky EH, Brundage BH, Dentre KM (1987) Primary pulmonary hypertension: a national prospective study. Ann Intern Med 107:216–223
Kuriyama K, Gamsu G, Stern RG, Cann CE, Herfkens RJ, Brundage BH (1984) CT-determined pulmonary artery diameters in predicting pulmonary hypertension. Invest Radiol 19:16–22
Lin LI (1989) A concordance correlation coefficient to evaluate reproducibility. Biometrics 45:255–268
Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B, Beauchet A, Jardin F (2001) Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: Incidence, clinical implications, and prognosis. Crit Care Med 29:1551–1555
Michard F, Wolff MA, Herman B, Wysocki M (2001) Right ventricular response to high-dose almitrine infusion in patients with severe hypoxemia related to acute respiratory distress syndrome. Crit Care Med 29:32–36
Haimovici JB, Trotman-Dickenson B, Halpern EF, Dec GW, Ginns LC, Shepard JA, McLoud TC (1997) Relationship between pulmonary artery diameter at computed tomography and pulmonary artery pressures at right-sided heart catheterization. Acad Radiol 4:327–34
Dini FL, Nuti R, Barsotti L, Baldini U, Dell'Anna R, Micheli G (2002) Doppler-derived mitral and pulmonary venous flow variables are predictors of pulmonary hypertension in dilated cardiomyopathy. Echocardiography 19:457–465
Penning S, Robinson KD, Major CA, Garite TJ (2001) A comparison of echocardiography and pulmonary artery catheterization for evaluation of pulmonary artery pressures in pregnant patients with suspected pulmonary hypertension. Am J Obstet Gynecol 184:1568–1570
Marinelli WA, Weinert CR, Gross CR, Knoedler JP, Bury CL, Kangas JR, Leatherman JW (1999) Right heart catheterization in acute lung injury. An observational study. Am J Respir Crit Care Med 160:69–76
Hemmila MR, Rowe SA, Boules TN, Miskulin J, McGillicuddy JW, Schuerer DJ, Haft JW, Swaniker F, Arbabi S, Hirschl RB, Bartlett RH (2004) Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg 240:595–607
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Beiderlinden, M., Kuehl, H., Boes, T. et al. Prevalence of pulmonary hypertension associated with severe acute respiratory distress syndrome: Predictive value of computed tomography. Intensive Care Med 32, 852–857 (2006). https://doi.org/10.1007/s00134-006-0122-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0122-9