Abstract
Vasopressin is a potent vasopressor for improving organ perfusion during septic shock. The rationale for the use of vasopressin is its relative deficiency of plasma levels and hypersensitivity to its vasopressor effects during septic shock. Growing evidence suggests that low-dose (<0.04 U/min) vasopressin is safe and effective for the treatment of vasodilatory shock. Although it is being used more frequently, there are no randomized clinical trials comparing vasopressin as a first-line agent to commonly used vasopressors. However, vasopressin causes arterial smooth muscle cell contraction through a non-catecholamine receptor pathway, thus it represents an attractive adjunct to the management of septic shock, especially when catecholamines are ineffective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vasopressin was first characterized in 1895 by Oliver and Schaefer [1], who recognized that neurohypophyseal extracts had potent vasoconstrictive effects. Three decades later, it was renamed antidiuretic hormone based on its effects on the distal tubule of the kidney. Until recently, its utility for vasoconstriction outside of variceal hemorrhage and hepatorenal syndrome has been largely forgotten [2, 3, 4, 5]. Recent inclusion of vasopressin in the American Heart Association Adult Cardiac Life Support Guidelines [6] has caused a “rediscovery” of its vasopressor effects and a new role in cardiac arrest. Vasopressin is also useful for the treatment of septic shock, however few clinicians are aware of its mechanism of action or the data supporting its use.
Vasopressin under normal conditions
Synthesis and metabolism
Vasopressin is a nonapeptide with a molecular mass of 1084 Daltons. (Fig. 1) It is strongly basic (isoelectric point pH 10.9) due to the amidation of three carboxyl groups and its biologic activity is readily destroyed by oxidation or reduction of its disulfide bond. Prepro-vasopressin is encoded by the 2.5 kb vasopressin-neurophysin II gene on the long arm of chromosome 20 (20p13) [7, 8]. Pro-vasopressin is generated by the removal of the signal peptide from prepro-vasopressin and glycosylation in magnocellular neurons in the hypothalamus. Vasopressin precursors migrate along neuronal axons that terminate in the posterior hypophysis. (Fig. 2) Additional post-translational processing of pro-vasopressin occurs within neurosecretory vesicles yielding vasopressin and neurophysin, which are secreted from axon terminals in the posterior pituitary.
Central neural pathways important in vasopressin synthesis and release. Vasopressin is synthesized in the cell bodies of magnocellular neurons located in the paraventricular nuclei within the hypothalamus and released into the bloodstream after migration via the supraoptic-posterior hypophyseal tract
Most newly synthesized vasopressin is stored intracellularly, only 10–20% of the total hormonal pool within the posterior pituitary can be readily released. The time from synthesis to release of the hormone into the systemic circulation is about 1.5 h [9]. Once secreted into the circulation, vasopressin is accompanied, but not bound, by its carrier protein, neurophysin II, which does not appear to have any independent biological activity. The plasma half-life of vasopressin is short, about 5–15 m. Thus, plasma concentrations [normal: 1 pg/ml (10–12 m)] reflect recent release of active hormone. Clearance occurs through vasopressinases in the liver and kidneys and is concentration independent. Vasopressin is also found in large quantities in platelets. Accordingly, vasopressin concentration in platelet-rich plasma is approximately five- to six-fold higher than in platelet-depleted plasma.
Mechanisms of action
Vasopressin has several important physiological functions including water retention by the kidneys and constriction of vascular smooth muscle (Table 1). Vasopressin exerts its effects via interaction with a family of membrane-bound G protein-coupled vasopressin-specific receptors, V1 and V2. The V1 receptors are located on vascular smooth muscle cells whereas V2 receptors are on the basolateral surface of cells of the distal convoluted tubules and medullary collecting ducts. There are also V3 receptors that are located on the anterior hypophysis and pancreatic islet cells, in the latter they play a role in insulin secretion [10].
While both receptors function via activation of guanosine triphosphate-binding proteins, their second messengers are different [11]. V1 receptor-ligand interactions lead to activation of phospholipase C, which promotes hydrolysis of phosphatidylinositol-(4,5)-biphosphate and the formation of inositol (1,4,5)-triphosphate and diacylglycerol (Fig. 3). Inositol (1,4,5)-triphosphate (IP3) acts as a second messenger that interacts with its own receptor on the endoplasmic reticulum promoting mobilization of calcium from intracellular stores and, thereby, leading to vascular smooth muscle contraction [11]. The V2 receptor is coupled to adenyl cyclase, which produces cAMP (cyclic adenosine monophosphate). Subsequent activation of cAMP-dependent protein kinases (PK) such as PKA causes recruitment of water channel proteins (aquaporin-2 [AQP2], a member of AQP family proteins) from cytoplasmic vesicles into the luminal membrane of the renal tubule, thereby increasing permeability of the luminal cell membrane to water [12]. Vasopressin may also increase translocation of AQP2 to the membrane and opening of individual water channels [13]. Once APQ2s are recruited, the bulk of water flow across the collecting tubule proceeds through epithelial cells rather than via intracellular junctional complexes [14, 15].
Mechanisms of action of vasopressin at the cellular level. Both V1 and V2 vasopressin receptors are G protein-coupled receptors. A Stimulation of V1 receptor by vasopressin leads to dissociation of Gq. The α-subunit of Gq then stimulates PLCβ, which promotes hydrolysis of PIP2, leading to increase in the levels of DAG and IP3 intracellularly. The IP3 acts on its specific receptor (IP3R) on the endoplasmic reticulum membrane promoting release of calcium from intracellular stores. B Interaction of vasopressin with its V2 receptor causes dissociation of Gs into its subunits, among which the α-subunit stimulates adenyl cyclase. Activation of adenyl cyclase leads to an increase in cAMP, which then activates PKA and the insertion of the pre-synthesized water channel (AQP2) into membrane. AQP-2 aquaporin-2, DAG diacylglycerol, GTP guanosine triphosphate, GDP guanosine diphosphate, IP3 inositol (1,4,5)-triphosphate, IP3R inositol (1,4,5)-triphosphate receptor, PIP2 phosphatidylinositol (4,5)-biphosphate, PKA protein kinase Α, PLCβ phospholipase Cβ, V1 V1 vasopressin receptor, V2 V2 vasopressin receptor
Vascular bed dependent functions
In addition to its antidiuretic action, vasopressin has functions that depend on the sensitivity of a vascular bed to vasopressin; most notable of these are its effects on the systemic and pulmonary circulation.
Systemic circulation
On a molar basis, vasopressin is a more potent vasoconstrictor than angiotensin II or norepinephrine [16]. Vasopressin constricts systemic arteries via V1 receptors in a dose-dependent fashion. However, exogenous vasopressin has negligible vasopressor activity in healthy individuals, nor are patients with the syndrome of inappropriate antidiuretic hormone predisposed to hypertension [17, 18, 19]. The overall effect of vasopressin on systemic blood pressure is minimal at normal plasma concentrations, as vasoconstriction is generally counteracted by baroreceptor-mediated reduction in cardiac output [20]. Although, similar baroreflex-mediated effects offset the rise in blood pressure with other vasopressors, it is more pronounced with vasopressin [21].
The difference in pressor response between vasopressin and other vasopressors may reflect a centrally mediated effect via activation of brain V1 receptors causing a leftward shift of the heart rate-arterial pressure baroreflex response [21, 22, 23, 24, 25, 26]. The site involved in the modulation of the baroreflex control of heart rate by vasopressin appears to be area postrema, where there is high expression of V1 receptors [21, 26, 27, 28]. In contrast to vasopressin, catecholamines do not have an effect on area postrema and therefore do not cause a similar degree of baroreflex response [21, 27]. Accordingly, a supra-physiologic dose of vasopressin (approximately 50 times the normal) is usually required to cause significant increases in mean arterial blood pressure in normal animals and humans [29, 30].
While in vitro and animal studies show an increase in intracellular calcium concentration and inotropic effect after stimulation of myocardial V1 receptors [31, 32], vasopressin may have net negative inotropic and chronotropic effects due to increased vagal and decreased sympathetic tone as well as decreased coronary blood flow (a consequence of coronary vasoconstriction) when circulating levels of vasopressin are high [33].
The degree of vasoconstriction by vasopressin differs among vascular beds [34, 35]. Vasopressin is more potent in skin, skeletal muscle, adipose tissue and pancreas than in mesenteric, coronary and cerebral circulations [33, 36]. Diminished vasoconstrictor effect in coronary and cerebral circulations may be due to the paradoxical release of nitric oxide (NO) by vasopressin in these vascular beds [37]. Recently, a small study of intrabrachial administration of vasopressin reported a dose-dependent biphasic change (vasoconstriction followed by vasodilation) of forearm blood vessels in humans [38]. Sustained infusion was associated with preservation of the vasodilatory effect, which was presumed to be mediated by NO, however, tachyphylaxis to the constrictive effects of vasopressin was noted. The receptor subtype responsible for vasodilation is uncertain, however the V2 receptor agonist, 1-desamino[8-D-arginine]vasopressin (DDAVP) decreases peripheral vascular resistance and causes facial flushing in humans and peripheral vasodilation in dogs. Similarly, inhibition of V2 receptors hinders the vasodilatory response of the renal afferent arteriole to vasopressin [39, 40]. Alternately, it has been suggested that endothelial oxytocin receptors may mediate vasopressin-induced NO production and vasodilation [41].
The effect of vasopressin on splanchnic perfusion is controversial. While earlier studies reported reduced mesenteric perfusion even at physiologic concentrations (as low as 10 pg/ml) [42, 43, 44, 45, 46, 47], more recent data using a vasopressin analog in endotoxemic animals suggest otherwise [48]. This discrepancy may be attributed to differences in volume status between studies. Asfar and colleagues challenged endotoxic animals with fluids prior to administration of the V1 specific analog, terlipressin [48]. Vasopressin increased systemic blood pressure without affecting gastrointestinal hemodynamics in fluid-challenged animals. These results suggest that maintenance of normal intravascular volume prevents vasopressin-induced reductions in splanchnic perfusion.
A similar discrepancy about the effects of vasopressin on gastrointestinal perfusion has been observed in human studies. Paralleling the results of the most recent animal study [48], Dünser and colleagues reported an improvement in gastrointestinal mucosal perfusion (assessed by gastric tonometry) in patients with septic shock during combined vasopressin and norepinephrine infusion compared to norepinephrine alone [49]. The differences between this study and earlier studies may be due to the use of higher concentrations and bolus application of vasopressin in earlier investigations [50].
Pulmonary circulation
Vasopressin vasodilates the pulmonary circulation decreasing pulmonary vascular resistance and pressure under both normal and hypoxic conditions as a consequence of V1 receptor-mediated release of NO from endothelial cells [41, 51, 52, 53]. Pulmonary artery vasodilation occurs with low concentrations of vasopressin [54]. Pulmonary vascular resistance does increase when extremely high levels of plasma vasopressin are achieved (>300 pg/ml) [55]. Unlike other vasoactive agents, such as epinephrine [56], vasopressin does not appear to alter ventilation perfusion relationships [57] in patients who undergo cardiopulmonary resuscitation, thus, this finding may not be relevant in the setting of vasodilatory shock.
Other functions
Vasopressin acts within the central nervous system to lower body temperature and facilitate memory consolidation and retrieval [58, 59]. It also heightens hypothalamic sensitivity to corticotropin-releasing hormone, thereby increasing adrenocorticotropic hormone (ACTH) release and cortisol production [60, 61]. This effect is likely carried out through NO and cGMP (cyclic guanosine monophosphate) via central V3 receptors, which were previously thought to be V1 receptor subtypes [62]. Vasopressin does not appear to affect oxytocin release [63].
Activation of V1 receptors by high levels of vasopressin leads to platelet aggregation [39, 64]. Extra-renal V2 receptors appear to mediate the release of several coagulation factors (Factor VIIIc, and von Willebrand factor) in response to the administration of DDAVP, which is a selective V2-agonist (antidiuretic/vasoconstrictive ratio 4000:1) [65, 66]. It has been suggested, but not proven, that DDAVP decreases peripheral vascular resistance and, consequently, systemic blood pressure and increases plasma renin activity via vascular V2 receptors [67], although such receptors remain to be identified on endothelial cells.
Regulation of secretion
Vasopressin secretion is regulated by both serum osmolality (osmoregulation) and blood pressure (baroregulation) and is released in response to a variety of stimuli (hemorrhage, hypoxia, hypertonic saline) in normal humans and animals. (Table 2) Both in normal humans and experimental animals, vasopressin release is attenuated or eliminated by pretreatment with glucocorticoids [68]. This downregulation of vasopressin release is mediated by a direct effect of glucocorticoids on the hypothalamus and/or neurohypophysis [69]. Vasopressin and corticotropin-releasing hormone co-localize in neurohypophyseal parvocellular neurons projecting to the median eminence and the neurohypophyseal portal blood supply of the anterior pituitary. Levels of both vasopressin and corticotropin-releasing hormone in these neurons are inversely related to plasma glucocorticoid levels. However, vasopressin appears to be considerably less sensitive to negative feedback than the corticotropin-releasing factor-ACTH system. Since neurohypophyseal vasopressin is involved in the control of ACTH secretion, it is likely that the modulation of neurohypophyseal vasopressin by glucocorticoids is part of the overall regulation of ACTH secretion.
Under normal conditions, vasopressin secretion is regulated primarily by changes in plasma osmolality [70, 71] (Fig. 4). In healthy adults, the osmotic threshold for vasopressin secretion ranges from 275 to 290 mosmol/kg (average about 280 mosmol/kg). When plasma osmolality is less than 280 mosmol/kg, plasma vasopressin levels range from 0.5 to 2 pg/ml (less than 4 pg/ml) [72]. In general, a 1 mosmol/kg rise in plasma osmolality should increase plasma vasopressin levels by 0.38 pg/ml and urinary osmolality by 100 mosmol/kg [13, 73, 74]. Maximally to suppress plasma vasopressin (<0.25 pg/ml) and maximally dilute the urine (<100 mosmol/kg), total body water needs to increase only by 2% (5.6 mosmol/kg). In contrast, a 2% decrease in total body water will result in doubling the plasma vasopressin level (i.e., from 1 to 2 pg/ml). Maximal urine concentration is achieved at a plasma osmolality of about 290–292 mosmol/kg and a plasma vasopressin level of 5–6 pg/ml. As a rule of thumb, a 1 pg/ml rise in plasma vasopressin increases urine osmolality by about 200 mosmol/kg.
Intravascular volume-mediated regulation of vasopressin release is regulated by baroreceptors in the left atrium, carotid sinus and aortic arch [73]. However, the minimal effect of small changes in blood volume and pressure on vasopressin secretion contrasts sharply with the extraordinary sensitivity of the osmoregulatory system. Under resting conditions or when stretched, baroreceptors inhibit vasopressin secretion. Decreased activity due to low blood pressure decreases baroreceptor neuronal output and results in the release of vasopressin from the hypothalamus. Atrial baroreceptors respond to smaller changes in blood volume than do arterial receptors and likely play a dominant role in eliciting vasopressin secretion [73, 75, 76]. This is particularly true for left atrial baroreceptors, which are more sensitive than those in the right atrium [13, 73].
There is an exponential inverse relationship between plasma vasopressin levels and the percent decline in mean arterial pressure in the setting of acute hypotension [13]. Small reductions in blood pressure (5–10% from baseline) usually have little or no effect on plasma vasopressin whereas a 20–30% drop results in hormone levels several fold higher than those required to produce maximal antidiuresis. Vasopressin response to acute reductions in blood volume is not well defined but appears to be quantitatively and qualitatively similar to the response to blood pressure [77, 78, 79]. Volume depletion in both experimental animals and humans produces little elevation in plasma vasopressin levels until the blood volume decreases by more than 8–10% [73, 80]. Further volume depletion results in exponential increases in plasma vasopressin levels. For example, a 10–15% fall in effective blood volume usually doubles hormone levels, whereas a 20% decline results in 20–30 fold increases in serum vasopressin levels. While less is known about the influence of acute elevations in blood volume or pressure, both appear to suppress vasopressin secretion [81].
In animals, reduction of left atrial pressure decreases the osmotic threshold and increases the sensitivity for osmotic release of vasopressin. In contrast, high left atrial pressure raises the threshold and dampens the sensitivity of osmoregulation [13, 73]. Fluid loading can suppress vasopressin secretion even in the presence of hyponatremia [13, 73] Changes in the osmotic set point in response to volume-mediated stimuli can be abolished by opioid antagonists [13, 73].
Due to interdependence between the osmo- and baro-regulation of vasopressin secretion, under conditions of moderate hypovolemia osmoregulation is preserved and renal water excretion is maintained, albeit at a lower plasma osmolality [70, 71, 82, 83]. As hypovolemia worsens, plasma vasopressin concentrations reach extremely high values and baroregulation overrides osmoregulation. In the elderly, osmoreceptor sensitivity is enhanced whereas baroregulation is blunted.
Vasopressin during septic shock
Pathophysiology of septic shock
Septic shock is characterized by physiologically inappropriate vasodilation leading to organ hypoperfusion despite adequate fluid resuscitation [84]. The mechanisms of hypotension during septic shock are multifactorial and include relative hypovolemia, ineffective intravascular volume and myocardial dysfunction. Blood return to the right ventricle is typically diminished because of relative hypovolemia due to a combination of loss of intravascular volume from capillary leak and increased venous capacitance. Blood return to the left ventricle is also compromised due to increased pulmonary vascular resistance.
Excessive vasodilation during septic shock occurs despite increased catecholamine levels and activation of the renin-angiotensin-aldosterone system [85, 86, 87, 88]. Hyposensitivity of α-adrenergic receptors to catecholamines due to tissue hypoxia and acidosis also contributes to vasodilation.
The membrane potential of arterial smooth muscle cells is regulated by adenosine triphosphate (ATP) sensitive K+-channels, which are important regulators of arterial tone [89, 90]. The opening of K+-channels closes voltage-dependent Ca2+-channels, decreasing intracellular calcium levels leading to smooth muscle relaxation and vasodilation [91]. Septic shock is associated with activation of these K+-ATP channels [89, 90]. Activation of the inducible form of NO synthase and deficiency of vasopressin also contribute to the vasodilation of septic shock.
Current management
In addition to identification of the nidus infection, judicious fluid administration to compensate for effective hypovolemia due to vasodilation is an important early step in the resuscitation of patients with septic shock [92]. This is an especially salient concern because absolute hypovolemia may develop as intravascular volume loss due to “third spacing” from capillary leak and increased insensible losses are common during sepsis. However, the assessment of volume status and adequacy of the fluid resuscitation is challenging and often based on clinical grounds. Therefore, it is not unusual to “under-resuscitate” these patients. In fact, Rivers et al. reported that the requirement for adequate fluid resuscitation in septic shock is frequently more than 5 L of crystalloids when invasive monitoring techniques are utilized [92].
Persistent hypotension with evidence of organ hypoperfusion despite volume resuscitation necessitates vasopressor agents. Vasopressors such as dopamine, norepinephrine, phenylephrine and epinephrine, alone or in combination, are typically used to treat septic shock. In some patients, these agents prove ineffective in maintaining adequate organ perfusion due to attenuated vasopressor response [93, 94, 95]. Similarly, there is a decreased response to the potent endogenous vasopressors, endothelin-1 and angiotensin II [96]. Differences in responsiveness to catecholamines among individuals are commonly observed and may represent differences of volume status, duration of septic shock (late versus early), phenotypic variations in responsiveness to endotoxin and other inflammatory mediators, and possibly downregulation and/or impairment of catecholamine receptors [94, 97, 98]. While numerous studies report the attributes of vasopressor regimens for the treatment of hypotension, it is important to bear in mind that there are no data that convincingly demonstrate a survival benefit with the use of any particular catecholamine or combination of catecholamines in septic shock.
Vasopressin physiology during septic shock
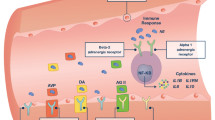
Two unique attributes of vasopressin make it well suited for the management of septic shock: (1) there often exists a relative deficiency of vasopressin and (2) the sensitivity of the systemic circulation to vasopressin during septic shock is increased (Table 3).
Vasopressin is important in maintaining arterial blood pressure during hypotension. Indeed, most forms of shock are associated with appropriately high levels of vasopressin (Fig. 5). Vasoconstrictive properties of vasopressin are important, especially when intravascular volume or arterial blood pressure is threatened as inhibition of V1 receptors causes marked hypotension in subjects with arterial underfilling [16, 17, 29]. The primary stimulus for vasopressin release in hypotensive states is baroreceptor-mediated [99]. It is noteworthy that exogenous vasopressin does not result in a marked pressor response when administered to volume-depleted, hypotensive patients, perhaps because the V1 receptors on vascular smooth muscle are already occupied by endogenous hormone [23, 100, 101].
Endotoxin stimulates vasopressin release directly, independent of baroreceptor activity [102]. Experimental endotoxemia is associated with a prompt rise in vasopressin levels as early as 15 min after its administration [45, 103]. In addition, acute phase cytokines [i.e. interleukin-1β (IL-1β), IL-6, tumor necrosis-α] enhance vasopressin production [104, 105, 106].
Plasma vasopressin levels demonstrate a biphasic pattern during septic shock, in both animals and humans, that is characterized by a significant rise in early septic shock. Conversely, vasopressin levels in late septic shock are inappropriately low for the degree of hypotension. This decline begins as early as 6 h after the diagnosis of septic shock and results in relative deficiency within 36 h [107] In a recent study by Sharshar et al., all patients were noted to have levels below 10 pg/ml within 24 h of the diagnosis of septic shock [107]. It is noteworthy that this relative deficiency contributes to diminished vasoconstriction (10–100 pg/ml) but does not affect antidiuretic action (0–7 pg/ml).
Low vasopressin levels are most likely due to impaired vasopressin secretion, rather than increased metabolism as vasopressinase levels remain undetectable during established septic shock [90, 108]. A single study of three patients reported undetectable plasma vasopressinase levels, which were attributed to renal and hepatic dysfunction commonly seen in septic patients [108]. Proposed mechanisms for reduced serum vasopressin during sepsis include the exhaustion of pituitary stores caused in response to baroreceptor-mediated release, autonomic dysfunction, inhibitory effects of increased norepinephrine and increased release of NO in the posterior pituitary, (which may downregulate vasopressin production) [90, 109, 110]. In fact, sepsis may lead to hypothalamic dysfunction/failure and NO mediated reductions of vasopressin [111].
The other unique attribute of vasopressin is increased sensitivity to its vasoconstrictive effects during septic shock [110]. Although the precise mechanism of this marked sensitivity to vasopressin during septic shock is not known at this time, it is probably multifactorial. Low plasma concentrations of vasopressin during septic shock make its V1 receptors available for binding by exogenously administered hormone. In contrast, exogenous catecholamines must compete for receptor-binding sites with endogenous catecholamines, which already occupy these receptors and lead to receptor desensitization/downregulation. Alternately, increased sensitivity may result from altered baroreflexes during septic shock. Loss of autonomic nervous regulation has been reported in hyperdynamic states such as septic shock and portal hypertension [112, 113]. There is a dissociated cardiovascular response to vasopressin in states of dysautonomia preventing the negative inotropic effect that normally offsets its vasopressor effect [23, 114]. Conceivably, sepsis may alter V1 receptor response in area postrema and alter the normal baroreflex response.
Another explanation for increased sensitivity to the pressor effects of vasopressin may be alterations in receptor expression and/or signal transduction. Interestingly, increased response to vasopressin during sepsis appears to occur in the face of decreased vasopressin receptor density [115, 116]. In fact, in both in vitro and in vivo models, sepsis reduces vasopressin receptor levels [115, 116]. This effect was dependent on NO in vitro [115] and mediated by pro-inflammatory cytokines in an NO-independent manner in vivo [116]. In these models, sepsis did not alter downstream receptor signaling systems (G proteins, IP3) [115].
Other factors contributing to increased sensitivity to vasopressin include potentiation of the vasoconstrictive effects of catecholamines [117] and vasopressin-mediated direct inactivation of K+-ATP channels in a dose-dependent manner [118]. Vasopressin enhances the sensitivity of the vasculature to the effects of catecholamines, potentiating the contractile effect of catecholamines, electrical stimulation and KCl in the arteries [119, 120, 121]. This effect is most likely mediated by prostaglandins as it can be inhibited by cortisol and lithium. Vasopressin also stimulates synthesis of the most potent vasopressor known, endothelin-I [122, 123]. Vasopressin’s effect on K+-ATP channels is particularly interesting as these channels are important regulators of arterial tone and play a key role in the pathogenesis of septic shock and probably in decreased responsiveness to catecholamines [89, 90, 124]. Vasopressin also attenuates endotoxin and IL-1β-stimulated generation of NO [120, 125] and directly decreases intracellular concentrations of cGMP, the second messenger of NO [126, 127]. In the setting of persistent lactic acidosis, cGMP levels are high and likely contribute to the peripheral vasodilation and subsequent persistent hypotension during septic shock. Whatever the cause, heightened sensitivity probably compensates for decreased serum levels and reduced receptor density during septic shock.
The presence and contribution of adrenal insufficiency and the need for steroid therapy in septic shock are unresolved controversies. Vasopressin may provide another favorable effect by increasing cortisol levels. Pharmacologic doses of vasopressin in animals and humans induce a prompt rise in plasma cortisol levels. Theoretically, depressed levels of vasopressin may be a contributor to the relative adrenal insufficiency found in some patients with septic shock.
Differences between vasopressin and other vasopressors
There are myriad differences between vasopressin and catecholamines. While catecholamines show vasopressor effects through α-adrenergic receptors, vasopressin acts on V1 receptors located on the vascular endothelium (Table 4). There is decreased vasopressor activity to catecholamines during septic shock [88, 93, 94, 128, 129], whereas there is increased sensitivity of V1 receptors during septic shock. Furthermore, the pressor effects of vasopressin are preserved during hypoxia and acidosis [130], while there is resistance to α-adrenergic catecholamines.
Another important difference between vasopressin and catecholamines is extrarenal V2 receptor-mediated vasodilation in selected vascular beds [40, 131]. Vasodilation occurs at low concentrations and appears to be NO-mediated [51, 54]. Interestingly, arteries of the circle of Willis are more sensitive to the vasodilatory effects of vasopressin than other intracranial and extracranial arteries [132].
Vasopressin also differs from catecholamines in terms of its effects on the kidneys. Besides its antidiuretic effects and osmoregulatory properties, vasopressin causes a paradoxical diuretic effect in patients with hepatorenal syndrome, congestive heart failure [133] and early septic shock (<24 h) [134]. Proposed mechanisms of this diuretic effect include relative resistance of renal vasculature to the vasoconstrictive effects of vasopressin [135], downregulation of V2 receptors [136], NO-mediated afferent arteriolar vasodilation [137, 138] and oxytocin receptor-activated natriuresis. At low doses, vasopressin causes efferent arteriolar vasoconstriction with relatively little effect on afferent arteriole (NO-mediated) [137, 138], thereby increasing renal perfusion pressure [138]. Vasopressin also releases atrial natriuretic peptide [139], which may be an indirect mechanism of its diuretic effect. It is not clear whether increased urine output is due to an improvement in renal function, as the creatinine does not change.
Like catecholamines, higher doses of vasopressin (>0.04 U/min) cause a dose-dependent fall in renal blood flow, glomerular filtration rate and sodium excretion [140, 141]. Afferent arteriole and medullary vessels appear to be the most sensitive parts of the renal vasculature. A V1 receptor antagonist can block the vasoconstrictor action of vasopressin on the afferent arteriole. Interestingly, even norepinephrine-induced vasoconstriction of the afferent arteriole can be abolished by treatment with vasopressin if the V1 receptor is blocked.
Clinical evidence for the use of vasopressin in septic shock
Despite available experimental data and increased enthusiasm for vasopressin in the management of septic shock, there are no clinical data to suggest any superiority (i.e., outcome benefits) over conventional pressors for the treatment of septic shock. Available studies about vasopressin in the management of septic shock consist of case series [110, 142], retrospective analyses [134, 143] and several small, randomized, controlled trials [49, 144, 145, 146]. Similar to vasopressin, terlipressin, a vasopressin analog, has also been shown to improve hemodynamics during septic shock in a case series [147] (Table 5). Vasopressin is effective in restoring blood pressure, decreasing the need for catecholamines in septic shock and also other forms of vasodilatory shock, such as late phase hemorrhagic shock [148], post-cardiotomy [143, 149, 150, 151, 152, 153], post left-ventricular assist device placement [154], solid organ transplantation [155, 156, 157] and milrinone-induced hypotension [158] (Table 6).
In all case series and randomized clinical trials, vasopressin has been shown to improve systemic blood pressure without significant adverse effects on cardiac function or pulmonary hemodynamics [49, 110, 134, 142, 143, 144, 145]. In some studies there was improvement in cardiac output [143, 153, 159, 160], probably due to the decreased use of norepinephrine, attenuation of endotoxin and IL-1β stimulated generation of NO [120], and increased intracellular calcium in myocardial cells [31, 32]. In some patients with septic shock, after hemodynamic stability had been achieved with vasopressin, attempts to discontinue it were unsuccessful [145]. A combination of vasopressin and norepinephrine appears to be more effective than norepinephrine alone in treating hemodynamic instability during vasodilatory shock [49].
Despite a growing body of evidence for the use of vasopressin in patients who remain hypotensive despite escalating doses of catecholamines, no data is available about its role as a first-line vasopressor. Given that earlier achievement of a normal hemodynamic status may improve outcome in septic shock [92], it would make sense to administer vasopressin early in the disease before refractory hypotension develops, to prevent end-organ damage. Doing so may allow diminution of the amounts of other vasopressors, which could potentially forestall development of insensitivity to catecholamines. However, lack of clinical evidence on its use early in the course of disease along with no proof of outcome benefit render it too premature to make such recommendations at this point.
Vasopressin dose during septic shock
Exogenous vasopressin can generate plasma concentrations similar to that expected for a particular degree of hypotension and causes a marked pressor response provided intravascular volume is adequate [110]. Infusion of vasopressin at 0.01 U/min raises plasma vasopressin levels to approximately 30 pg/ml, which are slightly higher than the levels reported in patients with cardiogenic shock (about 23 pg/ml) [29, 110]. Raising the infusion rate to 0.04 U/min increases plasma levels to 100 pg/ml [29, 110], which is substantially higher than the levels found during cardiogenic shock [110] and the degree of hypotension [161, 162]. Similarly, studies of low-dose vasopressin (0.01–0.04 U/min) in septic shock can also produce plasma levels that are appropriate for the degree of hypotension.
Volume-resuscitated, septic patients usually respond to vasopressin with a rise in blood pressure. However, there remain several unanswered questions, such as which criteria should be used for titration of the vasopressin dose in order to get optimal benefits from the treatment, what should be the target blood pressure and its role (or perhaps relative contraindication) in low output septic shock. It is also not clear whether vasopressin infusion should be started at a specific dose (i.e., 0.01 U/min) and titrated according to the hemodynamic response, as in the case of conventional vasopressors. It is also not known whether plasma vasopressin levels should be checked to determine relative deficiency of vasopressin before this is administered. It is not possible to determine whether an individual patient has relatively low vasopressin levels based on clinical information only [107]. At this time, routine testing of vasopressin levels does not appear to be feasible due to time requirement for processing the test (about 1 week). Clinical response to a trial of vasopressin is likely to be the best option in determining who would respond to this treatment.
Concerns about vasopressin use
The strong vasoconstrictive properties of vasopressin raise concerns about hypoperfusion in several vascular beds. Among these, the splanchnic circulation is perhaps the one of most concern since gut hypoperfusion may be a contributor to the development of multiorgan dysfunction syndrome. As reviewed above, the data regarding vasopressin’s effects on splanchnic perfusion are conflicting. Although vasopressin does not appear to worsen gastrointestinal perfusion in humans when volume status is optimized [49], the possibility of splanchnic hypoperfusion cannot be excluded. Similarly, there remain concerns regarding myocardial ischemia, which was reported at high doses (10–25 times the dose currently used for septic shock) or delivered via central venous catheter [163, 164].
Available data suggest that the risk of myocardial ischemia from low-dose vasopressin (<0.04 U/min) is small, even when it is administered via a central venous catheter [144, 145]. However, in a retrospective study by Holmes et al., there was increased mortality in patients with septic shock treated with vasopressin when compared to historical controls [134]. Although the majority of patients died due to refractory shock and multiorgan system failure, there was an increased incidence of cardiac arrests, which could potentially be attributed to vasopressin use. One patient developed pulseless electrical activity following a drop in cardiac output with vasopressin at 0.03 U/min. However, it is important to note that the dose of vasopressin was more than 0.05 U/min in four out of six patients who had cardiac arrest. Given the lack of large, randomized, controlled studies addressing the potential adverse effects of vasopressin on the heart, clinicians must remain cautious when using vasopressin, particularly in patients with underlying cardiovascular disease. While nitroglycerin was recommended with high-dose vasopressin in the management of variceal bleeding, there are no data suggesting a need for nitroglycerin with low-dose vasopressin.
Vasopressin is a potent vasoconstrictor of skin vessels at high doses [165, 166]. Extravasation of even small amounts of vasopressin can cause local skin necrosis [167]. Thus, administration via peripheral vessels should be avoided if possible. Bilirubin levels may increase during vasopressin infusion [49]. Although a direct cause and effect relationship has not been demonstrated and no clear mechanisms are known, liver function tests should be followed closely during vasopressin infusion.
Complications related to “low”-dose vasopressin appear to be infrequent and minor and can generally be prevented by avoiding bolus administration and infusion rates greater than 0.04 U/min.
Terlipressin
Terlipressin is a non-selective, synthetic analog of vasopressin that has a slightly greater affinity for vascular V1 receptors than vasopressin (V1/V2 receptor ratio of 2.2 versus 1 for vasopressin) [168]. It has been utilized in Europe in the management of variceal hemorrhage with improved outcome and has been suggested to become first-line therapy due to its improved tolerance compared to endoscopic intervention [2, 4]. It has also been successfully used in the management of hepatorenal syndrome with even greater treatment success when used in conjunction with albumin [3, 5, 169].
Terlipressin may have some advantages over vasopressin. It is less expensive than vasopressin and its long half-life (about 6 h) makes single dose boluses possible, whereas vasopressin is given as an infusion for several days. A single dose of terlipressin (1–2 mg) increases systemic blood pressure within 10–20 min in patients with septic shock. This improvement in blood pressure is sustained for at least 5 h [147]. This is an important attribute of terlipressin as rebound hypotension following discontinuation of vasopressin infusion is a common phenomenon in septic shock [144]. However, bolus infusion may be limited due to potential adverse effects such as increased pulmonary vascular resistance and uncontrolled rise in blood pressure and, therefore, continuous infusion may be preferable [170, 171].
In an experimental model of sepsis, terlipressin increased mean arterial blood pressure in control and endotoxic animals, albeit with a greater increase in the latter [172]. Pulmonary vascular resistance did not change in the control group but increased in the septic animals. It is not clear whether this was due to the reduction in cardiac output or direct vasoconstrictive effects of vasopressin. Interestingly, terlipressin decreased oxygen delivery and consumption in both groups, possibly due to decreased oxygen demand (rather than a decrease in cardiac output). This might be due to antipyretic effects via central V1 receptor and anti-inflammatory effects via release of catecholamines and subsequent activation of β2-adrenergic receptors [173].
Conclusion
The available data regarding the use of vasopressin to sustain blood pressure and organ perfusion in septic shock are, in general, encouraging. Its catecholamine receptor-independent mechanism of action positions vasopressin as a highly useful vasoconstrictor that should be considered early in the course of septic shock when catecholamine infusion rates are being escalated following volume resuscitation. We believe that this approach offers the possibility of forestalling the development of catecholamine unresponsive shock.
References
Oliver G, Schaefer EA (1895) On the physiological action of extract of pituitary body and certain other glandular organs. J Physiol 18:277–279
Escorsell A, Ruiz del Arbol L, Planas R, Albillos A, Banares R, Cales P, Pateron D, Bernard B, Vinel JP, Bosch J (2000) Multicenter randomized controlled trial of terlipressin versus sclerotherapy in the treatment of acute variceal bleeding: the TEST study. Hepatology 32:471–476
Ortega R, Gines P, Uriz J, Cardenas A, Calahorra B, De Las Heras D, Guevara M, Bataller R, Jimenez W, Arroyo V, Rodes J (2002) Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology 36:941–948
Combier E, Levacher S, Letoumelin P, Joseph A, Pourriat JL, de Pouvourville G (1999) Cost-effectiveness analysis of the terlipressin-glycerin trinitrate combination in the pre-hospital management of acute gastro-intestinal haemorrhage in cirrhotic patients. Intensive Care Med 25:364–370
Antoniades C, Auzinger G (2003) Terlipressin and albumin for the hepatorenal syndrome. Hepatology 37:946, Author’s reply 946
Anonymous (2000) Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Part 6: Advanced cardiovascular life support: Section 6: Pharmacology II: agents to optimize cardiac output and blood pressure. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation 102:I-129–135
Rao VV, Loffler C, Battey J, Hansmann I (1992) The human gene for oxytocin-neurophysin I (OXT) is physically mapped to chromosome 20p13 by in situ hybridization. Cytogenet Cell Genet 61:271–273
Riddell DC, Mallonee R, Phillips JA, Parks JS, Sexton LA, Hamerton JL (1985) Chromosomal assignment of human sequences encoding arginine vasopressin-neurophysin II and growth hormone releasing factor. Somat Cell Mol Genet 11:189–195
Sklar AH, Schrier RW (1983) Central nervous system mediators of vasopressin release. Physiol Rev 63:1243–1280
Lee B, Yang C, Chen TH, al-Azawi N, Hsu WH (1995) Effect of AVP and oxytocin on insulin release: involvement of V1b receptors. Am J Physiol 269:E1095–1100
Birnbaumer M (2000) Vasopressin receptors. Trends Endocrinol Metab 11:406–110
Deen PM, Verdijk MA, Knoers NV, Wieringa B, Monnens LA, van Os CH, van Oost BA (1994) Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science 264:92–95
Berl T, Robertson GL (2000) Pathophysiology of water metabolism. In: Brenner BM (ed) Brenner & Rector’s The Kidney. Saunders, Philadelphia, pp 866–924
Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S (1993) Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361:549–552
Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW (1993) Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci U S A 90:11663–11667
Reid IA, Schwartz J (1984) Role of vasopressin in the control of blood pressure. In: Martini L, Ganong WF (eds) Frontiers in neuroendocrinology. Raven Press, New York, pp 177–197
Wagner HWJ, Braunwald E (1956) The pressor effect of the antidiuretic principle of the posterior pituitary in orthostatic hypotension. J Clin Invest 35:1412–1418
Ohnishi K, Saito M, Nakayama T, Hatano H, Okuda K (1987) Effects of vasopressin on portal hemodynamics in patients with portal hypertension. Am J Gastroenterol 82:135–138
Morton JJ, Padfield PL (1986) Vasopressin and hypertension in man. J Cardiovasc Pharmacol 8(Suppl 7):S101–106
Abboud FM, Floras JS, Aylward PE, Guo GB, Gupta BN, Schmid PG (1990) Role of vasopressin in cardiovascular and blood pressure regulation. Blood Vessels 27:106–115
Undesser KP, Hasser EM, Haywood JR, Johnson AK, Bishop VS (1985) Interactions of vasopressin with the area postrema in arterial baroreflex function in conscious rabbits. Circ Res 56:410–417
Luk J, Ajaelo I, Wong V, Wong J, Chang D, Chou L, Reid IA (1993) Role of V1 receptors in the action of vasopressin on the baroreflex control of heart rate. Am J Physiol 265:R524–529
Cowley AW Jr, Monos E, Guyton AC (1974) Interaction of vasopressin and the baroreceptor reflex system in the regulation of arterial blood pressure in the dog. Circ Res 34:505–514
Cox BF, Hay M, Bishop VS (1990) Neurons in area postrema mediate vasopressin-induced enhancement of the baroreflex. Am J Physiol 258:H1943–1946
Webb RL, Osborn JW Jr, Cowley AW Jr (1986) Cardiovascular actions of vasopressin: baroreflex modulation in the conscious rat. Am J Physiol 251:H1244–251
Tribollet E, Ueta Y, Heitz F, Marguerat A, Koizumi K, Yamashita H (2002) Up-regulation of vasopressin and angiotensin II receptors in the thalamus and brainstem of inbred polydipsic mice. Neuroendocrinology 75:113–123
Peuler JD, Edwards GL, Schmid PG, Johnson AK (1990) Area postrema and differential reflex effects of vasopressin and phenylephrine in rats. Am J Physiol 258:H1255–1259
Xue B, Gole H, Pamidimukkala J, Hay M (2003) Role of the area postrema in angiotensin II modulation of baroreflex control of heart rate in conscious mice. Am J Physiol Heart Circ Physiol 284:H1003–1007
Mohring J, Glanzer K, Maciel JA Jr, Dusing R, Kramer HJ, Arbogast R, Koch-Weser J (1980) Greatly enhanced pressor response to antidiuretic hormone in patients with impaired cardiovascular reflexes due to idiopathic orthostatic hypotension. J Cardiovasc Pharmacol 2:367–376
Cowley AW Jr, Switzer SJ, Guinn MM (1980) Evidence and quantification of the vasopressin arterial pressure control system in the dog. Circ Res 46:58–67
Xu YJ, Gopalakrishnan V (1991) Vasopressin increases cytosolic free [Ca2+] in the neonatal rat cardiomyocyte. Evidence for V1 subtype receptors. Circ Res 69:239–245
Fujisawa S, Iijima T (1999) On the inotropic actions of arginine vasopressin in ventricular muscle of the guinea pig heart. Jpn J Pharmacol 81:309–312
Laszlo FA, Laszlo F Jr, De Wied D (1991) Pharmacology and clinical perspectives of vasopressin antagonists. Pharmacol Rev 43:73–108
Garcia-Villalon AL, Garcia JL, Fernandez N, Monge L, Gomez B, Dieguez G (1996) Regional differences in the arterial response to vasopressin: role of endothelial nitric oxide. Br J Pharmacol 118:1848–1854
Moursi MM, van Wylen DG, D’Alecy LG (1985) Regional blood flow changes in response to mildly pressor doses of triglycyl desamino lysine and arginine vasopressin in the conscious dog. J Pharmacol Exp Ther 232:360–368
Liard JF, Deriaz O, Schelling P, Thibonnier M (1982) Cardiac output distribution during vasopressin infusion or dehydration in conscious dogs. Am J Physiol 243:H663–669
Vanhoutte PM, Katusic ZS, Shepherd JT (1984) Vasopressin induces endothelium-dependent relaxation of cerebral and coronary, but not of systemic, arteries. J Hypertens Suppl 2:S421–422
Affolter JT, McKee SP, Helmy A, Jones CR, Newby DE, Webb DJ (2003) Intra-arterial vasopressin in the human forearm: pharmacodynamics and the role of nitric oxide. Clin Pharmacol Ther 74:9–16
Bichet DG, Razi M, Lonergan M, Arthus MF, Papukna V, Kortas C, Barjon JN (1988) Hemodynamic and coagulation responses to 1-desamino[8-D-arginine] vasopressin in patients with congenital nephrogenic diabetes insipidus. N Engl J Med 318:881–887
Liard JF (1992) cAMP and extrarenal vasopressin V2 receptors in dogs. Am J Physiol 263:H1888–1891
Thibonnier M, Conarty DM, Preston JA, Plesnicher CL, Dweik RA, Erzurum SC (1999) Human vascular endothelial cells express oxytocin receptors. Endocrinology 140:1301–1309
Cronenwett JL, Baver-Neff BS, Grekin RJ, Sheagren JN (1986) The role of endorphins and vasopressin in canine endotoxin shock. J Surg Res 41:609–619
Avontuur JA, Bruining HA, Ince C (1995) Inhibition of nitric oxide synthesis causes myocardial ischemia in endotoxemic rats. Circ Res 76:418–425
Wilson MF, Brackett DJ, Archer LT, Hinshaw LB (1980) Mechanisms of impaired cardiac function by vasopressin. Ann Surg 191:494–500
Brackett DJ, Schaefer CF, Tompkins P, Fagraeus L, Peters LJ, Wilson MF (1985) Evaluation of cardiac output, total peripheral vascular resistance and plasma concentrations of vasopressin in the conscious, unrestrained rat during endotoxemia. Circ Shock 17:273–284
Heyndrickx GR, Boettcher DH, Vatner SF (1976) Effects of angiotensin, vasopressin and methoxamine on cardiac function and blood flow distribution in conscious dogs. Am J Physiol 231:1579–1587
Schmid PG, Abboud FM, Wendling MG, Ramberg ES, Mark AL, Heistad DD, Eckstein JW (1974) Regional vascular effects of vasopressin: plasma levels and circulatory responses. Am J Physiol 227:998–1004
Asfar P, Pierrot M, Veal N, Moal F, Oberti F, Croquet V, Douay O, Gallois Y, Saumet JL, Alquier P, Cales P (2003) Low-dose terlipressin improves systemic and splanchnic hemodynamics in fluid-challenged endotoxic rats. Crit Care Med 31:215–220
Dunser MW, Mayr AJ, Ulmer H, Knotzer H, Sumann G, Pajk W, Friesenecker B, Hasibeder WR (2003) Arginine vasopressin in advanced vasodilatory shock: a prospective, randomized, controlled study. Circulation 107:2313–2319
Shelly MP, Greatorex R, Calne RY, Park GR (1988) The physiological effects of vasopressin when used to control intra- abdominal bleeding. Intensive Care Med 14:526–531
Okamura T, Ayajiki K, Fujioka H, Toda N (1999) Mechanisms underlying arginine vasopressin-induced relaxation in monkey isolated coronary arteries. J Hypertens 17:673–678
Russ RD, Walker BR (1992) Role of nitric oxide in vasopressinergic pulmonary vasodilatation. Am J Physiol 262:H743–747
Evora PR, Pearson PJ, Schaff HV (1993) Arginine vasopressin induces endothelium-dependent vasodilatation of the pulmonary artery. V1-receptor-mediated production of nitric oxide. Chest 103:1241–1245
Okamura T, Toda M, Ayajiki K, Toda N (1997) Receptor subtypes involved in relaxation and contraction by arginine vasopressin in canine isolated short posterior ciliary arteries. J Vasc Res 34:464–472
Wallace AW, Tunin CM, Shoukas AA (1989) Effects of vasopressin on pulmonary and systemic vascular mechanics. Am J Physiol 257:H1228–1234
Tang W, Weil MH, Gazmuri RJ, Sun S, Duggal C, Bisera J (1991) Pulmonary ventilation/perfusion defects induced by epinephrine during cardiopulmonary resuscitation. Circulation 84:2101–2107
Loeckinger A, Kleinsasser A, Wenzel V, Mair V, Keller C, Kolbitsch C, Recheis W, Schuster A, Lindner KH (2002) Pulmonary gas exchange after cardiopulmonary resuscitation with either vasopressin or epinephrine. Crit Care Med 30:2059–2062
Pittman QJ, Wilkinson MF (1992) Central arginine vasopressin and endogenous antipyresis. Can J Physiol Pharmacol 70:786–790
Perras B, Pannenborg H, Marshall L, Pietrowsky R, Born J, Lorenz Fehm H (1999) Beneficial treatment of age-related sleep disturbances with prolonged intranasal vasopressin. J Clin Psychopharmacol 19:28–36
Antoni FA (1993) Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol 14:76–122
Tucci JR, Espiner EA, Jagger PI, Lauler DP, Thorn GW (1968) Vasopressin in the evaluation of pituitary-adrenal function. Ann Intern Med 69:191–202
Bugajski J, Gadek-Michalska A, Olowska A, Borycz J, Glod R (1997) Role of nitric oxide in the vasopressin-induced corticosterone secretion in rats. J Physiol Pharmacol 48:805–812
Moos F, Freund-Mercier MJ, Guerne Y, Guerne JM, Stoeckel ME, Richard P (1984) Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J Endocrinol 102:63–72
Haslam RJ, Rosson GM (1972) Aggregation of human blood platelets by vasopressin. Am J Physiol 223:958–967
Mannucci PM, Aberg M, Nilsson IM, Robertson B (1975) Mechanism of plasminogen activator and factor VIII increase after vasoactive drugs. Br J Haematol 30:81–93
Richardson DW, Robinson AG (1985) Desmopressin. Ann Intern Med 103:228–239
Hirsch AT, Dzau VJ, Majzoub JA, Creager MA (1989) Vasopressin-mediated forearm vasodilation in normal humans. Evidence for a vascular vasopressin V2 receptor. J Clin Invest 84:418–426
Raff H (1987) Glucocorticoid inhibition of neurohypophysial vasopressin secretion. Am J Physiol 252:R635–644
Papanek PE, Sladek CD, Raff H (1997) Corticosterone inhibition of osmotically stimulated vasopressin from hypothalamic-neurohypophysial explants. Am J Physiol 272:R158–162
Dunn FL, Brennan TJ, Nelson AE, Robertson GL (1973) The role of blood osmolality and volume in regulating vasopressin secretion in the rat. J Clin Invest 52:3212–3219
Wade CE, Keil LC, Ramsay DJ (1983) Role of volume and osmolality in the control of plasma vasopressin in dehydrated dogs. Neuroendocrinology 37:349–353
Cowley AW Jr, Cushman WC, Quillen EW Jr, Skelton MM, Langford HG (1981) Vasopressin elevation in essential hypertension and increased responsiveness to sodium intake. Hypertension 3:I-93–100
Robinson AG, Verbalis JG (2003) Posterior pituitary gland. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS (eds) Williams Textbook of Endocrinology. Saunders, Philadelphia, pp 281–330
Robertson GL, Shelton RL, Athar S (1976) The osmoregulation of vasopressin. Kidney Int 10:25–37
Lee ME, Thrasher TN, Keil LC, Ramsay DJ (1986) Cardiac receptors, vasopressin and corticosteroid release during arterial hypotension in dogs. Am J Physiol 251:R614–620
Goetz KL, Wang BC, Sundet WD (1984) Comparative effects of cardiac receptors and sinoaortic baroreceptors on elevations of plasma vasopressin and renin activity elicited by haemorrhage. J Physiol (Paris) 79:440–445
Hammer M, Olgaard K, Schapira A, Bredgaard Sorensen M, Jensen K, Bonde-Petersen F (1988) Hypovolemic stimuli and vasopressin secretion in man. Acta Endocrinol (Copenh) 118:465–473
Leimbach WN Jr, Schmid PG, Mark AL (1984) Baroreflex control of plasma arginine vasopressin in humans. Am J Physiol 247:H638–644
De Lima J, Caillens H, Beaufils M, Ardaillou R (1981) Effects of furosemide-induced plasma volume reduction on plasma antidiuretic hormone in normal and hypertensive subjects. Clin Nephrol 15:246–251
Callahan MF, Ludwig M, Tsai KP, Sim LJ, Morris M (1997) Baroreceptor input regulates osmotic control of central vasopressin secretion. Neuroendocrinology 65:238–245
Goldsmith SR, Cowley AW Jr, Francis GS, Cohn JN (1984) Effect of increased intracardiac and arterial pressure on plasma vasopressin in humans. Am J Physiol 246:H647–651
Robertson GL, Athar S (1976) The interaction of blood osmolality and blood volume in regulating plasma vasopressin in man. J Clin Endocrinol Metab 42:613–620
Schrier RW, Berl T, Anderson RJ (1979) Osmotic and nonosmotic control of vasopressin release. Am J Physiol 236:F321–332
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Sylvester JT, Scharf SM, Gilbert RD, Fitzgerald RS, Traystman RJ (1979) Hypoxic and CO hypoxia in dogs: hemodynamics, carotid reflexes and catecholamines. Am J Physiol 236:H22–28
Benedict CR, Rose JA (1992) Arterial norepinephrine changes in patients with septic shock. Circ Shock 38:165–172
Cumming AD, Driedger AA, McDonald JW, Lindsay RM, Solez K, Linton AL (1988) Vasoactive hormones in the renal response to systemic sepsis. Am J Kidney Dis 11:23–32
Chernow B, Roth BL (1986) Pharmacologic manipulation of the peripheral vasculature in shock: clinical and experimental approaches. Circ Shock 18:141–155
Landry DW, Oliver JA (1992) The ATP-sensitive K+ channel mediates hypotension in endotoxemia and hypoxic lactic acidosis in dog. J Clin Invest 1992:2071–2074
Landry DW, Oliver JA (2001) The pathogenesis of vasodilatory shock. N Engl J Med 345:588–595
Nelson MT, Quayle JM (1995) Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 268:C799–822
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Chernow B, Rainey TG, Lake CR (1982) Endogenous and exogenous catecholamines in critical care medicine. Crit Care Med 10:409–416
Tsuneyoshi I, Kanmura Y, Yoshimura N (1996) Nitric oxide as a mediator of reduced arterial responsiveness in septic patients. Crit Care Med 24:1083–1086
Fink MP, Homer LD, Fletcher JR (1985) Diminished pressor response to exogenous norepinephrine and angiotensin II in septic, unanesthetized rats: evidence for a prostaglandin-mediated effect. J Surg Res 38:335–342
Hollenberg SM, Tangora JJ, Piotrowski MJ, Easington C, Parrillo JE (1997) Impaired microvascular vasoconstrictive responses to vasopressin in septic rats. Crit Care Med 25:869–873
Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA (2000) TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet 25:187–191
Paya D, Stoclet JC (1995) Involvement of bradykinin and nitric oxide in the early hemodynamic effects of lipopolysaccharide in rats. Shock 3:376–379
Thrasher TN, Keil LC (2000) Systolic pressure predicts plasma vasopressin responses to hemorrhage and vena caval constriction in dogs. Am J Physiol Regul Integr Comp Physiol 279:R1035–1042
Wolfer RS, Lovell NH, Brunner MJ (1994) Exogenous arginine vasopressin does not enhance carotid baroreflex control in the conscious dog. Am J Physiol 266:R1510–1516
Robinson JL (1986) Effect of vasopressin and phenylephrine on arterial pressure and heart rate in conscious dogs. Am J Physiol 251:H253–260
Kasting NW, Mazurek MF, Martin JB (1985) Endotoxin increases vasopressin release independently of known physiological stimuli. Am J Physiol 248:E420–424
Wilson MF, Brackett DJ, Tompkins P, Benjamin B, Archer LT, Hinshaw LB (1981) Elevated plasma vasopressin concentrations during endotoxin and E. coli shock. Adv Shock Res 6:15–26
Mastorakos G, Weber JS, Magiakou MA, Gunn H, Chrousos GP (1994) Hypothalamic-pituitary-adrenal axis activation and stimulation of systemic vasopressin secretion by recombinant interleukin-6 in humans: potential implications for the syndrome of inappropriate vasopressin secretion. J Clin Endocrinol Metab 79:934–939
Chikanza IC, Petrou P, Chrousos G (2000) Perturbations of arginine vasopressin secretion during inflammatory stress. Pathophysiologic implications. Ann N Y Acad Sci 917:825–834
Zelazowski P, Patchev VK, Zelazowska EB, Chrousos GP, Gold PW, Sternberg EM (1993) Release of hypothalamic corticotropin-releasing hormone and arginine-vasopressin by interleukin 1 beta and alpha MSH: studies in rats with different susceptibility to inflammatory disease. Brain Res 631:22–26
Sharshar T, Blanchard A, Paillard M, Raphael JC, Gajdos P, Annane D (2003) Circulating vasopressin levels in septic shock. Crit Care Med 31:1752–1758
Sharshar T, Carlier R, Blanchard A, Feydy A, Gray F, Paillard M, Raphael JC, Gajdos P, Annane D (2002) Depletion of neurohypophyseal content of vasopressin in septic shock. Crit Care Med 30:497–500
Goldsmith SR (1998) Vasopressin deficiency and vasodilation of septic shock. Circulation 97:292–293
Landry DW, Levin HR, Gallant EM, Ashton RC, Seo S, D’Alessandro D, Oz MC, Oliver JA (1997) Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation 95:1122–1125
Rivier C (2003) Role of nitric oxide in regulating the rat hypothalamic-pituitary-adrenal axis response to endotoxemia. Ann N Y Acad Sci 992:72–85
Godin PJ, Fleisher LA, Eidsath A, Vandivier RW, Preas HL, Banks SM, Buchman TG, Suffredini AF (1996) Experimental human endotoxemia increases cardiac regularity: results from a prospective, randomized, crossover trial. Crit Care Med 24:1117–1124
Moreau R, Hadengue A, Soupison T, Mechin G, Assous M, Roche-Sicot J, Sicot C (1990) Abnormal pressor response to vasopressin in patients with cirrhosis: evidence for impaired buffering mechanisms. Hepatology 12:7–12
Montani JP, Liard JF, Schoun J, Mohring J (1980) Hemodynamic effects of exogenous and endogenous vasopressin at low plasma concentrations in conscious dogs. Circ Res 47:346–355
Patel S, Gaspers LD, Boucherie S, Memin E, Stellato KA, Guillon G, Combettes L, Thomas AP (2002) Inducible nitric-oxide synthase attenuates vasopressin-dependent Ca2+ signaling in rat hepatocytes. J Biol Chem 277:33776–33782
Bucher M, Hobbhahn J, Taeger K, Kurtz A (2002) Cytokine-mediated downregulation of vasopressin V(1A) receptors during acute endotoxemia in rats. Am J Physiol Regul Integr Comp Physiol 282:R979–984
Bartelstone HJ, Nasmyth PA (1965) Vasopressin potentiation of catecholamine actions in dog, rat, cat and rat aortic strip. Am J Physiol 208:754–762
Wakatsuki T, Nakaya Y, Inoue I (1992) Vasopressin modulates K(+)-channel activities of cultured smooth muscle cells from porcine coronary artery. Am J Physiol 263:H491–496
Hamu Y, Kanmura Y, Tsuneyoshi I, Yoshimura N (1999) The effects of vasopressin on endotoxin-induced attenuation of contractile responses in human gastroepiploic arteries in vitro. Anesth Analg 88:542–548
Kusano E, Tian S, Umino T, Tetsuka T, Ando Y, Asano Y (1997) Arginine vasopressin inhibits interleukin-1 beta-stimulated nitric oxide and cyclic guanosine monophosphate production via the V1 receptor in cultured rat vascular smooth muscle cells. J Hypertens 15:627–632
Salzman AL, Vromen A, Denenberg A, Szabo C (1997) K(ATP)-channel inhibition improves hemodynamics and cellular energetics in hemorrhagic shock. Am J Physiol 272:H688–694
Balakrishnan SM, Gopalakrishnan V, McNeill JR (1997) Endothelin contributes to the hemodynamic effects of vasopressin in spontaneous hypertension. Eur J Pharmacol 334:55–60
Levin ER (1996) Endothelins as cardiovascular peptides. Am J Nephrol 16:246–251
Takakura K, Taniguchi T, Muramatsu I, Takeuchi K, Fukuda S (2002) Modification of alpha1 -adrenoceptors by peroxynitrite as a possible mechanism of systemic hypotension in sepsis. Crit Care Med 30:894–899
Umino T, Kusano E, Muto S, Akimoto T, Yanagiba S, Ono S, Amemiya M, Ando Y, Homma S, Ikeda U, Shimada K, Asano Y (1999) AVP inhibits LPS- and IL-1beta-stimulated NO and cGMP via V1 receptor in cultured rat mesangial cells. Am J Physiol 276:F433–441
Nambi P, Whitman M, Gessner G, Aiyar N, Crooke ST (1986) Vasopressin-mediated inhibition of atrial natriuretic factor-stimulated cGMP accumulation in an established smooth muscle cell line. Proc Natl Acad Sci U S A 83:8492–8495
Nambi P, Whitman M, Aiyar N, Crooke ST (1988) Inhibition of formation of cyclic AMP and cyclic GMP by vasopressin in smooth-muscle cells is insensitive to pertussis toxin. Biochem J 254:449–453
Taguchi H, Heistad DD, Chu Y, Rios CD, Ooboshi H, Faraci FM (1996) Vascular expression of inducible nitric oxide synthase is associated with activation of Ca(++)-dependent K+ channels. J Pharmacol Exp Ther 279:1514–1519
Roth BL, Spitzer JA (1987) Altered hepatic vasopressin and alpha 1-adrenergic receptors after chronic endotoxin infusion. Am J Physiol 252:E699–702
Fox AW, May RE, Mitch WE (1992) Comparison of peptide and nonpeptide receptor-mediated responses in rat tail artery. J Cardiovasc Pharmacol 20:282–289
Walker BR (1986) Role of vasopressin in the cardiovascular response to hypoxia in the conscious rat. Am J Physiol 251:H1316–323
Suzuki Y, Satoh S, Oyama H, Takayasu M, Shibuya M (1993) Regional differences in the vasodilator response to vasopressin in canine cerebral arteries in vivo. Stroke 24:1049–1053, discussion 1053–1054
Eisenman A, Armali Z, Enat R, Bankir L, Baruch Y (1999) Low-dose vasopressin restores diuresis both in patients with hepatorenal syndrome and in anuric patients with end-stage heart failure. J Intern Med 246:183–190
Holmes CL, Walley KR, Chittock DR, Lehman T, Russell JA (2001) The effects of vasopressin on hemodynamics and renal function in severe septic shock: a case series. Intensive Care Med 27:1416–1421
Cross RB, Trace JW, Vattuone JR (1974) The effect of vasopressin upon the vasculature of the isolated perfused rat kidney. J Physiol 239:435–442
Aiyar N, Nambi P, Crooke ST (1990) Desensitization of vasopressin sensitive adenylate cyclase by vasopressin and phorbol esters. Cell Signal 2:153–160
Rudichenko VM, Beierwaltes WH (1995) Arginine vasopressin-induced renal vasodilation mediated by nitric oxide. J Vasc Res 32:100–105
Edwards RM, Trizna W, Kinter LB (1989) Renal microvascular effects of vasopressin and vasopressin antagonists. Am J Physiol 256:F274–278
Gutkowska J, Jankowski M, Lambert C, Mukaddam-Daher S, Zingg HH, McCann SM (1997) Oxytocin releases atrial natriuretic peptide by combining with oxytocin receptors in the heart. Proc Natl Acad Sci U S A 94:11704–11709
McVicar AJ (1988) Dose-response effects of pressor doses of arginine vasopressin on renal haemodynamics in the rat. J Physiol 404:535–546
Harrison-Bernard LM, Carmines PK (1994) Juxtamedullary microvascular responses to arginine vasopressin in rat kidney. Am J Physiol 267:F249–256
Landry DW, Levin HR, Gallant EM, Seo S, D’Alessandro D, Oz MC, Oliver JA (1997) Vasopressin pressor hypersensitivity in vasodilatory septic shock. Crit Care Med 25:1279–1282
Dunser MW, Mayr AJ, Ulmer H, Ritsch N, Knotzer H, Pajk W, Luckner G, Mutz NJ, Hasibeder WR (2001) The effects of vasopressin on systemic hemodynamics in catecholamine-resistant septic and postcardiotomy shock: a retrospective analysis. Anesth Analg 93:7–13
Tsuneyoshi I, Yamada H, Kakihana Y, Nakamura M, Nakano Y, Boyle WA 3rd (2001) Hemodynamic and metabolic effects of low-dose vasopressin infusions in vasodilatory septic shock. Crit Care Med 29:487–493
Malay MB, Ashton RC Jr, Landry DW, Townsend RN (1999) Low-dose vasopressin in the treatment of vasodilatory septic shock. J Trauma 47:699–703, discussion 703–705
Patel BM, Chittock DR, Russell JA, Walley KR (2002) Beneficial effects of short-term vasopressin infusion during severe septic shock. Anesthesiology 96:576–582
O’Brien A, Clapp L, Singer M (2002) Terlipressin for norepinephrine-resistant septic shock. Lancet 359:1209–1210
Morales D, Madigan J, Cullinane S, Chen J, Heath M, Oz M, Oliver JA, Landry DW (1999) Reversal by vasopressin of intractable hypotension in the late phase of hemorrhagic shock. Circulation 100:226–229
Argenziano M, Chen JM, Choudhri AF, Cullinane S, Garfein E, Weinberg AD, Smith CR Jr, Rose EA, Landry DW, Oz MC (1998) Management of vasodilatory shock after cardiac surgery: identification of predisposing factors and use of a novel pressor agent. J Thorac Cardiovasc Surg 116:973–980
Argenziano M, Chen JM, Cullinane S, Choudhri AF, Rose EA, Smith CR, Edwards NM, Landry DW, Oz MC (1999) Arginine vasopressin in the management of vasodilatory hypotension after cardiac transplantation. J Heart Lung Transplant 18:814–817
Rosenzweig EB, Starc TJ, Chen JM, Cullinane S, Timchak DM, Gersony WM, Landry DW, Galantowicz ME (1999) Intravenous arginine-vasopressin in children with vasodilatory shock after cardiac surgery. Circulation 100:II-182–106
Morales DL, Gregg D, Helman DN, Williams MR, Naka Y, Landry DW, Oz MC (2000) Arginine vasopressin in the treatment of 50 patients with postcardiotomy vasodilatory shock. Ann Thorac Surg 69:102–106
Dunser MW, Mayr AJ, Stallinger A, Ulmer H, Ritsch N, Knotzer H, Pajk W, Mutz NJ, Hasibeder WR (2002) Cardiac performance during vasopressin infusion in postcardiotomy shock. Intensive Care Med 28:746–751
Argenziano M, Choudhri AF, Oz MC, Rose EA, Smith CR, Landry DW (1997) A prospective randomized trial of arginine vasopressin in the treatment of vasodilatory shock after left ventricular assist device placement. Circulation 96:II-286–290
Chen JM, Cullinane S, Spanier TB, Artrip JH, John R, Edwards NM, Oz MC, Landry DW (1999) Vasopressin deficiency and pressor hypersensitivity in hemodynamically unstable organ donors. Circulation 100:II-244–246
Iwai A, Sakano T, Uenishi M, Sugimoto H, Yoshioka T, Sugimoto T (1989) Effects of vasopressin and catecholamines on the maintenance of circulatory stability in brain-dead patients. Transplantation 48:613–617
Yoshioka T, Sugimoto H, Uenishi M, Sakamoto T, Sadamitsu D, Sakano T, Sugimoto T (1986) Prolonged hemodynamic maintenance by the combined administration of vasopressin and epinephrine in brain death: a clinical study. Neurosurgery 18:565–567
Gold JA, Cullinane S, Chen J, Oz MC, Oliver JA, Landry DW (2000) Vasopressin as an alternative to norepinephrine in the treatment of milrinone-induced hypotension. Crit Care Med 28:249–252
Overand PT, Teply JF (1998) Vasopressin for the treatment of refractory hypotension after cardiopulmonary bypass. Anesth Analg 86:1207–1209
Eyraud D, Brabant S, Nathalie D, Fleron MH, Gilles G, Bertrand M, Coriat P (1999) Treatment of intraoperative refractory hypotension with terlipressin in patients chronically treated with an antagonist of the renin-angiotensin system. Anesth Analg 88:980–984
Minaker KL, Meneilly GS, Youn GJ, Landsberg L, Stoff JS, Robertson GL, Rowe JW (1991) Blood pressure, pulse and neurohumoral responses to nitroprusside-induced hypotension in normotensive aging men. J Gerontol 46:M151–154
Robertson GL (1976) The regulation of vasopressin function in health and disease. Recent Prog Horm Res 33:333–385
Sirinek KR, Levine BA (1988) High-dose vasopressin for acute variceal hemorrhage. Clinical advantages without adverse effects. Arch Surg 123:876–880
Gimson AE, Westaby D, Hegarty J, Watson A, Williams R (1986) A randomized trial of vasopressin and vasopressin plus nitroglycerin in the control of acute variceal hemorrhage. Hepatology 6:410–413
Greenwald RA, Rheingold OJ, Chiprut RO, Rogers AI (1978) Local gangrene: a complication of peripheral Pitressin therapy for bleeding esophageal varices. Gastroenterology 74:744–746
Mogan GR, Wormser GP, Gottfried EB (1980) Infected gangrene. A serious complication of peripheral vasopressin administration. Am J Gastroenterol 73:426–429
Kahn JM, Kress JP, Hall JB (2002) Skin necrosis after extravasation of low-dose vasopressin administered for septic shock. Crit Care Med 30:1899–1901
Bernadich C, Bandi JC, Melin P, Bosch J (1998) Effects of F-180, a new selective vasoconstrictor peptide, compared with terlipressin and vasopressin on systemic and splanchnic hemodynamics in a rat model of portal hypertension. Hepatology 27:351–356
Moreau R, Durand F, Poynard T, Duhamel C, Cervoni JP, Ichai P, Abergel A, Halimi C, Pauwels M, Bronowicki JP, Giostra E, Fleurot C, Gurnot D, Nouel O, Renard P, Rivoal M, Blanc P, Coumaros D, Ducloux S, Levy S, Pariente A, Perarnau JM, Roche J, Scribe-Outtas M, Valla D, Bernard B, Samuel D, Butel J, Hadengue A, Platek A, Lebrec D, Cadranel JF (2002) Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: a retrospective multicenter study. Gastroenterology 122:923–930
Scharte M, Meyer J, Van Aken H, Bone HG (2001) Hemodynamic effects of terlipressin (a synthetic analog of vasopressin) in healthy and endotoxemic sheep. Crit Care Med 29:1756–1760
Medel J, Boccara G, Van de Steen E, Bertrand M, Godet G, Coriat P (2001) Terlipressin for treating intraoperative hypotension: can it unmask myocardial ischemia? Anesth Analg 93:53–55
Westphal M, Stubbe H, Sielenkamper AW, Borgulya R, Van Aken H, Ball C, Bone HG (2003) Terlipressin dose response in healthy and endotoxemic sheep: impact on cardiopulmonary performance and global oxygen transport. Intensive Care Med 29:301–308
Guirao X, Kumar A, Katz J, Smith M, Lin E, Keogh C, Calvano SE, Lowry SF (1997) Catecholamines increase monocyte TNF receptors and inhibit TNF through beta 2-adrenoreceptor activation. Am J Physiol 273:E1203–1208
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the American Heart Association, HL-66211, and the Evanston Northwestern Healthcare Research Institute.
Rights and permissions
About this article
Cite this article
Mutlu, G.M., Factor, P. Role of vasopressin in the management of septic shock. Intensive Care Med 30, 1276–1291 (2004). https://doi.org/10.1007/s00134-004-2283-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2283-8