Abstract
To determine whether the national soil heavy metal standards (GB 15618-2018) are applicable to some carbonate and non-carbonate zones in Southwest China, rice and rhizosphere soil samples were collected in Chongqing and analyzed for heavy metal contents, pH, and other chemical parameters. In addition, regression analysis was also used to predict the risk threshold of soil heavy metals. The Cd risk screening value in GB 15618-2018 was strict for alkaline soils (pH > 7.5) as compared to those revealed in carbonate and non-carbonate areas, while the calculated pollution threshold for Cd in acidic soils (pH ≤ 5.5) in the non-carbonate area was lower than that in GB 15618-2018. Therefore, to improve the applicability of the evaluation results, a soil-crop system evaluation is recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Heavy metals in soil have become one of the main factors affecting ecological quality, agricultural product quality, and human health due to their strong migration and degradation characteristics as well as their toxic effect on organisms (Huang et al. 2018; Sun et al. 2019; Xing et al. 2019). According to the 2014 National Survey Report on soil pollution, inorganic pollutants, such as Cd, Ni, Cu, As, Hg, and Pb, were the main pollutants. Indeed, the polluted areas were mainly found in the southwestern and central parts of China. Therefore, there is an urgent need to address the safe use of contaminated farmland and guarantee the safe production of crops (Chen et al. 2017; Wang et al. 2021a, b).
The heavy metal contents of soils are mainly influenced by the parent materials of soils in Southwest China, where carbonate rocks are widely distributed (Song et al. 2019; Wen et al. 2020). Gu et al. (2019a) studied the contamination degree of heavy metals in soils and crops in a carbonate rock area in Guangxi Zhuang Autonomous Region and showed that Cd contents in soil and rice exceeded the national soil heavy metal standard by 60.16 and 32.93%, respectively. Wang et al. (2019) studied the corresponding relationship between Cd contents in soil and two crop species (rice and corn) in southeast Chongqing and revealed a higher exceeding Cd rate in soil than that in crops. Ma et al. (2021) studied the speciation of Cd in the soil of Hengxian, Guangxi Zhuang Autonomous Region, and found that Cd in soil was mainly in a residual state, with low bioavailability and relatively safe rice production. According to the Action Plan on Prevention and Control of Soil Pollution, the farmland soil environmental quality classifications have been performed in many regions, based on the full content of heavy metals in the soil, to manage cultivated land resources (Dai 2018). However, China has a complex geological background, with spatial variation in soil properties depending on geological conditions, more particularly in the carbonate rock area. Due to the special soil formation process in the carbonate area, the migration capacity of heavy metals in soil is weak, and the bioavailability of heavy metals in soil is relatively low (Ruan et al. 2015; Zhang et al. 2019a, b; Yao et al. 2021). It is inevitable that “one size for all” problems will occur in the evaluation of heavy metal pollution in soil, based on the current Soil Environmental Quality-Risk Standard for Soil Contamination of Agricultural Land (GB 15618-2018) (MEE 2018), leading to a mismatch between the evaluation results of soil heavy metal contamination and crop safety, thus decreasing the safe utilization rate of cultivated land.
In view of this, this study aims to compare heavy metal contents (Cd, Hg, Pb, As, and Cr) in soil and rice in carbonate and non-carbonate rock areas and to construct a linear regression equation for heavy metal uptake by staple crop rice. In addition, based on the National Food Safety Standard for Maximum Levels of Contaminants in Foods (GB 2762-2017) (NHFPC 2017), the risk threshold of heavy metals in soil was deduced. The key factors affecting the heavy metal uptake by rice were assessed, and management control practices were proposed to provide a reference for the construction of heavy metal pollution evaluation methods for agricultural soils, resulting in improved food safety production and safe cultivated land uses.
Materials and Methods
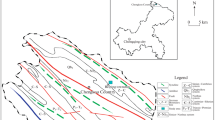
Qianjiang District of Chongqing (108°28 ′E–108°56′ E, 29°04 ′N–29°52′ N) is located in West Wuling Mountain, with a 45 km wide from east to west and 90 km long from north to south, covering an area of 2402 km2. It is adjacent to Xianfeng County and Lichuan City in Hubei Province in the northeast and northwest, reaching Youyang and Pengshui in the south and west, respectively. The study area is located in the southern part of the Qianjiang District, where exposed strata include Permian, Silurian, and Triassic, while the main lithological classes are limestone and sandstone.
In this study, rice samples were collected relatively uniformly, according to the spatial distribution of paddy fields in the study area. In addition, rhizosphere soil samples were collected from the same fields. In total, 30 and 35 sets of samples were collected from carbonate rock and non-carbonate rock areas, respectively. The spatial distribution of the sampling points is shown in Fig. 1. The Checkerboard, PLUM point, Diagonal, and Serpentine methods were used first for multipoint sampling in the sampling plot (SEPA 2014), according to the actual situation of fields, and then combined in equal amounts to obtain mixed samples.
Soil samples were shade-dried under natural conditions and often rubbed during the drying process to avoid cementation. Gravel and plant roots were removed from the soil. The dried samples were gently tapped with a mallet before analyses to restore the natural grain size of soil samples. After drying, 500 g of soil samples were first sieved using a 2 mm sieve, then bottled and sent to the laboratory for further analyses. Some samples were taken by the division method for soil pH measurement, while the others were ground using an agate ball mill and sieved using a 0.075 mm-mesh (200 mesh) to analyze other soil parameters. The rice samples were air-dried and threshed under natural conditions. The air-drying site was clean and free of contamination. In addition, the rice seeds were ground to 60 meshes using an agate mortar and placed in numbered bags.
In total, 12 national first-level standard substance (GSS1-12) control samples and repeated samples were used for accuracy control (△LgC) and precision (RD%) of the physicochemical analyses, according to the following formulas:
where Ci and Cs are the sample and standard concentration values, respectively; A1 and A2 are the analysis results of the basic analytical and duplicate samples, respectively.
In accordance with sample analytical schemes (Zhang 2005), an ion-selective electrode method (ISE) was adopted to determine soil pH. Organic carbon (Corg) concentration was measured by a volumetric technique. In addition, we applied X-ray fluorescence spectrometry (XRF, PW2440, Philips Co., Dutch) to test the concentrations of CaO, SiO2, TFe2O3, K2O, S and MgO. Inductively coupled plasma mass spectrometry (ICP-MS, Thermo Electron Co X-SERIES, USA) was used to analyze the concentrations of Hg, Cd, Pb, Cr, Zn and Mn. The concentration of As dissolved by aqua regia was tested by atomic fluorescence spectroscopy (AFS, Haiguang Instruments Co., Ltd, Beijing). It was important to ensure that the success rate of the various indicators in Table 1 reached more than 98% and that each quality index reached or exceeded the requirements 5of DZ/T 0258-2014 Multi-target Area Code for Geochemical Investigation. The accuracy and precision results of the analytical methods used for soil and rice samples are reported in Table 1.
By calculating the biological enrichment of the rice samples, the coefficient (BAF) was determined to analyze the risk of absorption and accumulation of heavy metals from the soil by rice (Chopra and Pathak 2015). Bioaccumulation Factor (BAF) represents the ratio between the heavy metal contents in rice and those in the rhizosphere soil, which can reflect the uptake degree of heavy metals by rice. Indeed, BAF is generally used to characterize the bioavailability of heavy metal elements (Gu et al. 2019b). Higher BCF values suggest a higher degree of heavy metal uptakes by rice. BAF was calculated using the following formula:
where BAF indicates the enrichment coefficient of a heavy metal element; Crice (mg kg−1) denotes the heavy metal content in rice; Csoil (mg kg−1) denotes the heavy metal content in the rhizosphere soil.
Multiple linear regression (MLR) is a widely used predictive model that can be built using various explanatory variables (Abdullah et al. 2019). The stepwise regression method was used to build an optimal linear regression model by selecting the most important explanatory variables showing the most statistically significant effects on the prediction results. Indeed, this method has also proven to be more effective in practice for achieving high prediction accuracy. In addition, the stepwise regression method can deal with the multicollinearity between chemical variables used as explanatory variables in the MLR model to a certain extent (You and Yan 2017).
In order to calculate the soil risk thresholds for heavy metals based on the rice safety, the predicted content of heavy metals in the rice, calculated based on the root-rice prediction model, should be accurately predicted. To eliminate the influence of measurement units on the MLR model, BAFs and chemical indicator values observed in the soil were log-transformed with a base of 10 (Gu et al. 2019b). In addition, stepwise regression analysis was performed using SPSS 25.0 to select the significantly chemical influencing factors of the soil and obtain regression equations for heavy metal absorption in rice in carbonate and non-carbonate rock areas. The final regression equation is:
where i indicates the soil heavy metal; a, b, c, …, n are regression coefficients. Soil properties used in this study were pH, CaO, SiO2, MgO, TFe2O3, and K2O.
The normalized mean error (NME) and the normalized root mean square error (NRMSE) were determined to assess the accuracy of the predictive models (Vries et al. 2011), according to the following formulas:
where ē and ō are the mean values of the predicted and measured, respectively; ei and oi represent the predicted and measured values of the ith sample, respectively; N is the sample numbers.
NME < 0 and NME > 0 indicate an underprediction and overprediction of the model, respectively. Whereas NRMSE indicates the deviation degree between the predicted and measured values, which is a good indicator of the prediction accuracy of the model (Janssen and Heuberger 1995; Vries et al. 2011).
Data sorting was performed using Microsoft Excel 2010 (Microsoft Corporation, USA), while the spatial structure analysis of the elements and the nugget coefficient of the soil heavy metal elements were performed using GS + 9.0 (Gamma Design Software Co., USA). All data were visualized using ArcGIS 10.2 (Environmental Systems Research Institute Inc., USA), Excel 2010, and CorelDRAW X8(Corel Corporation, Canada).
Results and Discussion
The pH values observed in carbonate and non-carbonate zones varied from 5.30 to 8.23 and from 4.69 to 8.23, with mean values of 7.55 and 6.09, respectively. The results of heavy metal and other chemical parameter content in soils in carbonate rock and the carbonate rock areas showed that the coefficient of variation of soil Cd in the carbonate rock area was 0.58, indicating high variation and spatial variability (Table 2). In addition, the soil Cd contents were unevenly distributed in the non-carbonate area with a high variation level. The coefficient of variation of soil Hg was high (1.59) due to the presence of abnormally high-value points. Therefore, Hg outliers were removed by adding or subtracting 3 times the standard deviation of Hg content in the soil, resulting in a coefficient of variation of 0.34, which was close to that observed in the non-carbonate area, belonging to a medium variation level (Fig. 2). On the other hand, the nugget coefficient can reflect the spatial correlation of elements. Nugget coefficient values less than 0.25, in the range of 0.25–0.75, and above 0.75 suggest strong, moderate, and weak spatial autocorrelation between variables, respectively. In fact, the Nugget coefficient is mainly affected by both structural and random factors (Cambardella et al. 1994). The results showed that the nugget coefficients of soil Cd in carbonate and non-carbonate areas were 0.26 and 0.48, respectively (Fig. 3), indicating that the spatial autocorrelation of soil Cd in carbonate rock area was strong and was greatly influenced by the parent soil material (Qin et al. 2020; Yang et al. 2016). However, the non-carbonate area was influenced by both human activities and geological background. Other elements, namely As and Cr, were mainly influenced by the parent soil material, showing strong spatial autocorrelations, confirming their inheritance to the parent material. These findings were consistent with those reported by Yu et al. (2018) in the carbonate area of Enshi, Hubei province.
The exceeding rates of soil heavy metals were determined in this study according to the screening and control values of soil heavy metal pollution risk in GB 15618-2018. The results indicated that Cd was the main heavy metal element exceeding the screening value of GB 15618-2018 in carbonate and non-carbonate rock areas, with proportion rate values of 23.33 and 11.43%, respectively, and there was no point exceeding the control value. These results indicate that soil Cd might be safe for agricultural products, although there was a risk for crop growth and the ecological environment of the soil. Whereas no exceeding rates for other heavy metals were observed. The average contents of heavy metals in soils were higher in the carbonate rock area than in the non-carbonate rock area (Table 2), which may be due to the secondary enrichment of heavy metals in the soil surface layer during the soil-forming process of limestone (Xia et al. 2020). This result may also be one of the reasons for the higher exceeding rate of Cd in the carbonate rock area than that in the non-carbonate area. Liu et al. (2010) revealed a loss in soluble minerals during the soil formation process, while insoluble iron and manganese oxides, organic matters, and clay minerals were constantly accumulated in limestone, which has a strong adsorption capacity of heavy metals (e.g., Cd). The more developed the soil is, the higher the content and stability of heavy metals in the soil (e.g., Cd). Therefore, it is necessary to assess the safety threshold of heavy metals in the soils of carbonate and non-carbonate areas based on crop safety.
The content characteristics of heavy metals in rice in carbonate rock and carbonate rock areas are summarized in Table 3. The results showed that the Cd and As contents in rice in the carbonate rock area were lower than those observed in the non-carbonate rock area. Indeed, the exceeding rate values of Cd in rice in carbonate rock and the carbonate rock areas were 10 and 20%, respectively, indicating a difference between the Cd absorption levels in rice and those observed in soil parent materials. These findings were, indeed, consistent with those reported by Li et al. (2021) in karst and non-karst areas of Guangxi Zhuang in the Autonomous Region, showing that soil carbonate reduced Cd bioavailability in karst areas, while rice plants grown on acidic soils in the non-karst area accumulated higher Cd in their grains, demonstrating different biological activity of heavy metals among soil parent materials. Although the soil's heavy metal contents in the carbonate rock area were higher, the bioavailability was low. On the contrary, the soil's heavy metal contents in the non-carbonate rock area were lower, while the bioavailability was high (He et al. 2017).
The BAFs of heavy metals in rice in different soil parent materials were calculated, and the results are shown in Fig. 4. As can be seen from Fig. 4, the BAFs of Pb, Hg, Cr, and As in rice were all lower than 0.2 suggesting low absorption degrees of these elements in rice and no-distinct ecological risk. In addition, there was a small difference in the absorption degree between Pb, Hg, Cr, and As in rice in various soil parent materials. According to the results, the absorption degree of Cd in rice was significantly higher than that of other elements. The BAF of Cd ranges in rice were 0.009–0.980 and 0.007–1.060, with average values of 0.170 and 0.440, in carbonate rock and non-carbonate rock areas, respectively. The results suggested higher absorption degrees of Cd in rice in the non-carbonate rock area, explaining the lower soil Cd content (0.30 mg·kg−1) in the non-carbonate rock area and higher exceeding rate of Cd in rice (20%) compared to that observed in the carbonate rock area (10%).
The absorption of Cd, Hg, Pb, As, and Cr by rice was affected not only by the total concentrations of these heavy metals in soil but also by the soil pH, organic matter content, and oxide contents (CaO, SiO2, MgO, TFe2O3, and K2O) (Gu et al. 2019a). Potassium (K) and magnesium (Mg) are the main components of clay minerals, which exhibit strong adsorption effects on heavy metal ions and can rapidly convert heavy metal ions from soluble to adsorption states, reducing the bioavailability of soil heavy metals (Chen et al. 2016). In cultivated soil with rice under long-term flooding, the redox potential of the soil is usually low, and sulfur (S) is often reduced to S2−, forming metal sulfide precipitation with Cd2+ and Pb2+, reducing the activity of heavy metals (Zhao et al. 2011). Soil pH is an important factor affecting the migration and transformation of soil heavy metals. Indeed, under acid conditions, the solubility of some solid salt increases, which intensifies the exchange of soil heavy metal ions and reduces their adsorption in the soil, thus increasing the bioavailability of heavy metals. With increasing soil pH, the soil colloid negative charge increases, and heavy metal ions are firmly adsorbed in the solid phase of the soil, thus decreasing their absorption by crops (Zhang et al. 2006). Cd2+ and Ca2+ are characterized by similar ionic radius and exhibit competitive relationships with negatively charged adsorption potential in the soil (Zhang et al. 2019a, b). In addition, the soil CaO content affects the soil pH, thus affecting the activity of heavy metals (Mcbride et al. 1997). Iron oxide in various forms in the soil is insoluble, resulting in the formation of oxide nodules and hydroxide colloids and both can effectively immobilize the soil's heavy metal ions. The majority of the manganese oxide forms in the soil are amorphous due to the lower zero charge and larger specific surface area of Mn, however, it is more effective than Fe in adsorbing heavy metals. During the soil evolution process, iron and manganese oxide nodules with high heavy metal contents (e.g., Cd) can be formed, which have stable properties and low biological activity under supergene conditions (Zhou et al. 2019). Soil organic matter, as an important component of solid soil parts, mainly originates from the decomposition of animal residues, plant litter, and microbes. The surface of soil organic matter contains rich negatively charged functional groups characterized by strong adsorption effects on the soil heavy metal ions and is one of the main factors controlling the heavy metal activities in the soil (Ning et al. 2016).
The regression analysis results of the heavy metal uptakes by rice in different soil-forming parent material zones are reported in Table 4. The correlation coefficients R obtained using the prediction models for rice seed BAFs in carbonate and non-carbonate zones ranged from 0.78 to 0.90, thus demonstrating relatively good prediction accuracies of the rice seed models. The NME results demonstrated that the predicted BAF values of Cd, Pb, As, and Cr in rice in the carbonate area were slightly underpredicted, while the predicted BAF value of Hg in rice was slightly overpredicted. On the other hand, the predicted BAF of Cd, Pb, and Cr in rice in non-carbonate rock areas was slightly underpredicted, while the predicted BAF of Hg and Cr in rice was slightly overpredicted. The NRMSE values were all below 1, indicating relatively good prediction accuracy of the MLR model.
Based on the heavy metal content standards for rice in the GB 2762-2017 standards, and combined with indices for rhizosphere soil, the regression equations (Table 4) were used to calculate the soil risk thresholds for heavy metals based on the rice safety. The calculated thresholds were then compared to the soil heavy metal risk screening values of GB 15618-2018. The obtained results are shown in Fig. 5.
As can be seen from Fig. 5, the risk threshold for Hg, Pb, As, and Cr in two different soil parent materials showed slight differences from the safety limits given by the national standard at different pH range values, e.g., pH value less than 5.5 or 5.5–6.5, only the calculated safety threshold for Cr in the non-carbonate rock region (300 mg·kg−1, 310 mg·kg−1) is about higher 0.2 times than the safety limit in GB 15617-2018 (250 mg kg−1), while the difference between the calculated safety threshold and the safety threshold in GB 15617-2018 is less than 0.2 times in the other pH ranges, indicating that these heavy metals are relatively stable in the soil. The risk screening value of the national standard is suitable for the safety evaluation of Hg, Pb, As, and Cr in the soils in Qianjiang District. According to the results obtained, the risk threshold for soil Cd in the carbonate rock area, determined using the regression equation, was higher than that found in the non-carbonate rock area. Studies have shown that during the weathering and pedogenesis process of carbonate rocks, the soil Cd can be adsorbed by clay minerals, organic matters, and metal manganese oxides, which have lower activities. While the Cd content in the soil increases with the development of the soil, gradually decreasing the bioavailability of Cd in the soil (Guo et al. 2019). Compared with the national standard, it was found that the calculated risk thresholds for Cd at different soil pH ranges in the carbonate rock area were significantly higher than the screening values of the national standard, indicating that the safety limits of the national standard were rigorous for the carbonate rock area, which is consistent with the results found by Wang et al. (2019) in the Guizhou karst geomorphologic area. In addition, the calculated risk threshold for soil Cd in the non-carbonate area was slightly higher than that of the national standard at a soil pH value greater than 5.5 (slightly acidic and alkaline soils). However, the calculated risk threshold of soil Cd was 0.2 mg·kg−1 at a soil pH value lower than 5.5, which was lower than the national standard screening value. This finding suggests a highly mobile state of Cd in highly acidic soil, resulting in significant Cd uptake by crops. However, the calculated safety limits of different heavy metals changed to some extent with changing soil pH values, suggesting the significant impact of the soil pH on heavy metal activities, more particularly Cd. Under acidic conditions, the bioavailability of Cd in the soil is high, which has a significant impact on the safety of crops (Wang et al. 2021a, b). On the other hand, the correlation analysis revealed a significant negative correlation coefficient between soil pH and BAF for the Cd content in rice in the non-carbonate rock area (R = − 0.92; p < 0.01) (Fig. 6). The BAF of Cd in rice decreased with increasing pH, more significantly at pH values greater than 7, reaching a BAF value of about 0.1 and demonstrating low biological activity of Cd. This finding suggests that the soil pH is a key factor controlling the Cd uptake by rice.
Numerous studies showed that dissolved oxygen contents in acidic soils are significantly reduced, under the flooding condition, decreasing oxidizing substances in soils (Sebei et al. 2018; Hu et al. 2010; Wang et al. 2020), while soil pH value close to the neutral value leads to decrease in the redox potential of the soil, forming a reduction environment. The soil heavy metals (e.g., Cd) can form stable and insoluble precipitates, thus decreasing the activity of the heavy metals in the soil and the rates of heavy metal uptakes by crops. In addition, the use of a soil conditioner and optimization of the tillage system and fertilization use can increase the soil pH value above 5.5, thus resulting in improved soil structure, reduced heavy metal activities in the soil, and sustainable production capacity of the soil. Indeed, commonly used soil conditioners include lime nitrogen, calcium soil conditioner, and calcium magnesium phosphate fertilizer (Qin et al. 2018; Li et al. 2018). Therefore, it is suggested to implement water control measures in the study area and adjust the soil pH by extending the flooding time of paddy fields. In addition, applying alkaline soil conditioners to strongly acidic soils can effectively reduce Cd activities in the soil and ensure safe crop production.
Based on the safety limits for rice reported in GB 2762-2017, the risk thresholds for soil heavy metals in carbonate and non-carbonate rock areas at different pH range values were calculated and compared with the soil heavy metal risk screening values in GB 15618-2018 to investigate the applicability of GB 15618-2018 in the evaluation of soil heavy metal contamination in some carbonate and non-carbonate rock areas in Southwest China. This study can provide references for assessing the safe use of agricultural soils.
References
Abdullah S, Nasir NHA, Ismail M, Ahmed AN, Jarkoni MNK (2019) Development of Ozone Prediction Model in Urban Area. Int J Innov Technol Explor Eng 8(10):2263–2267. https://doi.org/10.35940/ijitee.J1127.0881019
Cambardella CA, Moorman TB, Novak JM, Parkin TB, Karlen DL, Turco RF, Konopka AE (1994) Field-scale variability of soil properties in central Iowa soils. Soil Sci Soc Am J 58(5):1501–1511. https://doi.org/10.2136/sssaj1994.03615995005800050033x
Chen H, Yuan X, Li T, Hu S, Ji J, Wang C (2016) Characteristics of heavy metal transfer and their influencing factors in different soil-crop systems of the industrialization region China. Ecotox Environ Safe 126:193–201. https://doi.org/10.1016/j.ecoenv.2015.12.042
Chen NC, Zheng YJ, He XF, Li X, Zhang X (2017) Analysis of the report on the national general survey of soil contamination. J Agro Environ Sci 36(9):1689–1692
Chopra AK, Pathak C (2015) Accumulation of heavy metals in the vegetables grown in wastewater irrigated areas of Dehradun, India with reference to human health risk. Environ Monit Assess 187(7):445. https://doi.org/10.1007/s10661-015-4648-6
Dai Y (2018) Talking about my country's soil pollution and its prevention and control in combination with "Ten Articles of Soil". https://doi.org/10.16317/j.cnki.12-1377/x.2018.06.075 (in Chinese)
Gu QB, Yu T, Yang ZF, Ji JF, Hou QY, Wang L, Wei X, Zhang Q (2019a) Prediction and risk assessment of five heavy metals in maize and peanut: a case study of Guangxi. China. Environ Toxicol Phar 70:103199. https://doi.org/10.1016/j.etap.2019.103199
Gu QB, Yang ZF, Yu T, Ji JF, Hou QY, Zhang QZ (2019b) Application of ecogeochemical prediction model to safely exploit seleniferous soil. Ecotoxicol Environ Safe 177:133–139. https://doi.org/10.1016/j.ecoenv.2019b.03.084
Guo C, Wen YB, Yang ZF, Li W, Guan DX, Ji JF (2019) Factors controlling the bioavailability of soil cadmium in typical karst areas with high geogenic background. Nanjing Univ Nat Sci 55(4):678–687. https://doi.org/10.13232/j.cnki.jnju.2019.04.018 (in Chinese)
He S, Lu Q, Li W, Ren Z, Zhou Z, Feng X, Zhang Y, Li Y (2017) Factors controlling cadmium and lead activities in different parent material-derived soils from the pearl river basin. Chemosphere 182:509–516. https://doi.org/10.1016/j.chemosphere.2017.05.007
Hu K, Yu H, Feng WQ, Qin YS, Lan LL, Miu ML, Wang CQ, Tu S (2010) Effect of different fertilizers of secondary, micro-and beneficial elements on soil pH and Cd availability under waterlogged condition. Southwest China J Agric Sci 23(4):1188–1193. https://doi.org/10.3724/SP.J.1077.2010.10305
Huang Y, Wang LY, Wang WJ, Li TQ, He ZL, Yang XE (2018) Current status of agricultural soil pollution by heavy metals in China: a meta-analysis. Sci Total Environ 651:3034–3042. https://doi.org/10.1016/j.scitotenv.2018.10.185
Janssen PHM, Heuberger PSC (1995) Calibration of process-oriented models. Ecol Model 83:55–66. https://doi.org/10.1016/0304-3800(95)00084-9
Li X, Lin DS, Liu Y, Jiao WX, Zhang L (2018) Effects of different soil conditioners on cadmium control in cadmium-contaminated paddy fields. J Agro Environ Sci 37(7):1511–1520. https://doi.org/10.11654/jaes.2018-0802
Li C, Yang ZF, Yu T, Hou QY, Liu X, Wang J, Zhang QZ, Wu TS (2021) Study on safe usage of agricultural land in karst and non-karst areas based on soil Cd and prediction of Cd in rice: a case study of Heng County Guangxi. Ecotox Environ Safe 208:111505. https://doi.org/10.1016/j.ecoenv.2020.111505
Liu KH, Fang YT, Yu FM, Liu Q, Li FR, Peng SL (2010) Soil acidification in response to acid deposition in three subtropical forests of subtropical China. Pedosphere 20:399–408. https://doi.org/10.1016/S1002-0160(10)60029-X
Ma HH, Peng M, Guo F, Liu F, Tang SQ, Yang ZF, Zhang FG, Zhou YL, Yang K, Li K, Liu XQ (2021) Factors affecting the translocation and accumulation of cadmium in a soil-crop system in a typical karst area of Guangxi Province China. Environ Sci 42(3):1514–1522. https://doi.org/10.13227/j.hjkx.202007138
Mcbride M, Sauve S, Hendershot W (1997) Solubility control of Cu, Zn, Cd and Pb in contaminated soils. Eur J Soil Sci 48(2):337–346. https://doi.org/10.1111/j.1365-2389.1997.tb00554.x
MEE (Ministry of Ecology and Environment of the People’s Republic of China) (2018) Soil Environmental quality–risk control standards for soil contamination of agricultural land (GB 15618–2018). Chinese National Standard Agency, Beijing (in Chinese)
MLR (Ministry of Land and Resources) (2016) Specification of land quality geochemical assessment (DZ/T0295-2016). Geological Publishing House, Beijing (in Chinese)
NHFPC National Health and Family Planning Commission (2017) National Food Safety Standard: Maximum Limit of Contaminants in Food (GB 2762–2017). Standards Press of China, Beijing (in Chinese)
Ning CC, Wang JB, Cai KZ (2016) The effects of organic fertilizers on soil fertility and soil environmental quality: a review. Ecol Environ Sci 25(1):175–181. https://doi.org/10.16258/j.cnki.1674-5906.2016.01.026
Qin Y, Shi AYY, Xu L, Xu Y, Li J, Zhang Z, Gu CK, Li FD (2018) Bibliometric analysis of heavy metal passivation technology based on invention patents. J Agric Res Environ 35(4):283–291. https://doi.org/10.13254/j.jare.2017.0239 (in Chinese)
Qin JX, Fu W, Zheng GW, Deng B, Wu TS, Zhao XJ, Lu BK, Qin YX (2020) Selenium distribution in surface soil layer of karst area of Guangxi and Its affecting factors: a case study of wuming county. Acta Pedol Sin 57(5):1299–1310. https://doi.org/10.11766/trxb201909120327
Ruan YL, Li XD, Li YY, Chen P, Lian B (2015) Heavy metal pollution in agricultural soils of the karst areas and its harm to human health. Earth Environ 43(1):92–97. https://doi.org/10.14050/j.cnki.1672-9250.2015.01.013 (in Chinese)
Sebei A, Helali M, Oueslati W, Abdelmalek-Babbou C, Chaabani F (2018) Bioavailability of Pb, Zn, Cu, Cd, Ni and Cr in the sediments of the Tessa River: a mining area in the North-West Tunisia. J Afr Earth Sci 137:1–8. https://doi.org/10.1016/j.jafrearsci.2017.09.005
SEPA (State Environmental Protection Administration) (2014) The technical specification for soil environmental monitoring (HJ/T 166–2004). China Environment Publising Group, Beijing (in Chinese)
Song B, Wang FP, Zhou L, Pang R, Wu Y, Chen TB (2019) Prediction model for cadmium concentrations in rice grain under the geochemical background of a cadmium anomaly area in Guangxi. J AgroEnviron Sci 38(12):2672–2680. https://doi.org/10.11654/jaes.2019-0723 (in Chinese)
Sun L, Guo DK, Liu K, Meng H, Zheng YJ, Yuan FQ, Zhu HG (2019) Levels, sources, and spatial distribution of heavy metals in soils from a typical coal industrial city of Tangshan China. CATENA 175:101–109. https://doi.org/10.1016/j.catena.2018.12.014
Vries DW, McLaughlin MJ, Groenenberg JE (2011) Transfer functions for solid-solution partitioning of cadmium for Australian soils. Environ Pollut 159:3583–3594. https://doi.org/10.1016/j.envpol.2011.08.006
Wang R, Zhang FL, Xu SS, Zhang YW (2019) The method of dividing the value of soil heavy metal pollution risk screening: taking Cd as an example. Environ Sci 40(11):5082–5089. https://doi.org/10.13227/j.hjkx.201904132 (in Chinese)
Wang R, Hu XL, Zhang YW, Yu F, Zhu HS, Li Y (2020) Bioavailability and influencing factors of soil Cd in the major farming areas of Chongqing. Environ Sci 41(4):353–359. https://doi.org/10.13227/j.hjkx.201910229 (in Chinese)
Wang R, Deng H, Jia ZM, Yan MS, Jiao Z, Jin XD, Wang JB, Yu F (2021a) Characteristics of cadmium enrichment and pollution evaluation of soil-crop system in typical karst area. Environ Sci 42(2):941–951. https://doi.org/10.13227/j.hjkx.202008085 (in Chinese)
Wang XL, Liu HY, Zhou XY, Luo K, Yu EJ, Ran XZ (2021b) Study on the risk threshold for soil cadmium, based on potato quality in a high geological background area. J Agro-Environ Sci 2:355–363. https://doi.org/10.11654/jaes.2020-0988 (in Chinese)
Wen YB, Li W, Yang ZF (2020) Enrichment and source identification of Cd and other heavy metals in soils with high geochemical background in the karst region, southwestern China. Chemos. https://doi.org/10.1016/j.chemosphere.2019.125620
Xia XQ, Ji JF, Yang ZF, Han HJ, Huang CL, Li Y, Zhang W (2020) Cadmium risk in the soil-plant system caused by weathering of carbonate bedrock. Chemos 254:126799. https://doi.org/10.1016/j.chemosphere.2020.126799
Xing WQ, Zheng YL, Scheckel KG, Luo Y, Li L (2019) Spatial distribution of smelter emission heavy metals on farmland soil. Environ Monit Assess 191(2):1–12. https://doi.org/10.1007/s10661-019-7254-1
Yang Q, Hou QY, Gu QB, Yu T, Yang ZF (2016) Study of Geochemical Characteristics and Influencing Factors of Soil Selenium in the Typical Soil Profiles in Wuming County of Guangxi. Geoscience 30(2):455–462 (in Chinese)
Yao CB, Zhou MZ, Xiong KN, Zhang D, Yang Y, Zhang XR, Yang LS (2021) Contents of heavy metals in soils and crops in the demonstration area of karst rocky desertification control of the Karst Plateau-Gorge. China Environ Sci 41(1):316–326. https://doi.org/10.19674/j.cnki.issn1000-6923.2021.0038 (in Chinese)
Yu T, Yang ZF, Wang R, Zeng QL, Wang WL (2018) Characteristics and sources of soil selenium and other elements in typical high selenium soil area of Enshi. Soils 50(6):1119–1125. https://doi.org/10.13758/j.cnki.tr.2018.06.009 (in Chinese)
You SB, Yan Y (2017) Stepwise regression analysis and its application. Stati Decis 14:31–35. https://doi.org/10.13546/j.cnki.tjyjc.2017.14.007 (in Chinese)
Zhang Q (2005) A complete of analytical schemes and analytical data monitoring systems for determination of 54 components in multi-purpose geochemical mapping. Quat Sci 25:292–297 (in Chinese)
Zhang HM, Lv JL, Xu MG, Liu HX, Gong CY (2006) Review of studies on cadmium adsorption by soils. Soil Fertil Sci China 6:8–12 (in Chinese)
Zhang JZ, Sugir ME, Li YY, Yuan L, Zhou M, Lv P, Yu ZM, Wang LM, Zhou DX (2019a) Effects of vermicomposting on the main chemical properties and bioavailability of Cd/Zn in pure sludge. Environ Sci Pollut R 26(20):20949–20960. https://doi.org/10.1007/s11356-019-05328-2
Zhang JC, Zeng XP, Zhang ZM, Wen XM, Zhang QH, Lin CH (2019b) Characteristics and evaluation of speciation of heavy metals in forest soils of karst. Res Soil Water Conser 26(6):347–352. https://doi.org/10.13869/j.cnki.rswc.2019.06.046
Zhao Z, Jiang X, Wu YG, Zhan YZ, Chen CX, Jin XC, Cao CL (2011) Distribution characteristics of acid volatile sulfide and bioavailability evaluation of heavy metals in sediments of Lake Taihu. Acta Sci Circumstantiae 31(12):2714–2722 (in Chinese)
Zhou H, Deng Y, Lin L, CaoM ZL (2019) Stabilization of arsenic-contaminated soils using Fe-Mn oxide under different water conditions. Environ Sci 40(8):3792–3798. https://doi.org/10.13227/j.hjkx.201903119
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Li, X., Zhou, X. et al. Exploring the Risk Thresholds of Soil Heavy Metals in Carbonate and Non-carbonate Rock Areas: The Case of Qianjiang District in Chongqing, China. Bull Environ Contam Toxicol 109, 910–919 (2022). https://doi.org/10.1007/s00128-022-03580-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-022-03580-w