Abstract
Polycyclic aromatic hydrocarbons (PAHs) are persistent toxic substances that have ubiquitous presence in water, air, soil, and sediment environments. The growth of PAH toxicities and related ecotoxicology risk in estuary sediment has a serious concern. Present study examined the PAHs concentration, sources, and ecological risk from selected sites in Subarnarekha River estuary (SRE) sediment deposits. The sum of toxic 16 PAHs was ranged from 36.8 to 670.8 ng/g (mean = 223.46 ± 196.35 ng/g). The total PAH concentration varied significantly among the sampling sites (range 511.3 ng/g to 233.8 ng/g) based on allochthonous contaminant loads. Among the 16 compounds, Phen had the highest concentration (40.18 ng/g), followed by Pye (31.86 ng/g), Flur (29.36 ng/g), and NA (19.33 ng/g). Most of the sampling sites contained abundant 3-ring and 4–5-ring PAHs. Based on diagnostic ratios and PCA analysis petroleum combustion, biomass, and coal-burning have been identified as the major sources. The PAHs had high mutagenic equivalent factor and toxic equivalent factor values posing great ecological threats and health risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Water quality is one of the most critical issues in any aquatic environment. The impact of anthropogenic activities and natural processes such as volcanic activity and biological processes on human health and ecology are the main focus in most of the studies on aquatic systems. Rapid industrialization and population growth are the two significant factors for environmental contamination. Organic pollutants have a significant environmental concern because of their multi-faceted impacts on ecosystem health, human health and wellbeing, and associated socio-economic implications (Botwe et al. 2017). As an important category of organic contaminants, Polycyclic aromatic hydrocarbons (PAHs) represent semi-volatile persistent hydrocarbons having more than one aromatic fused ring of carbon and hydrogen atoms. They have earned significant environmental concern owing to their ubiquitous presence, recalcitrance, bioaccumulation potential, carcinogenic, mutagenic and endocrine disrupting effects (Ravindra et al. 2008; Mitra et al. 2019). International Agency for Research on Cancer (IARC) has reported that 16 individual PAH compounds have been registered as important pollutants by the US EPA while selecting seven of them as potential human carcinogenic pollutants (Zheng et al. 2016). The type of PAHs, their molecular structure, and exposure routes are responsible for PAHs' toxicity (Douben 2003). In linear or angular arrangements, PAHs have two or more aromatic rings. It can also be graded as high and low molecular weight PAHs according to their molecular weight (Abdollahi et al. 2013). The high molecular weight PAHs (4 or higher ring PAHs) are more lipophilic, less unstable, and less water-dissolved than low molecular weight PAHs (3 or lesser ring PAHs). The HMW-PAH has feasible carcinogenic effects on humans (Hale et al. 2012; Tavakoly Sany et al. 2014).
PAHs are released into the environment from both natural and anthropogenic sources. Diverse anthropogenic activities including incomplete burning of fossil fuels (e.g. coal, petrol) and biomass (organic garbage, grass, or plant materials), oil spills, municipal and urban runoff, wastewater discharges cause PAH pollution (Zakaria et al. 2002; Okuda et al. 2010;). However, some natural processes, such as volcanic eruptions and forest fires, can also contribute copiously to the natural budget of the PAH inventory (Ma et al. 2010; Tobiszewski and Namiesnik 2012). Due to their distinct chemical attributes, PAHs' affinity to abiotic and biotic components of air, water, soil/sediment environments vary to a great extent (Guo et al. 2009; Pérez-Fernández et al. 2015). PAHs can persist in water for a very long period of time because of their low sensitivity to photooxidation in water than in air attributed to hydrophobicity and turbulence-induced dilution effects. The distribution, transformation and fate of different PAHs are regulated by their relative solubility and hydrophobicity (Scott et al. 2012). In an aquatic environment, PAHs are readily adsorbed onto particulate matter, consequently remaining in higher concentrations in sediments and lower in surface water and water columns (Scott et al. 2012; Giesy et al. 2016). Thus the sediment behaves as the primary sink and reservoir for PAHs in the aquatic environment.
Although plenty of studies of PAHs distribution in rivers and coastal/estuarine sediments have been conducted in the more industrialized regions in the world, there remains a paucity of information on environmental distribution PAHs and their sources apportionment across the estuary in India where climatic regimes of the region characterized by frequent and intense rainstorms play a significant role in washing out land-based and airborne contaminants to the aquatic environments more effectively than moderate and high latitudes. Under this perspective the present study was undertaken with the following objectives: (1) to determine concentrations of USEPA's 16 priority pollutant PAHs across the Subarnarekha River estuary, India; (2) to identify the potential sources apportionment of these PAHs by diagnostic ratios and principal component analysis (PCA); (3) to assess the source-specific potential ecological risks posed by PAHs derived from identified sources employing the toxic equivalent factor (TEQ) and mutagenic equivalent factor (MEQ).
Materials and Methods
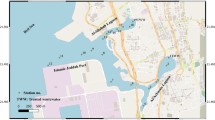
The current work was performed along the estuary of the Subarnarekha River (SRE) (87 × 18′ E 21 × 33′ N). The Subarnarekha River travels a total flow of 395 km (245 mi) in length. The river originates near Nagri village (23° 18′ 02′′ N, 85° 11′ 04′′ E) and is fed by precipitation. It flows through the 470 km long state of Jharkhand, West Bengal, and Orissa and eventually falls into the Bay of Bengal at the port of Kirtania, Baleswar district, Orissa (21° 33′ 18′′ N, 87° 23′ 31′′ E). The total drainage system area (basin size) of the Subarnarekha River is 18,950 km2 (7317 m2). Forests, farmland, industrial areas, and mining areas form the dominant land cover. The river climate is of an arid and sub-humid to humid sub-arid type.
The Subarnarekha River flows through the significant mineralized and industrial areas of India, passing through industrial belt of Jharkhand, which has TATA Steel, TATA motors, and other Jamshedpur and Adityapur group-related companies in its fold. It is often highly polluted, especially during dry periods, due to industrial emissions and effluents. The present study was carried out at the estuary of the Subarnarekha River.
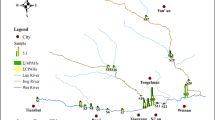
The sediment samples were collected from three sampling zones along the SRE: four representatives from the freshwater zone (Fw), three samples from the brackish water zone (Bw), and four samples from the estuary zone (Ez) (Fig. 1) in summer (March to early May) 2019 using a stain-less steel van Veen grab sampler (AISI 316 station steel, the grab = 8 mm arms, designed from the square tube, 60 × 60 × 4 mm with 12 mm reinforcements). The sampler was equipped with four lids that allowed easy removal of undisturbed part of the sample without emptying the whole selection. The samples were collected on a weekly basis. The sampling time was from morning 9 AM to 10 AM. The samples were randomly collected in triplicate from 4 to 6 cm depth at each sampling point. The triplicate sediment samples were adequately mixed and immediately packed in pre-cleaned (with Milli-Q water having resistivity = 11,818.2 MΩ cm) aluminum containers. With icebox prevention, the collected samples were kept and transferred to the Environmental Research Laboratory, Department of Chemistry, NIT Jamshedpur within 10 h and stored in a refrigerator at − 18°C until extraction.
PAHs in sediment samples were extracted following standard solvent extraction protocols. The air-hydrated sediment samples were homogenized and delivered through 60 standard lattice sieves. The dry sediment sample (2 g) mixed with 0.5 g anhydrous Na2SO4 in 10 mL of Dichloromethane (DCM) was sonicated for 2 h trailed by centrifugation. Then 3 mL of supernatant was filtered through 2 g of silica gel column with 11 mL 1:1(v/v) elution of hexane and DCM (Chen et al. 2007). The evaporation of solvent fractions was carried out by rotary evaporator (Hei-VAP Core—hand lift model with G1 diagonal glassware) and substituted by acetonitrile with an absolute volume of 2 mL. The deuterium-labeled PAHs (Naphthalene-D8, Phenanthrene-D10, and Chrysene-D12) were used as surrogate standards. Afterward, below a thin stream of clean nitrogen, the eluent solvent was concentrated to 20 μL. To quantify the internal standard, analyte hexamethyl benzene was transferred before investigation (Khuman et al. 2018). The USEPA's 16 priority pollutant PAHs namely Naphthalene (NA), Acenaphthene (AC), Acenapthylene (ACY), Fluorene (Fluo), Phenanthrene (Phen), Anthracene (AN), Fluoranthene (Flur), Pyrene (Pye), Benzo(a)Anthracene (BaA), Chrysene (CHRY), Benzo(b)Flouranthene (BbF), Benzo(k)Flouranthene (BkF), Benzo(a)Pyrene(BaP), Dibenzo [a,h]anthracene (DBA), Benzo[g,h,i]perylene (BgP) and Indeno[1,2,3-cd]pyrene (IN) were analysed from Agilent 7890B Gas chromatograph (GC-FID) of the sample sediment extracts using a HP-5 MS capillary column (30 m × 0.32 mm × 0.25 µm) with 7 inch enclosure. In splitless mode, 1 µL of each sample was injected, and the presence of the target compounds was determined by matching the retention time of the compounds with that of an authentic standard. High purity nitrogen gas was used as carrier gas at a flow rate of 1.83 mL/min. The initial oven temperature was set at 70°C for 1 min and then increased by keeping for 20 min to 300°C at a rate of 5°C/min. The injector and transfer line temperatures were held at 300°C and 325°C, respectively.
The standard solution of 16 PAHs in acetonitrile (ID-3697900), deuterium-labeled PAHs, and hexamethyl benzene (CAS Number-87-85-4, make: Sigma-Aldrich, USA) was used for analysis in the present study. The procedural blank samples, sample duplicates, samples spiked with surrogate standards: Phenanthrene-D10 (CAS Number -1517-22-2), Naphthalene-D8 (CAS Number -1146-65-2), and Chrysene-D12 (CAS Number -1719-03-5) were used. Hexamethyl benzene was used as an internal standard for all PAHs calibration for quality control testing. The recovery percentage of 16 PAHs in sediment samples was approximately 65%–105%. The detection limits of the Method Detection Limits (MDLs) of the target PAHs have been determined as three times the standard deviation (sd) plus the mean target compound concentrations in blank samples. The detection limit (LOD) range for compounds of PAHs was from 0.02 to 0.18 ng/g. Concentration values below the detection limits for the method were considered to be < LOD.
The toxic equivalent quotient (TEQ) and mutagenic equivalent quotient (MEQ) were calculated using Eqs. (1) and (2) in sediment samples of SRE. Potential mutagenic and carcinogenic toxicities of the high molecular weight PAHs were assessed and ranked relative to benzo[a]pyrene (BaP), the best known most studied PAH. In Eqs. (1) and (2), Cn stands for each PAHs congeners (n) concentration, TEFn represents each PAHs congeners (n) toxic equivalent factor, and MEFn stands for individual PAHs congeners (n) mutagenic equivalent factor, respectively. These were calculated by multiplying with their mutagenic equivalent factor (MEQ) or toxic equivalent factor (TEQ) with the summation of all sediment samples' carcinogenic PAHs concentration (Lerda 2011; Adeniji et al. 2019).
The corresponding parameter values are given in Table 2.
Results and Discussion
Sixteen individual PAHs were investigated in the sediment samples. Total PAHs are reported as a sum of 16 congeners and ranged from 36.8 to 670.8 ng/g dry weight (Table 1), with a mean ± standard deviation value of 223.46 ± 196.35. The level of PAH contamination in sediments from SRE was compared with previously reported deposits of different regions of the world (Table 1). The PAHs level observed in the present study is higher than reported in sediments of Yellow River, China (range 31.0–133.0 ng/g) (Li et al. 2006), and Bohai Bay, China (range 24.6–280.6 ng/g) (Wang et al. 2015). However, PAHs contamination in the present study was lower than Mithi River, India (range 1206.0–4735.0 ng/g) (Singare 2015), and the Yellow Sea, China (range 148.3–907.5 ng/g). Being in a similar field the results obtained in this study match with Priolo Bay, Italy (range 56.4–847.1 ng/g) (Di Leonardo et al. 2014). The levels of Σ16PAHs in sediments of station Ez were the highest and ranged between 181.2 and 511.3 ng/g followed by station Fw, which ranged between 95.7 and 343 ng/g, station Bw, 55.6 to 64.9 ng/g (Fig. 3).
The level of 3-ring PAH Phen was found to be higher (range 10.6–144.7 ng/g, mean: 40.18 ± 39.99 ng/g), which was followed by the 4-ring PAHs Pye (range 8.1–97.3 ng/g, mean: 31.86 ± 29.53 ng/g) and Flur (range 8.1–96.2 ng/g, mean: 29.36 ± 25.64 ng/g). However, the 5-ring PAH DBA's range (< DL-2.3) ng/g with a mean (1.7 ± 0.33) ng/g was found lower, followed by the 6-ring PAHs BgP (< DL-7.6) ng/g with a mean (3.49 ± 1.99) ng/g and IN (< DL-8.6) ng/g with a mean (3.06 ± 2.68) ng/g (Fig. 2).
With a total of 16PAHs 511.3 ng/g, sample site S8 had the highest PAH concentration, followed by S9 at 454.3 ng/g and S1 at 343 ng/g. The sampling site S6 with a total Σ16PAHs 58.8 ng/g and S7 with 55.6 ng/g were detected with a lower PAH concentration. At sampling site S6, only seven PAH congeners were seen out of sixteen, and at site S7, nine PAHs were detected in the Bw zone. Overall, the higher concentration of PAHs was identified in the sediments of the estuary zone (Ez) (S8–S11) rather than the upstream sampling stations Fw (S1–S4) and Bw (S5–S7). This variation of PAHs concentration in the sediments of SRE might be related to several factors such as differential re-suspension and re-deposition of sedimentary PAHs, microbial degradation of PAHs, textural composition and depositional rate of sediments, and vertical mixing due to either physical or biological processes (Boonyatumanond et al. 2006). Depending upon the ring size, the PAHs can be categorized according to their molecular weight as high molecular weight PAHs (HMW) and low molecular weight PAHs (LMW-PAH) (Abdollahi et al. 2013). The high molecular weight PAHs (4–6 ring PAHs) are more lipophilic, less volatile, and less water-soluble and have a greater tendency to accumulate in the sediments than low molecular weight PAHs (2–3 ring PAHs) and have probable carcinogenic effects on human beings (Hale et al. 2012; Tavakoly Sany et al. 2014; Khuman et al. 2018). In all sampling sites, the (LMW-PAH) 3-ring and (HMW-PAH) 4–5-ring PAHs were abundant (Fig. 3). The 3-ring PAHs concentration was higher at sampling site S8, with 194.4 ng/g, followed by sampling site S9 with 93.8 ng/g, and S5 recorded the lowest concentration (15.4 ng/g) of 3-ring PAHs. The sampling site S9 also recorded a higher concentration of 210.3 ng/g 4-ring PAHs, followed by sampling site S1 with 195.3 ng/g, and S8 recorded 186.2 ng/g of 4-ring PAHs. The sampling site S7 was spotted with a lower concentration of 4-ring PAHs. The sampling site S9 registered a higher concentration of 121.8 ng/g 5-ring PAHs, followed by sampling site S8 with 73.1 ng/g. Furthermore, the sampling site S7 was spotted with a lower concentration of 5-ring PAHs (Fig. 3). The ring wise composition of PAHs in sediments shows that the 4-ring PAHs contribute 42% of the total PAHs concentration, followed by 3-ring (30%), 5-ring (16%), 2-ring (9%), and 6-ring PAHs (3%).
Principal component analysis (PCA) (Fig. 5) and three molecular ratios between isomers: AN/(AN + Phen), Flur/(Flur + Pye) and BaA/(BaA + CHRY) (Yunker et al. 2002; Zhang et al. 2004) (Fig. 4) were employed for source apportionment. The 45% ratios of Flur/(Flur + Pye) > 0.5 allowed to identify the possible sources like biomass and coal combustion sources, and 36% ratios of Flur/(Flur + Pye) between 0.4 and 0.5 suggested a source of petroleum combustion. The 91% ratios of AN/(AN + Phen) > 0.1 described a discharge source. The 100% ratios of BaA/(BaA + CHRY) > 0.35 allowed us to identify the possible sources of combustion (Fig. 4). No industrial activities were detected at the study sites. However, the Subarnarekha River flows through one of the most heavily industrialized areas of India. The area is known for ore mining and associated industrial activities. The city of Jamshedpur is a major industrial centre of Eastern India with > 1200 small and medium scale industries. All 16 PAHs were represented by 3 PC factors (PC1, PC2, and PC3), and the extracted eigenvectors for all PAHs showed 70% for PC1. This factor was heavily loaded with the 3-ring PAH Phen, 4-ring PAH Pye, and 5-ring PAH Bap and CHRY. PAHs like Pye and CHRY are the indicators for coal burning (Tavakoly Sany et al. 2014), and BaP is the marker for fuel combustion (Belis et al. 2011). PC2 showed 15% of the total alteration and was loaded with 4-ring PAH Flur and 5-ring PAH BkF. The high molecular PAHs like Flur and BkF may indicate pyrolysis and incomplete biomass combustion (Jiang et al. 2009). The PC3 contributed only 11% of the total loaded, and alteration was with 2-ring PAH NA (Fig. 5). The PAH NA is the main component of diesel fuels and gasoline, which may be formed by other incomplete combustion sources (Dong and Lee 2009; Soltani et al. 2015). The PCA and diagnostic ratios suggested that the origins of contamination of sediments in SRE are due to the combustion sources like gasoline burning and fuel and coal burning.
The toxicity assessment of sediments from SRE was carried out using the total concentration of 8 PAHs (BaA, CHRY, BbF, BkF, BaP, DBA, BgP, and IN) because of their unique carcinogenic potencies relative to PAHs (Ambade et al. 2020). The sum of TEQ and MEQ values of carcinogenic PAHs was 148.2 ng/g and 169.33 ng/g (Table 2). The total TEQ values calculated for sediment samples ranged from 0.24 to 85.6 ng/g. Bap had a higher TEQ value (85.6 ng/g) among 8 PAHs and accounted for 57% of the total TEQ value, whereas the BgP had the lowest (0.24 ng/g). The absolute MEQ values calculated for sediment samples ranged from 2.31 to 85.6 ng/g. The TEQ value for BaP was found higher (85.6 ng/g) and accounted for 50% of the total TEQ value, whereas the value for CHRY was the lowest (2.31 ng/g). The average TEQ and MEQ were 18.52 ng/g and 21.16 ng/g, respectively. The average values of TEQ and MEQ in the sediments of SRE were lower than in the sediments of Loire River, France (Bertrand et al. 2015) and Mithi River, India (Singare 2015). This indicates that the TEQ and MEQ values for SRE were low and contained less ecological risk. The risk level of PAHs contamination in sediments of SRE towards the biological community was analyzed by comparing the concentration of individual PAHs with the Effect Range Low (ERL) and Effect Range Median (ERM) values (Long and MacDonald 1998). We found that most PAHs level was less than the ERL value indicating no adverse effect on the aquatic ecosystem. However, the level of Fluoranthene at sampling site S1 was relatively higher than the ERM value. The living organisms at sampling sites S1 might have some risks due to PAH contamination. Furthermore, there were no PAHs detected whose level higher than the ERM value. However, the PAHs can cause adverse biological effects on individual species and alter ecosystem functioning by long-time exposure to PAHs (Akhbarizadeh et al. 2016).
This study provided essential data on levels, distribution, and probable sources of PAHs contamination in sediments of SRE. It reported a high concentration of PAHs at sampling sites S8, S9, S1, and S11 compared to other sampling sites due to higher port activities such as heavy traffic of boats, large ships, and wastewater discharges. Site S8 also showed intense industrial activities, vehicular emissions, and combustion processes compared to sampling sites S5, S6, and S7. Most of the sampling sites contained abundant 3-ring and 4–5-ring PAHs. The sediment profile recorded at sampling site S9 included the highest 4-ring PAHs, followed by S1 > S8 > S11. However, the sediments of sampling site S8 have the highest level of 3-ring PAHs, followed by S9. Likewise, the 5-ring PAHs were most abundant at site S9, followed by S8. The 4-ring PAHs were reported higher at all sampling sites and provided an incremental footprint of 42% of the total PAHs concentration followed by the 3-ring ones (30%). The calculated individual PAHs diagnostic ratios and PCA revealed that the basic causes of PAHs contamination were gasoline burning, biomass (including grass) burning, and coal combustion. This study's approved method is suggestive of the inclusive toxicity of the aquatic environment and offers an effective tool for identifying responsible chemical contaminations working in the background. It advises that mitigation strategies for PAHs in the Subarnarekha River river basin must include an emphasis on controlling and guiding exhaust discharge and coal burning. The assessed integrated lifetime cancer risks associated with such aquatic environment indicated that ingestion and dermal contact are prevailing routes of exposure to adults and children, presenting ‘moderate’ potential cancer risk. The assessed TEQ and MEQ values for sediments of SRE serve as an indicator of minor toxicity and low environmental risk. Finally, the results presented in this study warrant regular long-term monitoring of PAHs in the riverine ecosystem in order to identify the fate of PAHs and avert serious ecotoxicological risks that may crop up from the build-up of PAHs beyond the critical levels.
References
Abdollahi S, Raoufi Z, Faghiri I, Savari A, Nikpour Y, Mansouri A (2013) Contamination levels and spatial distributions of heavy metals and PAHs in surface sediment of Imam Khomeini Port, Persian Gulf. Iran Mar Pollut Bull 71:336–345
Adeniji AO, Okoh OO, Okoh AI (2019) Levels of polycyclic aromatic hydrocarbons in the water and sediment of buffalo river estuary, South Africa and their health risk assessment. Arch Environ Con Tox 76:657–669
Akhbarizadeh R, Moore F, Keshavarzi B, Moeinpour A (2016) Aliphatic and polycyclic aromatic hydrocarbons risk assessment in coastal water and sediments of Khark Island, SW Iran. Mar Pollut Bull 108:33–45
Belis CA, Cancelinha J, Duane M, Forcina V, Pedroni V, Passarella R, Tanet G, Douglas K, Piazzalunga A, Bolzacchini E, Sangiorgi G, Perrone MG, Ferrero L, Fermo P, Larsen BR (2011) Sources for PM air pollution in the Po Plain, Italy: I. Critical comparison of methods for estimating biomass burning contributions to benzo(a)pyrene. Atmos Environ 45:7266–7275
Bertrand O, Mondamert L, Grosbois C, Dhivert E, Bourrain X, Labanowski J, Desmet M (2015) Storage and source of polycyclic aromatic hydrocarbons in sediments downstream of a major coal district in France. Environ Pollut 207:329–340
Botwe BO, Kelderman P, Nyarko E, Lens PNL (2017) Assessment of DDT, HCH and PAH contamination and associated ecotoxicological risks in surface sediments of coastal Tema Harbour (Ghana). Mar Pollut Bull 115:480–488
Boonyatumanond R, Wattayakorn G, Togo A, Takada H (2006) Distribution and origins of polycyclic aromatic hydrocarbons (PAHs) in riverine, estuarine, and marine sediments in Thailand. Mar Pollut Bull 52:942–956
Chen Y, Zhu L, Zhou R (2007) Characterization and distribution of polycyclic aromatic hydrocarbon in surface water and sediment from Qiantang River, China. J Hazard Mater 141:148–155
Dong TTT, Lee BK (2009) Characteristics, toxicity, and source apportionment of polycylic aromatic hydrocarbons (PAHs) in road dust of Ulsan, Korea. Chemosphere 74(9):1245–1253
Douben PE (2003) PAHs: an ecotoxicological perspective. Wiley, Hoboken
Di Leonardo R, Mazzola A, Tramati CD, Vaccaro A, Vizzini S (2014) Highly contaminated areas as sources of pollution for adjoining ecosystems: the case of Augusta Bay (Central Mediterranean). Mar Pollut Bull 89(1–2):417–426
Guo W, He M, Yang Z, Lin C, Quan X, Men B (2009) Distribution, partitioning and sources of polycyclic aromatic hydrocarbons in Daliao River water system in dry season China. J Hazard Mater 164:1379–1385
Giesy JP, Tang Z, Zhao X (2016) Historical record of effects of human activities on absolute and relative concentrations of polycyclic aromatic hydrocarbons (PAHs) in Lake Chao, China. J Environ Sci 46:1–4
Hale SE, Lehmann J, Rutherford D, Zimmerman AR, Bachmann RT, Shitumbanuma V, O’Toole A, Sundqvist KL, Arp HPH, Cornelissen G (2012) Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ Sci Technol 46:2830–2838
Jiang Y-F, Wang X-T, Wang F, Jia Y, Wu M-H, Sheng G-Y, Fu J-M (2009) Levels, composition profiles and sources of polycyclic aromatic hydrocarbons in urban soil of Shanghai, China. Chemosphere 75:1112–1118
Khuman SN, Chakraborty P, Cincinelli A, Snow D, Kumar B (2018) Polycyclic aromatic hydrocarbons in surface waters and riverine sediments of the Hooghly and Brahmaputra Rivers in the Eastern and Northeastern India. Sci Total Environ 636:751–760
Lewis MA, Russell MJ (2015) Contaminant profiles for surface water, sediment, flora and fauna associated with the mangrove fringe along middle and lower eastern Tampa Bay. Mar Pollut Bull 95(1):273–282. https://doi.org/10.1016/j.marpolbul.2015.04.001
Lerda D (2011) Polycyclic aromatic hydrocarbons (PAHs) Factsheet, 4th edn. JRC technical notes, 66955–2011, pp. 6–13.
Li G, Xia X, Yang Z, Wang R, Voulvoulis N (2006) Distribution and sources of polycyclic aromatic hydrocarbons in the middle and lower reaches of the Yellow River, China. Environ Pollut 144(3):985–993
Li J, Dong H, Zhang D, Han B, Zhu C, Liu S, Liu X, Ma Q, Li X (2015) Sources and ecological risk assessment of PAHs in surface sediments from Bohai Sea and northern part of the Yellow Sea. China Mar Pollut Bull 96(1–2):485–490. https://doi.org/10.1016/j.marpolbul.2015.05.002
Long ER, MacDonald DD (1998) Recommended uses of empirically derived, sediment quality guidelines for marine and estuarine ecosystems. Hum Ecol Risk Assess Int J 4:1019–1039
Ma WL, Li YF, Qi H, Sun DZ, Liu LY, Wang DG (2010) Seasonal variations of sources of polycyclic aromatic hydrocarbons (PAHs) to a northeastern urban city, China. Chemosphere 79:441–447
Malik A, Verma P, Singh AK, Singh KP (2011) Distribution of polycyclic aromatic hydrocarbons in water and bed sediments of the Gomti River, India. Environ Monit Assess 172(1–4):529–545. https://doi.org/10.1007/s10661-010-1352-4
Minissi S, Caccese D, Passafiume F, Grella A, Eleonora C, Rizzoni M (1998) Mutagenicity (micronucleus test in Vicia faba root tips), polycyclic aromatic hydrocarbons and heavy metal content of sediments collected in Tiber River and its tributaries within the urban area of Rome. Mutat Res 420(1–3):77–84. https://doi.org/10.1016/S1383-5718(98)00142
Mitra S, Corsolini S, Pozo K, Audy O, Sarkar SK, Biswas JK (2019) Characterization, source identification and risk associated with polyaromatic and chlorinated organic contaminants (PAHs, PCBs, PCBzs and OCPs) in the surface sediments of Hooghly estuary, India. Chemosphere 221:154–165
Okuda T, Okamoto K, Tanaka S, Shen ZX, Han YM, Huo ZQ (2010) Measurement and source identification of polycyclic aromatic hydrocarbons (PAHs) in the aerosol in Xi’an, China, by using automated column chromatography and applying positive matrix factorization (PMF). Sci Total Environ 408:1909–1914
Pérez-Fernández B, Viñas L, Franco MÁ, Bargiela J (2015) PAHs in the Ría de Arousa (NW Spain): a consideration of PAHs sources and abundance. Mar Pollut Bull 95(1):155–165
Ravindra K, Sokhi R, Van GR (2008) Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos Environ 42:2895–2921
Sun JH, Wang GL, Chai Y, Zhang G, Li J, Feng J (2009) Distribution of polycyclic aromatic hydrocarbons (PAHs) in Henan Reach of the Yellow River, Middle China. Ecotoxicol Environ Saf 72:1614–1624
Scott HE, Aherne J, Metcalfe CD (2012) Fate and transport of polycyclic aromatic hydrocarbons in upland Irish headwater lake catchments. Sci World J. https://doi.org/10.1100/2012/828343
Singare PU (2015) Studies on polycyclic aromatic hydrocarbons in surface sediments of Mithi River near Mumbai, India: assessment of sources, toxicity risk and biological impact. Mar Pollut Bull 101(1):232–242
Soltani N, Keshavarzi B, Moore F, Tavakol T, Lahijanzadeh AR, Jaafarzadeh N, Kermani M (2015) Ecological and human health hazards of heavy metals and polycyclic Aromatic hydrocarbons (PAHs) in road dust of Isfahan metropolis, Iran. Sci Total Environ 505:712–723
Tavakoly Sany S, Hashim R, Salleh A, Rezayi M, Mehdinia A, Safari O (2014) Polycyclic aromatic hydrocarbons in coastal sediment of Klang Strait, Malaysia: distribution pattern, risk assessment and sources. PLoS ONE 9:e94907
Tobiszewski M, Namiesnik J (2012) PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut 162:110–119
Wang M, Wang C, Hu X, Zhang H, He S, Lv S (2015) Distributions and sources of petroleum, aliphatic hydrocarbons and polycyclic aromatic hydrocarbons (PAHs) in surface sediments from Bohai Bay and its adjacent river, China. Mar Pollut Bull 90(1–2):88–94
Yunker M, MacDonald R, Vingarzan R, Mitchell R, Goyette D, Sylvestre S (2002) PAHs in the Fraser River Basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515
Zakaria MP, Takada H, Tsutsumi S, Ohno K, Yamada J, Kouno E, Kumata H (2002) Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: a widespread input of petrogenic PAHs. Environ Sci Technol 36:1907–1918
Zhao X, Ding J, You H (2014) Spatial distribution and temporal trends of polycyclic aromatic hydrocarbons (PAHs) in water and sediment from Songhua River, China. Environ Geochem Health 36(1):131–143. https://doi.org/10.1007/s10653-013-9524-0
Zhang ZL, Huang J, Yu G, Hong HS (2004) Occurrence of PAHs, PCBs and organochlorine pesticides in the Tonghui River of Beijing, China. Environ Pollut 130:249–261
Zheng B, Wang L, Lei K, Nan B (2016) Distribution and ecological risk assessment of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River estuary and the adjacent area, China. Chemosphere 149:91–100
Acknowledgements
This study was financially supported by the SERB (Science and Engineering Research Board [Grant No. NITJSR/CHEM/RP/02]), India, to provide the GC/FID facilities to analyze the PAH samples. Mr. Shrikanta Shankar Sethi thanks BRNS (Board of Research in Nuclear Science) for providing him financial support during the study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ambade, B., Sethi, S.S., Giri, B. et al. Characterization, Behavior, and Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in the Estuary Sediments. Bull Environ Contam Toxicol 108, 243–252 (2022). https://doi.org/10.1007/s00128-021-03393-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03393-3