Abstract
Batch equilibrium phosphorus concentration (EPC0) methods were applied to determine phosphorus capacity of sediments from three agriculturally influenced tributaries in southern Ontario – Belle River, Big Creek, and Nissouri Creek. Aliquots of sediments were amended with soluble reactive phosphorus and incubated at four temperatures (5, 15, 25, and 35°C). Batches of sediments from each location and temperature were also subjected to a treatment; gamma (γ)-irradiated at 28 kGy over 24-h (sterilized) and compared to non-sterilized biotic samples. Treatment showed a significant effect on EPC0 in Belle River and Nissouri Creek but non-significant effect in Big Creek. Temperature showed a significant effect in Belle River, Nissouri Creek, and the biotic subset of Big Creek. While direction of shift was not consistent in all cases, the biotic subsets of all three locations showed a significant effect of temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Phosphorus (P) is an important nutrient to primary producers in aquatic environments, and contributes to eutrophication and harmful algal blooms in receiving lakes when added in excess (Pant and Reddy 2001; Lucci et al. 2010; Cooper et al. 2015; Emelko et al. 2016). For agricultural streams, a major source of P is from soil loss and fertilizer applications collectively, referred to as non-point sources (Pant and Reddy 2001; Lucci et al. 2010). Once P enters water it can settle as particulate forms, be carried downstream in solubilized form and/or undergo several biogeochemical reactions that further alter its fate, mobility, and sequestration in the environment (Sharpley et al. 2007).
The dynamic interaction of P in its various forms between sediments and soluble reactive phosphorus (SRP) in overlying water is referred to as the P buffering system (Novak et al. 2004; Weigelhofer et al. 2018). The P buffering system is directly related to the sorption and desorption capacity of sediments for SRP and has historically been considered to be influenced by geochemical and physical characteristics of the sediments (Novak et al. 2004; Emelko et al. 2016; Weigelhofer et al. 2018). However, the role that microbial organisms play in mediating sediment sorption capacity and the sediment buffering system is not fully established (Gibbons et al. 2014).
A common tool for describing and measuring the sediment buffering system is the equilibrium phosphorus concentration (EPC0) concept. The EPC0 specifies the overlying water SRP concentration at which no net sorption or desorption occurs with the underlying sediments (Novak et al. 2004). EPC0 is typically measured by batch vial equilibrium studies where water/sediment systems are amended with varying levels of SRP to determine by extrapolation the level of amendment where no net sorption onto sediment takes place. Sediments with very low EPC0 are considered to have high buffering capacity and are capable of scavenging dissolved P from water column when SRP exceeds EPC0. Sediments with high EPC0 have lower buffering capacity and can release dissolved SRP into water when the SRP is less than EPC0 (Pant and Reddy 2001; Smith et al. 2006; Lucci et al. 2010; Machesky et al. 2010). This can pose particular challenges to managing P-loads into receiving aquatic water bodies since reduction of SRP entering from the land can revert tributary sediments from sink to source after long term legacy P loadings are abated.
In order to examine the potential influence of microbial processes on the sediment buffering capacity, EPC0 was used as a surrogate measure of sediment buffering capacity for P. This study measured EPC0 in treated (sterilized) and non-treated (biotic) sediments obtained from three different agriculturally influenced tributaries. The treatment of the sediments was sterilization performed by gamma (γ-) irradiation. This method of sterilization is effective at destroying microorganisms but does not alter physical–chemical properties of sediments as may occur under autoclaving or chemical sterilization procedures (Lotrario et al. 1995; McNamara et al. 2003). Given that microbial processes are temperature dependent, comparisons of sterilized and biotic EPC0 measurements were also performed across a gradient of temperature. If sediment buffering capacity were governed by purely physical and chemical processes then we would expect no effect of sterilization on EPC0.
Materials and Methods
Three streams were sampled by obtaining a spade-full of the sediment from the top layer taken to approximately 5 cm deep and placing the sediments into a sealed plastic bucket. The locations include the Belle River (Lakeshore, Ontario, Canada), Big Creek (Comber, Ontario, Canada), and the Nissouri Creek (London, Ontario, Canada). Each stream is agriculturally stressed; however, the method of fertilization is varied such that the fields surrounding Big Creek are predominantly chemically fertilized, the fields surrounding Nissouri Creek are predominantly manure fertilized, and the fields surrounding Belle River are more evenly split between manure and chemical fertilization.
Subsamples of sediment from each location were sent to McMaster University (Hamilton, Ontario, Canada) for γ-radiation (hereon called “sterilized”). To do so, the two samples from each site were homogenized, and approximately one-third of the combined samples were placed into two zip-lock bags, sealed and subsequently placed into a 20 L bucket with the other sample sites prepared in the same manner. McMaster University placed the 20 L bucket next to a Cobalt-60 source giving a 28 kGy dose of gamma radiation over a 24-h period (Chen et al. 2013).
Prior to completing the experiments, small subsamples of the sediments were taken using cryogenic tubes and frozen at − 80°C for RNA extractions to ensure sterilization. RNA was extracted from these samples using Takara RNA extraction kits (Mountain View, California, USA). The RNA extracts were then run on an Agilent 2100 Bioanalyzer (Santa Clara, California, USA) to determine presence or absence of microbial community. The detection limit for the method is 5 ng RNA/μL of extractant.
Grain size distribution of sediments were performed as described by Szalinska et al. (2006). Comparison of each grain size fraction between the biotic and the sterilized sediments was performed using paired t-tests and a Bonferroni Corrections of p values for the number of tests. Total organic carbon percentage (%TOC) was completed using loss on ignition (LOI) on each sediment as per Drouillard et al. (2006). Comparison of the LOI in biotic and the sterilized sediments was done by paired t test.
Prior to starting the EPC0 batch experiment, stock solutions of 0.8 g/L P Potassium Dihydrogen Phosphate (KH2PO4) and 0.00571428 M of Calcium Chloride Dihydrate (CaCl2·2H2O) were prepared. For the multi-point batch equilibrium experiment aliquots of sediments (5–6 g) of each sediment type and treatment were added to sets of 7 labelled 50 mL centrifuge tubes with caps and stored at − 20°C prior to running. The CaCl2·2H2O solution and KH2PO4 solutions were added to the centrifuge tubes to generate initial SRP concentrations of 2000, 500, 150, 70, 30,15, and 4 µg/L P, respectively. The sealed centrifuge tubes were placed on an orbital shaker at 150 rpm and incubated at temperatures of 5, 15, 25, or 35°C. After 24 h the tubes were taken off the orbital shaker and centrifuged at 3000 rpm for 30 min. After centrifugation, the supernatant was filtered using 0.45 µm cellulose acetate syringe filters into acid washed polyethylene bottles and stored at − 20°C for analysis of SRP using a SmartChem 200 DA (Unity Scientific, Brookfield, CT). The reaction occurring in the SmartChem 200 DA is based on the phosphomolybdenum blue method and uses ammonium molybdate, potassium antimony titrate, sulfuric acid, ascorbic acid, and sodium dodecyl sulfate as reagents. The detection limit for the method is 1.9 μg/L.
Triplicate batch experiments were performed for each sediment type and sterilization treatment across each temperature. For each replicate the amount of SRP sorbed (mg/g) to sediment at the end of incubation was calculated according to Eq. 1:
where SRPi is the initial spiked SRP concentration (μg/L), SRPf is the final SRP concentration (μg/L) measured at the end of incubation, v is the volume (L), and mdry is the dry mass of the sediment (g). EPC0 is subsequently determined by interpolating the SRP concentration at the point where Psorb = 0. This was achieved using a line of best fit derived from a linear equation described by Eq. 2:
where m is the slope of the linear fit and c is a constant. Equation 2 was fit using Systat (San Jose, California, USA) software to obtain probability of slope as well as the fitting variables. EPC0 is solved as the value of SRPf where Psorb = 0, Eq. 3, by rearrangement of Eq. 2:
Statistical differences in EPC0 across sediment type, sterilization, treatment and temperature were analyzed by a general linear model (GLM) using Systat software (San Jose, California, USA). Assumptions of normality were tested using normal probability plots. When variable interactions were significant, GLM’s were repeated for data from each site separately. If variable interaction significance persisted in the site-specific GLMs then ANOVA’s were performed to test treatment effects at each temperature separately and liner regressions were used to test the temperature effect in sterilized or biotic samples separately.
Results and Discussion
The particle size distribution, %TOC, and RNA bioanalysis of sediment samples used in the experimental trials are shown in Table 1. For any given sediment location, there was no significant difference in the particle size distribution (p value > 0.9; all cases) or %TOC (p value > 0.5; all cases). Sterilization was determined successful as shown by statistical difference in RNA concentration (p value < 0.05 for each treatment type).
Previous studies have used γ-irradiation as this sterilization process is effective at removing microbial activity, while being minimally disruptive to the physical and chemical properties of sediments (Lotrario et al. 1995; McNamara et al. 2003). In the present study, neither sediment grain size distribution or %TOC were altered by irradiation. Further, the analysis of the sterilization showed a statistical difference in concentration between the biotic and the sterilized sediments. It is, therefore, appropriate to compare the sterilized and biotic sediments of the same site to explore microbial community effect on EPC0 values. The following will be an assessment of the trends in the calculated EPC0 values.
Three sample sets generated non-significant (p values > 0.05) linear regression (Belle River biotic incubated at 15°C set B, Belle River sterilized incubated at 25°C set A, and Nissouri Creek sterilized incubated at 15°C set C) between Psorbed and SRPf and were removed from data analysis. From the appropriately fitted isotherms, EPC0 values were calculated using the standardized method, as per Supplementary Information (SI Table 1). In addition to Eq. 2, Freundlich isotherms were explored but Freundlich equations yielded either Freundlich exponents with values of 1 or exponents that were well outside of theoretical ranges and were considered less parsimonious than linear regression fits.
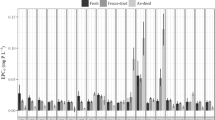
Figure 1 provides a general summary of the distribution of EPC0 values across the three sediment types after combining measurements from the different temperature treatments. Nissouri Creek had the largest overall magnitude of EPC0 relative to the other sediments that was not related to %TOC or grain size. Between sterilized and biotic treatments of a given sediment type significant differences (p value < 0.008) were observed for Belle River and Nissouri Creek but not for Big Creek (p value > 0.05). However, it was also noted that the direction of EPC0 shift varied for Belle River relative to Nissouri Creek, with the latter showing lower EPC0 values for sterilized sediments and the former elevated EPC0 values in the sterile samples. Sharpley et al. (2007) found that irradiation increased the EPC0 of agricultural ditch sediments by 40% which is consistent with the observation found for Belle River but not with Nissouri Creek which showed a similar 40% difference but in the opposite direction.
Figure 2 summarizes temperature effects on EPC0 for each sediment type and treatment separately. In order to generate a more comprehensive picture of the changes by site, treatment, and temperature, a GLM: LogEPC0 = Constant + Site + Treatment + Temperature + Site × Temperature +Treatment × Temperature + Site × Treatment was tested. However, the GLM yielded significant interactions (p value < 0.001) between treatment and site necessitating statistical testing for variable effects be performed for each sediment location separately. For Belle River, the Treatment × Temperature interaction was non-significant (p value = 0.085) and, therefore, was removed from the GLM. In the reduced model, both temperature (negative slope and p value < 0.05) and treatment (p value < 0.001) were significant. In the cases of Big Creek and Nissouri Creek, the Treatment × Temperature interaction were both significant (p value ≤ 0.001; each test) necessitating that each variable (temperature and treatment) be explored independently. To analyze the treatment effects on EPC0, ANOVAs were completed at each temperature separately for Big Creek and Nissouri Creek. For Big Creek a treatment effect only occurred at the 35°C (p value < 0.001) temperature, whereas for Nissouri Creek, treatment was significant across each temperature tested (p value ≤ 0.001; all tests).
Apart from the significant negative effects of temperature on EPC0 observed for both sterilized and biotic treatments in Belle River noted previously, temperature effects on EPC0 for Big Creek and Nissouri Creek were analyzed separately for each treatment. For Big Creek there was a significant negative temperature effect (p value < 0.01) for the biotic samples but non-significant (p value > 0.05) temperature effect for the sterilized samples. Nissouri Creek showed a significant negative effect (p value = 0.001) with temperature in the biotic samples and a significant positive effect (p value = 0.021) in the sterilized system. Despite these apparent system specific differences, it is worth noting that the overall temperature pattern of EPC0 remained broadly consistent for the sterilized treatments across sample sites. For Big Creek and Nissouri Creek, a minimum EPC0 occurred at 15°C, and in Belle River at 25°C. However, the biotic samples reacted very differently with temperature, dependent on the site. Belle River biotic EPC0 values tended to show a pattern similar to the temperature effect of sterilized samples, although with lower EPC0 values, whereas Big Creek and Nissouri Creek demonstrated a decreasing effect of temperature on biotic EPC0 values. These differences highlight the complexity in microbial community (biotic) responses across sites and temperatures. In Haggard et al. (1999) it was suggested that the P buffering capacity could vary temporally depending on seasonal biotic processes in each ecosystem. The temperature patterns observed in the present study support this.
Overall, the present study provides evidence to support the hypothesis that microbial communities alter EPC0 of sediments. However, the influence of microbial processes varied in unpredictable ways dependent on location of sediment collection and temperature. Although the exact mechanism by which microbial communities alter EPC0 are not fully known, we hypothesize a number of direct and indirect mechanisms might explain the observations. Direct mechanisms for change in EPC0 could involve direct scavenging of SRP by bacteria that would effectively decrease EPC0 (Boström et al. 1988) or through alteration of the rate of labile carbon metabolism that changes the sequestration capacity of this sediment compartment (Nottingham et al. 2015). Additional mechanisms may include biotic-mediated changes to mineral composition and/or availability of such minerals either through biotic uptake/sequestration of minerals or indirectly by metabolism induced changes in redox status (Boström et al. 1988; Huang et al. 2008). Huang et al. (2008) concluded that microorganisms play important roles in P release from sediment, including facilitating the use of Fe3+ as an electron acceptor to induce phosphate release, acting as a catalyst in the exchange of OH− and PO43−, and the overall mineralizing efficiency of moderately labile orthophosphate. The above mechanism requires a gradient in redox conditions and microbial consortia commonly found in undisturbed aquatic sediments. However, batch studies as employed by the present study are likely to disrupt fine scale redox gradients due to the constant shaking of samples during batch incubations. Thus, use of minimally disruptive methods including fluvarium and chamber methods as employed by Huang et al. (2008) and Sharpley et al. (2007) may be necessary to further uncover redox and microbial community interaction effects on EPC0. For two sites, EPC0 was depressed at one or more temperatures in biotic samples compared to sterilized samples implicating a greater propensity of live sediments to sorb P similar to what has been reported elsewhere (Sharpley et al. 2007). However, for Nissouri Creek, EPC0 values were elevated in biotic samples suggesting a greater propensity for sediments to release P. These differences were not related to %TOC or grain size characteristics. Nissouri Creek was notable for having a predominantly manure based fertilizer applications in its watershed which may influence the biotic community structure, however, further testing and validation of actual differences in microbial community composition coupled with fertilizer type amendment studies would be necessary to establish if fertilizer type was indeed responsible for the distinct EPC0 patterns observed in the present study.
References
Boström B, Andersen JM, Fleischer S, Jansson M (1988) Exchange of phosphorus across the sediment-water interface. Hydrobiologia 170:229–244. https://doi.org/10.1007/BF00024907
Chen M, Walshe G, Chi Fru E et al (2013) Microcosm assessment of the biogeochemical development of sulfur and oxygen in oil sands fluid fine tailings. Appl Geochem 37:1–11. https://doi.org/10.1016/j.apgeochem.2013.06.007
Cooper RJ, Rawlins BG, Krueger T et al (2015) Contrasting controls on the phosphorus concentration of suspended particulate matter under baseflow and storm event conditions in agricultural headwater streams. Sci Total Environ 533:49–59. https://doi.org/10.1016/j.scitotenv.2015.06.113
Drouillard K, Tomczak M, Reitsma S, Haffner GD (2006) A river-wide survey of polychlorinated biphenyls (PCBs), polycylic aromatic hydrocarbons (PAHs), and selected organochlorine pesticide residues in sediments of the detroit river – 1999. J Great Lakes Res 32:209–226. https://doi.org/10.3394/0380-1330(2006)32
Emelko MB, Stone M, Silins U et al (2016) Sediment-phosphorus dynamics can shift aquatic ecology and cause downstream legacy effects after wildfire in large river systems. Glob Chang Biol 22:1168–1184. https://doi.org/10.1111/gcb.13073
Gibbons SM, Jones E, Bearquiver A et al (2014) Human and environmental impacts on river sediment microbial communities. PLoS ONE 9:1–9. https://doi.org/10.1371/journal.pone.0097435
Haggard BE, Stanley EH, Hyler R (1999) Sediment-phosphorus relationships in three northcentral Oklahoma streams. Trans Am Soc Agric Eng 42:1709–1714. https://doi.org/10.13031/2013.13333
Huang TL, Ma XC, Cong H, Bing H, Chai BB (2008) Microbial effects on phosphorus release in aquatic sediments. Water Sci Technol 58:1285–1289. https://doi.org/10.2166/wst.2008.459
Lotrario JB, Stuart BJ, Lam T et al (1995) Effects of sterilization methods on the physical characteristics of soil: implications for sorption isotherm analyses. Bull Environ Contam Toxicol 54:668–675. https://doi.org/10.1007/BF00206097
Lucci GM, McDowell RW, Condron LM (2010) Evaluation of base solutions to determine equilibrium phosphorus concentrations (EPC0) in stream sediments. Int Agrophys 24:157–163
Machesky ML, Holm TR, Slowikowski JA (2010) Phosphorus speciation in stream bed sediments from an agricultural watershed: solid-phase associations and sorption behavior. Aquat Geochem 16:639–662. https://doi.org/10.1007/s10498-010-9103-2
McNamara NP, Black HIJ, Beresford NA, Parekh NR (2003) Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Appl Soil Ecol 24:117–132. https://doi.org/10.1016/S0929-1393(03)00073-8
Nottingham AT, Turner BL, Stott AW, Tanner EVJ (2015) Nitrogen and phosphorus constrain labile and stable carbon turnover in lowland tropical forest soils. Soil Biol Biochem 80:26–33. https://doi.org/10.1016/j.soilbio.2014.09.012
Novak JM, Stone KC, Szogi AA et al (2004) Dissolved phosphorus retention and release from a coastal plain in-stream wetland. J Environ Qual 33:394. https://doi.org/10.2134/jeq2004.3940
Pant HK, Reddy KR (2001) Phosphorus sorption characteristics of estuarine sediments under different redox conditions. J Environ Qual 30:1474. https://doi.org/10.2134/jeq2001.3041474x
Sharpley AN, Krogstad T, Kleinman PJ et al (2007) Managing natural processes in drainage ditches for nonpoint source phosphorus control. J Soil Water Conserv 62:197–206
Smith DR, Warnemuende EA, Haggard BE, Huang C (2006) Changes in sediment–water column phosphorus interactions following sediment disturbance. Ecol Eng 27:71–78. https://doi.org/10.1016/j.ecoleng.2005.10.013
Szalinska E, Drouillard K, Fryer B, Haffner GD (2006) Distribution of heavy metals in sediments of the Detroit River. J Great Lakes Res 32:442–454. https://doi.org/10.3394/0380-1330(2006)32
Weigelhofer G, Ramião JP, Pitzl B et al (2018) Decoupled water-sediment interactions restrict the phosphorus buffer mechanism in agricultural streams. Sci Total Environ 628–629:44–52. https://doi.org/10.1016/j.scitotenv.2018.02.030
Acknowledgements
This research was supported by the NSERC SPG Grant (401255464). The \( \gamma \)-irradiation was performed at the McMaster University Nuclear Reactor. The authors would like to thank the Environmental Genomics Facility (EGF) and the Organic Analytical and Nutrient Laboratory (OANL) at the Great Lakes Institute for Environmental Research (GLIER), University of Windsor. Final acknowledgments to the Weisener Lab and Drouillard Lab groups for their combined support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
DiCarlo, A.M., Weisener, C.G. & Drouillard, K.G. Evidence for Microbial Community Effect on Sediment Equilibrium Phosphorus Concentration (EPC0). Bull Environ Contam Toxicol 105, 736–741 (2020). https://doi.org/10.1007/s00128-020-03019-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-03019-0