Abstract
An automated on-line solid-phase extraction (SPE) combined with LC–MS/MS method was developed for determination of deoxynivalenol (DON), 3-acetyl-DON and 15-acetyl-DON in corn flour and wheat flour samples. The extraction solvent of the samples was injected into the automated on-line SPE system to remove matrix interference. After washing step, the targets were eluted from the SPE cartridge into liquid chromatography (LC) column. Several SPE parameters including injection volume, elution volume and eluting flow rate were assessed and optimized. Method validation was evaluated and good linearity was obtained (R2 > 99%) with the limit of detection of 0.1–0.2 μg/kg. Recoveries were evaluated in spiked corn flour and wheat flour samples at three concentrations and the values ranged from 86.5% to 99.7%. The benefit of the present method with automated on-line SPE system is the ability to inject directly pure extracts into LC–MS/MS, offering faster analyses and improving analysis efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mycotoxins are the secondary fungal metabolites, which are omnipresent in nature and contaminate cereal crops, posing potential health hazards to humans and animals (Mishra et al. 2020). It is reported that 25–50% of globally harvested crops have been contaminated with mycotoxins each year, and about 500 million people are exposed to natural toxins by consuming their staple diet (Ran et al. 2013; Mishra et al. 2020). Deoxynivalenol (DON), also known as vomitoxin, is a common mycotoxin produced as a secondary metabolite mainly by Fuasriumgraminearum and Fuasriumculmorum, mainly occurring in cereal grains such as wheat and maize (Rahmani et al. 2017; Tralamazza et al. 2016; Alizadeh et al. 2016). Its acetylated derivatives, 3-acetyl-deoxynivalenol (3-ADON) and 15-acetyl- deoxynivalenol (15-ADON), are intermediate fungal metabolites in the biosynthesis of DON and can occur together with DON in cereal staples, but at much lower levels (Wu et al. 2017; Gonçalves and Stroka 2016). These mycotoxins are stable during food processing, and have adverse effects including anorexia, immune-suppression, circulatory shock, and diarrhea on human and animal consumers (Liu et al. 2016; Wu and Zhang 2014; Gerez et al. 2015). To ensure consumers health, the European Union’s Commission stipulated maximum levels for DON (1250 μg/kg in cereals; 200 μg/kg in processed cereal-based foods and baby foods) (European Commission 2006; Olcer et al. 2014). The United States and China also set the maximum limits for DON in cereal products at 1000 μg/kg (Yuan et al. 2017).
DON and its derivatives have been commonly analyzed by high-performance liquid chromatography (HPLC), gas chromatography-mass spectrometry (GC–MS), liquid chromatography-mass spectrometry (LC–MS), LC–MS/MS, immunoassay, and microfluidics based on electrochemical profiling (Ran et al. 2013; Olcer et al. 2014; Rahmani et al. 2017; Broekaert et al. 2014). GC–MS and LC–MS are attractive instrumental analytical methods due to their fast speed, excellent sensitivity, and capacity of simultaneously determining multi-targets in a single run. However, analysis of DON and its derivatives using GC–MS needs clean-up and derivation steps, making this technique complicated and time consuming (Tang et al. 2018; Rodríguez-Carrasco et al. 2017; Ran et al. 2013). Recently, LC–MS and LC–MS/MS combined with off-line solid phase extraction (SPE) are widely used to determine DON and its derivatives without derivatization step (Zhang et al. 2016; Broekaert et al. 2014). The major drawbacks of off-line SPE are sample loss and contamination, time consuming, and high sample volume (Ferrer-Aguirre et al. 2016; Salazar-Beltrán et al. 2017). These problems can be well solved by on-line SPE method, which owns the advantages including high efficiency, time saving, low or no contamination, and minimal sample volume (Bourgogne et al. 2015; Rossi et al. 2017).
On-line SPE combined with LC–MS methods for measuring environmental phenols and parabens in serum (Ye et al. 2008), sulphonamides in eggs and pork (Fang et al. 2006), glyphosate in groundwater (Sanchís et al. 2012), phenols in urine (Zhou et al. 2014), and ochratoxin A in wine (Campone et al. 2018) have been developed. To our knowledge, there are few reports on the determination of DON and its derivatives, 3-acetyl-DON and 15-acetyl-DON, using on-line SPE combined with LC–MS method. In this study, we used a full-automated on-line SPE sample processor for preconcentration and purification of targets, which was integrated with LC–MS/MS, allowing simultaneous extraction and analysis of samples with increased efficiency, sensitivity and convenience. Parameters of the automated on-line SPE-LC–MS/MS affecting the extraction efficiency, accuracy and sensitivity were optimized. Under the optimal conditions, the developed method was validated according to ESP (USP 29/NF 24, 2006) and ICH (ICH Q2, 2005), and then applied at corn flour and wheat flour.
Materials and methods
DON (200 μg/mL), 3-ADON (50 μg/mL) and 15-ADON (50 μg/mL) in methanol were purchased from Sigma-Aldrich (St. Louis, MO, USA). 13C15-DON (stable isotope labelled internal standard, 25.2 μg/mL) and 13C17-3-ADON (stable isotope labelled internal standard, 25.3 μg/mL) in acetonitrile were purchased from Cerilliant (LGC Standards, Boras, Sweden). Methanol (LC–MS grade) and acetonitrile (LC–MS grade) were provided by Merck (Darmstadt, Germany). Ultrapure water (resistance of 18 MΩ × cm at 25 °C) was produced by a Milipore water purification system (Billerica, MA, USA). Other reagents were purchased from Tianjin Chemical Reagent Factory (Tianjin, China). All reagents were of the highest available purity and at least of analytical reagent grade.
A Symbiosis ™ Pico system (Spark Holland, Emmen, Netherlands) was the automated SPE device consisting of an Alias™ antosampler, a binary high-pressure gradient pump with the solvent selector, the column selector, one automated cartridge exchange unit, and a column oven. The control software was Spark Link workstation.
The automated SPE unit was coupled to an HPLC separation module using a reversed-phase C18 analytical column (4.6 mm × 250 mm, 5 μm, Agilent, USA) that was connected to a Triple Quad™ 6500 mass spectrometer equipped with an electrospray ionization (ESI) source (AB SCIEX, Foster City, CA, USA). An IKA MTS 2/4 digital microtiter shaker (IKA Werke, Staufen, Germany) and an ultrasonic cleaner (Elma Transonic T310, Singen, Germany) were also used.
The HLB SPE cartridge (10 mm × 1 mm, Waters, USA) was automatically picked from a 96 well cartridge plate for sample extraction procedure. After the SPE cartridge was conditioned and equilibration, 50 μL of sample extracts containing internal standard 13C15-DON and 13C17-3-ADON were loaded onto the SPE cartridges. Then the targets were eluted from the cartridge directly to the analytical column. Detailed on-line SPE conditions were shown in Table S1 (Supplementary Material). For LC separation, the column temperature was set at 35 °C, and the mobile phase comprised solvent A (0.1% ammonia solution, v/v) and solvent B (acetonitrile). The solvent gradient setting is summarised in Table S2 (Supplementary Material).
The mass spectrometry was operated in the multiple reaction monitoring (MRM) mode, and MS data were obtained using the negative ESI mode for all analytes. The optimized MS parameters were as follows: ion spray voltage, − 4500 V; curtain gas pressure, 30 psi; nebulizer gas pressure, 50 psi; auxiliary gas pressure, 55 psi; ion source temperature, 500 °C. The optimized MS parameters for DON, 3-ADON, 15-ADON and internal standard were given in Table 1. The results were analyzed using MultiQuant 3.0.1 software (AB SCIEX, Foster City, CA, USA).
Corn flour and wheat flour were purchased from local supermarkets (Hebei province, China) and the test sample was verified free of DON, 3-ADON and 15-ADON by HPLC–MS/MS. The sample preparation was carried out as described (Mateo et al. 2001) with some minor modifications.
To 2.0 g of corn flour or wheat flour, 13C15-DON (internal standard solution, 400 ng), 13C17-3-ADON (internal standard solution, 400 ng) DON, 3-ADON and 15-ADON were added. Then the samples were homogenized by vortex mixing for 1 min. After standing for 1 h at 4 °C, 20 mL of a mixture of acetonitrile–water (84:16, v/v) was added into the samples to extract the targets with the aid of ultrasonic vibration for 10 min. After centrifugation (4500 rpm for 10 min at 4 °C), 5 mL of the supernatant were selected to a new tube, and then dried under nitrogen flow at 45 °C. The resulting residues were dissolved in 5 mL of deionized water. Subsequently, the mixture was filtered using a membrane filter (pore size, 0.22 μm) and the filtrate was subjected to the on-line SPE procedure.
Results and Discussion
The automated on-line SPE combined with LC–MS/MS consists of a high-pressure dispenser module for solvent and samples, an automated cartridge exchange module, LC module for separation, and a tandem mass spectrometer (Fig. 1). After the washing step, the SPE cartridge was automatically moved onto the right clamp from the left clamp, where the target analytes were eluted into the LC column. In the meantime, a new SPE cartridge was placed onto the left side clamp, being ready for the next preconcentration and purification sequence.
MS/MS conditions were evaluated in this study by infusion of a standard solution of 500 ng/mL of each compound. Electrospray ionization in negative and positive mode was tested, indicating that the more abundant precursor ions for DON, 3-ADON, 15-ADON, 13C15-DON, and 13C17-3-ADON were obtained in negative mode. So the precursor ions, [M–H]−, were selected for MRM analysis. Other parameters such as declustering potential and collision energy were also studied, selecting the optimum conditions listed in Sect. 2.4.
The mobile phase system for chromatographic separation of the target compounds was optimized by direct injection method with the help of the high-pressure dispenser module. Methanol–water, acetonitrile–water, and acetonitrile-ammonia solution (0.1%, v/v) as mobile phase were test under gradient elution conditions. The last solvent gave more satisfactory results regarding peak shapes. Moreover, the addition of ammonia to the mobile phase obtained better ionization efficiency than water. Therefore, acetonitrile-ammonia solution (0.1%, v/v) was selected as mobile phase.
Critical factors affecting the efficiency of the on-line SPE including the injection volume, elution volume and eluting flow rate were assessed by evaluating the performance of the on-line SPE procedure.
Different the injection volume was carried out to investigated whether the sensitivity of this method, could be improved by increasing the sample injection volume using spiked blank matrix at 50 ng/mL. The results (Fig. S1, in Supplementary Material) shown that each curve were linear with a good correlation coefficient (R2 > 0.995) in the evacuated injection volume range (10–100 μL). The signal responses of DON, 3-ADON and 15-ADON increased proportionally with the injection volume and no matrix effects were obtained. In order to obtain good sensitivity and less matrix interference, the injected sample volume was set at 50 μL.
The effect of eluting flow rates (50 μL/min, 100 μL/min, 150 μL/min and 200 μL/min) was assessed by loading 50 μL of spiked blank matrix (50 ng/mL). The results showed that the chromatographic elution peaks and recoveries of the targets were good as the flow rate was 100 μL/min, avoiding the tailed phenomenon of chromatographic peak at low flow rate (50 μL/min). However, the recoveries were decreased when the eluting flow rate was more than 100 μL/min. To ensure the sensitivity and recovery of the method, the eluting flow rate of 100 μL/min was chosen during the on-line SPE procedure.
Elution volume was optimized by employing methanol as eluent. The results (Fig. 2) indicated that the targets response were not increased any more as more than 250 μL of eluent were applied. Therefore, 250 μL of methanol was adopted in this study.
Matrix effect (ME) is the interfering sample components during ionization, which can affect the sensitivity and accuracy of the LC–MS/MS analysis. To assess the degree of ME (enhancement or suppression of the analytical signals), DON and its derivative were spiked in the blank corn flour and wheat flour sample extracts. The ME (%) was defined as A (peak area of the spiked sample extracts)/B (peak area of standard solution) (Mi et al. 2014). The results (Table S3, in Supplementary Material) showed that the ME values of the present work were 95.89–100.03% and the ranges of relative standard deviations (RSDs) were 3.2–5.5%, indicating that no adversely affected by ME with the method was found. Therefore, the on-line SPE used here can remove co-extraction compounds during the washing step, ensuring the detection sensitivity and quantification accuracy of the target analyte.
The proposed method validation was carried out under USP (USP 29/NF 24, 2006) and ICH (ICH Q2, 2005) guideline, including linearity, selectivity, accuracy, detection limit and quantitation limit.
Working calibration solutions (0.1–5000 μg/kg) were prepared by adding DON, 3-ADON and 15-ADON into the black sample extract solutions, which contained internal standards 13C15-DON and 13C17-3-ADON with fixed concentration (200 μg/kg). Calibration curve of DON and its derivative were obtained by plotting peak area ratio of the analytes and internal standard (y) against the analytes concentrations (x). The regression equation and linear range were listed in Table 2, showing good linear correlation (R2 > 99%) between the target concentrations and the signals and wide linear range.
To estimate the sensitivity of the developed method, the limit of detection (LOD) and the limit of quantitation (LOQ) were calculated on the basis of a signal-to-noise ratio (S/N) of 3 and 10, respectively. The results of LOD and LOQ were reported in Table 2, indicating high sensitivity with LOQ in the range of 0.3–0.6 μg/kg for corn flour and wheat flour matrixes.
Wheat and corn are consumed as a staple food across the globe. The occurrence of DON and its derivative in grain production have been reported globally, and the mean contamination level in China were around 78.9 μg/kg and 240 μg/kg for corn and wheat, respectively (Mishra et al. 2020). Therefore, the recovery studies were carried out to assess the accuracy of the proposed method by processing corn flour and wheat samples spiked with DON, 3-ADON and 15-ADON at three different concentration levels (40 μg/kg, 100 μg/kg and 400 μg/kg). Each spiked sample was analyzed for six repetitions. As shown in Table 3, the recoveries of DON and its derivative from the spiked samples were 86.5–99.7% with RSDs ranging from 2.5 to 8.3%.
Precision of the method was also assessed by measuring RSDs of intra- and inter-day tests. Intra-day assay was carried out by analyzing three aliquots of spiked samples in one day. Inter-day assay was performed on five consecutive days with spiked samples. As shown in Table S4 (Supplementary Material), the RSDs values of intra-day and inter-day ranged from 2.9% to 8.4%, suggesting good stability and reproducibility of the on-line SPE-LC–MS/MS method.
Compared with the reported methods for analysis of DON, 3-ADON and (or) 15-ADON based on HPLC with air assisted-dispersive liquid–liquid microextraction (Rahmani et al. 2017), on-line SPE LC–MS/MS (Broekaert et al. 2014), off-line multifunctional column LC–MS/MS (Yoshinari et al. 2012), our developed method has low LOD value, wide linear range, and high recovery.
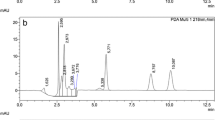
The specificity was defined as non-interference with the endogenous substances in the regions of target (Li et al. 2004), which was evaluated by the selective reaction monitoring (SRM) of DON, 3-ADON and 15-ADON, in blank samples and blank samples after spiking with the targets. The results were presented in Fig. 3, showing no interfering peaks in the chromatographic elution zone.
The method was applied to analyze 20 commercial grain flour samples (10 corn flour and 10 wheat flour) obtaining from local markets in Hebei province, China. The commercial wheat flour without the bran and the corn flour with the bran were produced using a mill. Analysis results were listed in Table S5 (Supplementary Material), indicating that 80%, 20% and 20% corn flour samples were contaminated by DON, 3-ADON, and 15-ADON, respectively, with the levels ranging from 1.4 to 3198.9 μg/kg. 60%, 0% and 0% wheat flour samples were contaminated by DON, 3-ADON and 15-ADON, respectively, with the levels ranging from 0.8 to 3245.1 μg/kg. These results showed sensing of DON and its derivative in grain flour were very important for ensuring the food safety. Moreover, these samples were also analyzed by GC–MS according to the paper [Ibáñez-Vea et al. 2011]. The analytical results were shown in Figs. S2 and S3 (Supplementary Material), indicating linear regression relationships of the on-line SPE-LC/MS and GC–MS data with good correlations. Therefore, this on-line SPE-LC/MS is suitable for detection of DON and its derivates in commercial grain flour samples.
In this paper, an automated on-line solid-phase extraction combined with LC–MS/MS method was developed to determine DON, 3-ADON and 15-ADON in corn flour and wheat flour samples. This method presents wide linearity range, good precision, low LODs (0.1–0.2 μg/kg) and high recoveries for the three analytes (86.5–99.7%). Importantly, the automated on-line solid-phase extraction and analytical procedure can reduce manual procedures, improving analysis efficiency. Therefore, this method could be used in routine supervision and analysis of vomitoxin in cereal foods.
References
Alizadeh A, Braber S, Akbari P, Kraneveld A, Garssen J, Fink-Gremmels J (2016) Deoxynivalenol and its modified forms: are there major differences? Toxins 8(11):334
Bourgogne E, Grivet C, Varesio E, Hopfgartner G (2015) Generic on-line solid phaseextraction sample preparation strategies for the analysis of drugs in biological matrices by LC-MS/MS. J Pharmaceut Biomed 102:290–298
Broekaert N, Devreese M, De Mil T, Fraeyman S, De Baere S, De Saeger S, De Backer P, Croubels S (2014) Development and validation of an LC-MS/MS method for the toxicokinetic study of deoxynivalenol and its acetylated derivatives in chicken and pig plasma. J Chromatogr B 971:43–51
Campone L, Piccinelli AL, Celano R, Pagano I, Russo M, Rastrelli L (2018) Rapid and automated on-line solid phase extraction HPLC-MS/MS with peak focusing for the determination of ochratoxin A in wine samples. Food Chem 244:128–135
European Commission (2006) Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. In: Commission, E. (ed.) pp. 2–8. Official Journal of the European Union L.
Fang GZ, He JX, Wang S (2006) Multiwalled carbon nanotubes as sorbent for on-line coupling of solid-phase extraction to high-performance liquid chromatography for simultaneous determination of 10 sulfonamides in eggs and pork. J Chromatogr A 1127(1–2):12–17
Ferrer-Aguirre A, Romero-González R, Vidal JM, Frenich AG (2016) Simple and quick determination of analgesics and other contaminants of emerging concern in environmental waters by on-line solid phase extraction coupled to liquid chromatography-tandem mass spectrometry. J Chromatogr A 1446:27–33
Gerez JR, Pinton P, Callu P, Grosjean F, Oswald IP, Bracarense APF (2015) Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp Toxicol Pathol 67(2):89–98
Gonçalves C, Stroka J (2016) Cross-reactivity features of deoxynivalenol (DON)-targeted immunoaffinity columns aiming to achieve simultaneous analysis of DON and major conjugates in cereal samples. Food Addit Contam A 33(6):1053–1062
Ibáñez-Vea M, Lizarraga E, González-Peñas E (2011) Simultaneous determination of type-A and type-B trichothecenes in barley samples by GC-MS. Food Control 22(8):1428–1434
ICH, Q2 (R1) Harmonised Tripartite Guideline (2005). Validation of analytical procedure: text and methodology. In: International Conference on Harmonization, Geneva.
Liu Y, Lu Y, Wang L, Chang F, Yang L (2016) Occurrence of deoxynivalenol in wheat, Hebei Province, China. Food Chem 197:1271–1274
Li X, Xiao H, Liang X, Shi D, Liu J (2004) LC-MS/MS determination of naringin, hesperidin and neohesperidin in rat serum after orally administrating the decoction of Bulpleurum falcatum L. and Fractus aurantii. J Pharmaceut Biomed 34(1):159–166
Mateo JJ, Llorens A, Mateo R, Jiménez M (2001) Critical study of and improvements in chromatographic methods for the analysis of type b trichothecenes. J Chromatogr A 918(1):99–112
Mi J, Li S, Xu H, Liang W, Sun T (2014) Rapid analysis of three β-agonist residues in food of animal origin by automated on-line solid-phase extraction coupled to liquid chromatography and tandem mass spectrometry. J Sep Sci 37(17):2431–2438
Mishra S, Srivastava S, Dewangan J, Divakar A, Rath SK (2020) Global occurrence of deoxynivalenol in food commodities and exposure risk assessment in humans in the last decade: a survey. Crit Rev Food Sci 8:1346–1374
Olcer Z, Esen E, Muhammad T, Ersoy A, Budak S, Uludag Y (2014) Fast and sensitive detection of mycotoxins in wheat using microfluidics based real-time electrochemical profiling. Biosens Bioelectron 62:163–169
Rahmani M, Ghasemi E, Sasani M (2017) Application of response surface methodology for air assisted-dispersive liquid-liquid microextraction of deoxynivalenol in rice samples prior to HPLC-DAD analysis and comparison with solid phase extraction cleanup. Talanta 165:27–32
Ran R, Wang C, Han Z, Wu A, Zhang D, Shi J (2013) Determination of deoxynivalenol (DON) and its derivatives: current status of analytical methods. Food Control 34(1):138–148
Rodríguez-Carrasco Y, Moltó JC, Mañes J, Berrada H (2017) Development of microextraction techniques in combination with GC-MS/MS for the determination of mycotoxins and metabolites in human urine. J Sep Sci 40(7):1572–1582
Rossi E, Errea MI, de Cortalezzi MMF, Stripeikis J (2017) Selective determination of Cr (VI) by on-line solid phase extraction FI-SPE-FAAS using an ion exchanger resin as sorbent: an improvement treatment of the analytical signal. Microchem J 130:88–92
Salazar-Beltrán D, Hinojosa-Reyes L, Ruiz-Ruiz E, Hernández-Ramírez A, Guzmán-Mar JL (2017) Determination of phthalates in bottled water by automated on-line solid phase extraction coupled to liquid chromatography with uv detection. Talanta 168:291–297
Sanchís J, Kantiani L, Llorca M, Rubio F, Ginebreda A, Fraile J, Garrido T, Farré M (2012) Determination of glyphosate in groundwater samples using an ultrasensitive immunoassay and confirmation by on-line solid-phase extraction followed by liquid chromatography coupled to tandem mass spectrometry. Anal Bioanal Chem 402(7):2335–2345
Tang K, Liu H, Li XZ, Hassan YI, Shao S, Zhou T (2018) An efficient gas chromatography-mass spectrometry approach for the simultaneous analysis of deoxynivalenol and its bacterial metabolites 3-keto-DON and 3-epi-DON. J Food Protect 81(2):233–239
Tralamazza SM, Bemvenuti RH, Zorzete P, de Souza GF, Corrêa B (2016) Fungal diversity and natural occurrence of deoxynivalenol and zearalenone in freshly harvested wheat grains from Brazil. Food Chem 196:445–450
USP 29/NF 24 the United States Pharmacopoeia (2006) 29 the Rev. and the National Formulary (pp. 1964–1966). United States Pharmacopoeial Convention Inc., Rockville
Wu Q, Kuča K, Humpf HU, Klímová B, Cramer B (2017) Fate of deoxynivalenol and deoxynivalenol-3-glucoside during cereal-based thermal food processing: a review study. Mycotoxin Res 33(1):79–91
Wu W, Zhang H (2014) Role of tumor necrosis factor-α and interleukin-1β in anorexia induction following oral exposure to the trichothecene deoxynivalenol (vomitoxin) in the mouse. J Toxicol Sci 39(6):875–886
Ye X, Tao LJ, Needham LL, Calafat AM (2008) Automated on-line column-switching HPLC-MS/MS method for measuring environmental phenols and parabens in serum. Talanta 76(4):865–871
Yoshinari T, Ohnishi T, Kadota T, Sugita-Konishi Y (2012) Development of a purification method for simultaneous determination of deoxynivalenol and its acetylated and glycosylated derivatives in corn grits and corn flour by liquid chromatography-tandem mass spectrometry. J Food Protect 75(7):1355–1358
Yuan J, Sun C, Guo X, Yang T, Wang H, Fu S, Li C, Yang H (2017) A rapid Raman detection of deoxynivalenol in agricultural products. Food Chem 221:797–802
Zhang H, Sun J, Zhang Y, Lu M, Sun L, Li W, Hu X, Wang B (2016) Retention of deoxynivalenol and its derivatives during storage of wheat grain and flour. Food Control 65:177–181
Zhou X, Kramer JP, Calafat AM, Ye X (2014) Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J Chromatogr B 944:152–156
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2017YFF0211300) and the Scientific Research Foundation for the Introduced Talent of Hebei Agricultural University (No. YJ201911).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, M., Li, Q., Zhang, Y. et al. Determination of Deoxynivalenol and Its Derivative in Corn Flour and Wheat Flour Using Automated On-line Solid-Phase Extraction Combined with LC–MS/MS. Bull Environ Contam Toxicol 107, 248–254 (2021). https://doi.org/10.1007/s00128-020-02920-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-02920-y