Abstract
Antibiotics in manure, soil, wastewater, and groundwater samples from the livestock and poultry farms in Xuzhou City were investigated in the present study. The concentrations of antibiotics in all matrices varied greatly among farms. Total concentrations of fluoroquinolones and macrolides were much higher than those of sulfonamides in manures and soil samples. Total concentrations of antibiotics in wastewater of livestock farms were higher than those of poultry farm. Josamycin (JM) and tilmicosin (TIL) accounted for more than 74% of the nine macrolides in all groundwater samples. Sulfamethizole (SMT), fleroxacin (FLE), cinoxacin (CIN) and JM were the main antibiotics detected in manure and soil samples, while sulfamethoxypyridazine (SMP), sulfameter (SME), SMT, FLE, JM and TIL accounted for a large proportion of antibiotics in surface and groundwater. The risk assessment of target antibiotics revealed that JM in wastewater showed relatively high RQs for aquatic organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Antibiotics are physiologically highly active substances and extensively used to treat bacteria-borne diseases and promote growth of animals (Wei et al. 2011). An estimated 210,000 tons of antibiotics are produced every year in China, of which 48% are used in the agricultural and livestock industries (Li et al. 2015). Previous studies showed that antibiotic concentrations in feces from various animals varied from the low mg/kg up to g/kg due to vastly different antibiotics use patterns (Kim et al. 2012; Berendsen et al. 2015; Chen et al. 2012).
Approximately 30%–90% of the parent compounds and their metabolites are excreted into the environment via animal wastes (Bound and Voulvoulis 2004). Active ingredients of antibiotics can find their way into the upper soil layer through animal manure fertilizers, transport to surface water via runoff and to groundwater via leaching, and finally enter into the food chain (Zhang et al. 2016). The fate of antibiotics in soils is greatly affected by their physicochemical properties. Due to the weak sorption to soil, sulfonamide antibiotics have been frequently detected in surface water, groundwater and even soil pore water (Wegst-Uhrich et al. 2014), while tetracyclines or fluoroquinolones may persist for several months to years in soil (Jechalke et al. 2014). A study reported that sulfamethoxazole and ciprofloxacin could accumulate in the Mexican soils in the sequestered form for 20 years during long-term irrigation with untreated wastewater (Philipp et al. 2012). Pan et al. (2011) examined the residual veterinary antibiotics in 126 swine manure samples with detection frequencies of 84.9%–96.8% for tetracyclines, 0.8%–51.6% for sulfonamides and 4.8% for macrolides. Seventeen veterinary antibiotics, including sulfonamides, quinolones, tetracyclines, macrolides and amphenicols, in 53 soil samples collected from four provinces of China were detected with the concentrations ranging from below detection limit to 415.00 mg/kg dry weight (Wei et al. 2019). Low concentrations of sulfonamide antibiotics could lead to developmental abnormalities in fish (Lin et al. 2014a), including decreased locomotion ability, disordered organ function and oxidative stress (Lin et al. 2014b). The activity of acetylcholine-esterase in muscles of Tra catfish (Pangasianodon hypophthalmus) was lower than that of control group when exposed to enrofloxacin (ENR) (Wang et al. 2009).
In these studies, antibiotics were separately reported from manure, soil, wastewater or groundwater, and few studies were conducted to simultaneously determine these compounds in different matrices. Previous report (Hu et al. 2010) showed the residues of antibiotics were widely detected in manure, soil, vegetables and groundwater from organic vegetable bases, northern China, which would pose a great pressure on the safety of ecosystems and human health in organic vegetable bases. The first objective of this study was to investigate the occurrence of sulfonamides, quinolones, and macrolides in manure, soil, wastewater, and groundwater from livestock and poultry farms. The second purpose was to evaluate the risk of antibiotics on the aquatic organisms.
Materials and Methods
Antibiotics standards with purities of > 98% were purchased from Sigma-Aldrich (St. Louis, MO, USA). The target antibiotics comprised of 12 sulfonamides (sulfabenzamide (SB), sulfacetamide (SA), sulfadimethoxine (SDM), sulfadoxine (SDO), sulfaguanidine (SG), sulfameter (SME), SMZ, sulfamethizole (SMT), SMX, sulfapyridine (SPD), sulfamethoxypyridazine (SMP), SDZ), nine fluoroquinolones (cinoxacin (CIN), difloxacin (DIF), ENR, fleroxacin (FLE), flumequine (FLU), lomefloxacin (LOM), sparfloxacin (SPA), ofloxacin (OFL), nalidixic acid (NAL)) and nine macrolides (azithromycin (AZI), clarithromycin (CLA), erythromycin (ERY), josamycin (JM), roxithromycin (ROX), spiramycin (SPI), tiamulin (TIA), lincomycin (LIN), tilmicosin (TIL)). HPLC grade acetonitrile and methanol were obtained from Fisher (New Jersey, USA). Ultrapure water was produced by a Milli-Q water purification system (Millipore, Billerica, MA, USA). The glass microfiber filters with pore size of 0.45 μm and a diameter of 47 mm were obtained from Whatman Corporation (Maidstone, UK). Oasis HLB cartridges (500 mg, 6 cc) were purchased from Waters Corporation (Milford, MA, USA).
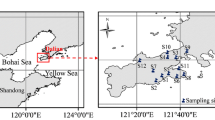
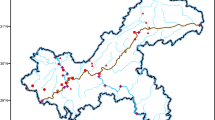
Samples from seven livestock and poultry farms (Table 1) located in Xuzhou City in China were collected in June 2017 (Fig. 1). Composite manure samples were prepared by mixing an equal amount of three discrete subsamples (depth 0–10 cm) in the corresponding farms on June 14, 2017. Similarly, five soil subsamples (depth 0–15 cm) distributed as the S-shaped pattern were collected from the farmland adjacent to the corresponding farms on June 14, 2017, and thoroughly mixed into one composite soil sample. Grab samples of wastewater were collected from the livestock and poultry farms, and grab samples of groundwater were taken from local wells at different depths (Table 1) on June 14, 2017.
Two grams of each freeze-dried manure and soil samples spiked with 50 ng of CIP-d8 and TMP-D3 internal standard were separately extracted with 15 mL acetonitrile and 15 mL 0.1 M EDTA-McIlvaine buffer solution (pH 4.0). The sample was successively shaken and ultrasonicated for 20 min, followed by centrifugation at 5000 rpm for 10 min. The extraction process was repeated twice and the supernatant was diluted to 200 mL with ultrapure water. All water samples were filtered through glass fiber membranes to remove suspended particulate matter. 200 mL of filtered water samples were adjusted to pH 3 with 1.0 M H2SO4, and 0.2 g Na2-EDTA was added. The water samples spiked with 50 ng of CIP-d8 and TMP-D3 internal standard were loaded onto the preconditioned Oasis HLB cartridges at a flow rate of 3–5 mL/min. The cartridges were washed with Milli-Q water (6 mL) and then pumped dry for 60 min. Analytes were eluted with 8 mL of methanol. The eluent solution was concentrated to nearly 0.1 mL under a gentle stream of high-purity nitrogen and then reconstituted to 1 mL with methanol. Samples were passed through a 0.22 μm nylon filter prior to instrumental analysis.
Quantification of target analytes was accomplished by an internal standard (CIP-d8 and TMP-D3) method. The linear calibration curves were obtained by injecting standards at concentrations ranging from 0.05 to 100 μg/L with R2 > 0.99 for each of the target antibiotic. The concentrations with signal-to-noise (S/N) of 3 and 10 were defined as the limit of detection (LOD) and limit of quantification (LOQ), which were estimated to be 0.01–0.08 and 0.05–0.2 ng/L, respectively. The spiking recovery rates of the target antibiotics (100 ng/L, n = 5) were in the range of 78.9–%%01.4%, 83.7%–116.4%, 72.1%–123.7% for manure, soil and water samples, respectively. The relative standard deviation was less than 10% and the reproducibility was good. The procedural blank was performed for each batch of samples to monitor the background contamination and system performance. Meanwhile, pure solvent (methanol) was injected periodically as a check for residual of antibiotics between the samples analyzed.
The environmental risk of antibiotics in the water environment was assessed according to the risk quotient (RQ) of individual compound, which was calculated by dividing the measured environmental concentration (MEC) by the predicted no-effect concentration (PNEC) (Nie et al. 2015). The PNEC was extrapolated by dividing the median effective concentrations (EC50) with an assessment factor of 1000 for acute toxicity and 100 for chronic toxicity (Leung et al. 2012), which were estimated by the USEPA model, ECOSAR (Hans et al. 2003). The risk levels were classified into low risk (0.01 ≤ RQ < 0.1), medium risk (0.1 ≤ RQ < 1), and high risk (RQ > 1) (Weihai i et al. 2013).
Results and Discussion
Figure 2 indicated that the total concentrations of 12 SAs in different animal manures followed the order of horse-manure (19.95 μg/kg) > chicken-manure (11.65 μg/kg) > swine-manure (11.15 μg/kg) > cattle-manure (10.30 μg/kg) > goat-manure (8.95 μg/kg) > duck-manure (8.10 μg/kg) and goose-manure (8.10 μg/kg). SAs are not tending to adsorb to soils and thus may be further released into wastewater and groundwater through runoff or leaching (Wegst-Uhrich et al. 2014). Among the 12 SAs, SMP, SMT and SME were detected in all manures, and SMT was the most abundant which is one of the most commonly used SAs in food-producing animals (Pan et al. 2011). The relatively low levels of SAs may attribute to the restrictions of their use in breeding industry.
Among the 9 FQs, at least four quinolones were detected in all manure samples in the present study (Fig. 2b). The lowest total concentration of 18.20 μg/kg and the highest total concentration of 104.55 μg/kg were detected in cattle-manure and goose-manure, respectively. High detection frequency of FLE was found in all manures except for the chicken manure with the maximum concentration of 38.85 μg/kg in swine-manure. CIN and SPA were the dominant antibiotics in chicken-manure at 15.80 and 7.40 μg/kg. The maximum concentrations of CIN and LOM were both found in goose-manure at 44.35 and 33.65 μg/kg. As for 9 MACs (Fig. 2c), the minimum and maximum total concentrations were detected in cattle-manure (10.20 μg/kg) and swine-manure (167.70 μg/kg), respectively. Neither ERY nor TIA was detected in all manure samples. JM was detected with the highest concentrations in all manures ranging from 7.50 to 121.95 ng/L, suggesting that JM was one of the most widely used MACs in the study area.
Among the 12 SAs, the minimum and maximum total concentrations were found in soil samples from the cattle (2.75 μg/kg) and duck farms (15.50 μg/kg), respectively (Fig. 3a). SMP, SMT and SME were detected in all soil samples, and SMT accounted for the highest proportion except for the soil from goose farm, which was attributed to its high concentrations in manure samples. SMZ was detected at relatively high concentrations in soil samples from horse, duck and goose farms. Boxall et al. (Boxall et al. 2002) reported that the distribution coefficient (Kd) values of SAs were 0.9–18.1 mL/g, indicating that SAs were easy to move downward to deeper soil.
At least 6 (CIN, DIF, FLE, LOM, SPA and OFL) out of the 9 FQs were detected in all soil samples (Fig. 3b). The minimum and maximum total concentrations were detected in soil samples from chicken and horse farms at 5.95 and 42.25 μg/kg, respectively. FLE and CIN were the dominant antibiotics with the maximum concentrations of 15.50 and 28.70 μg/kg in soil sample from duck and swine farm, respectively. Riaz et al. (2017) reported that strong sorption of FQs on soil was significantly influenced by pH and cation exchange capacity of soil, resulting in its increase persistence. It might be an important reason why concentrations of FQs were higher than other antibiotics in soil samples. The maximum total concentration of MACs (27.15 μg/kg) was detected in soil sample from horse farm (Fig. 3c). The highest proportion of MACs was JM, followed by TIL. MACs would circulate in the food chain through plants grown on contaminated soil, thus pose potential adverse effect on the health of creatures through inhibiting drug metabolism in the liver (Wei et al. 2019).
The maximum total concentration of 12 SAs (596.34 ng/L) was detected in wastewater from goat farm, followed by cattle farm (493.01 ng/L), swine farm (283.33 ng/L), horse farm (73.99 ng/L) and duck farm (10.33 ng/L) (Fig. 4a). SA, SDM, SDO, SME, SMZ and SMT were detected in all wastewater from livestock farms (cattle, goat, swine), while SMX was only detected in wastewater from duck farm at 0.33 ng/L. SMP was found in all wastewater samples, which was in accordance with its occurrence in manure samples. The maximum concentrations of SDZ (164.05 ng/L) and SMZ (49.35 ng/L) were respectively detected in wastewater from cattle and horse farm, indicating their extensive use to treat cattle and horse diseases (Yang et al. 2018).
Among the 9 FQs, DIF, FLE, FLU, SPA and NAL could be detected in all wastewater samples. FLE accounted for the largest proportion, followed by SPA (Fig. 4b). OFL was only detected in wastewater from cattle farm at 8.05 ng/L. CIN was detected in wastewater from livestock farms (cattle, goat, swine) with concentrations of 14.67, 10.15 and 64.33 ng/L, respectively. The maximum and minimum total concentrations of 9 MACs were detected in wastewater from swine and goat farm at 1138.33 and 126.34 ng/L, respectively (Fig. 4c). JM was detected with highest concentration except for the wastewater from goat farm, in which the concentration of LIN was the highest (76.67 ng/L).
As shown in Fig. 5, only a few antibiotics were detected at ng/L in groundwater, which was consistent with a previous report on the occurrence of antibiotics in groundwater affected by livestock waste (Bartelt-Hunt et al. 2011). The maximum total concentration of 12 SAs (27.24 ng/L) was detected in groundwater from duck farm (Fig. 5a). At least one sulfonamide antibiotic was detected in all the groundwater samples, and SMX and SPD were found below the limit of quantitation. The highest concentrations of SMP (10.62 ng/L) and SMT (21.13 ng/L) were observed in groundwater from duck and chicken farms, respectively. SDM was detected in groundwater only from goat farm at 0.83 ng/L. SAs have been detected in groundwater samples in many places around the world, such as Korea, Germany, Spain, Taiwan and USA (Sui et al. 2015). The widespread occurrence of SAs in groundwater could be attributed to their weak sorption to soil, low biodegradability and strong mobility (Strauss et al. 2011).
The highest total concentration of 9 FQs (29.83 ng/L) was detected in groundwater from chicken farm (Fig. 5b), in which the concentration of FLE was the highest (26.61 ng/L). Both CIN (6.83 ng/L) and OFL (0.41 ng/L) were detected only in groundwater from goose farm. DIF, LOM, SPA and NAL were detected in at least four groundwater samples. The maximum total concentration of 9 MACs (23.02 ng/L) was detected in groundwater from cattle farm (Fig. 5c). TIL and JM accounted for more than 74% of the 9 MACs in all groundwater samples, in which ERY and TIA were all found below the limit of quantitation. AZI was detected in all groundwater samples with relatively low concentration ranging from 0.21 to 0.63 ng/L. The reported concentration of AZI in surface and ground water was from 0.025 to 0.14 μg/L (Grujić et al. 2009), and AZI can eventually be transferred to drinking water in which AZI was reported to be 5–9.5 ng/L in Spain (Rosa, et al. 2014).
Antibiotics could cause hazardous ecological and health impacts in aquatic environment and pose potential risk to the food chain, even at low concentrations (Bouki et al. 2013). Exposure to antibiotics may cause adverse effects on the reproductive system in the early life stages of different organisms (Klaus 2009). Moreover, a previous study (Erik et al. 2011) found that subtherapeutic concentrations up to several 100-fold below minimal inhibitory concentrations (25 and 0.46 ng/mL of tetracycline and ciprofloxacin, respectively) can also induce antibiotics resistance genes (ARGs). Although the concentration of antibiotics in this study is relative low, they may be pseudo-persistent owing to a cycle of bioaccumulation, partial transformation, and deposition in soils and waters (Carvalho and Santos 2016).
The influence of antibiotics on the aquatic organisms was evaluated by the RQs of individual compound as shown in Fig. 6. Algae and daphnid were generally more susceptible to antibiotics than fish, which suggested that antibiotics were more likely harmful to algae and daphnid in aquatic environment (Li et al. 2014). All RQs were less than 0.1 in the range of 10−2 to 10−9, indicating the low risk to aquatic organisms at different trophic levels. However, JM in wastewater showed relatively high RQs ( > 0.01) for aquatic organisms, implying a relatively low ecological risk. SME, SMZ, SMT, SMP, FLU and TIL were shown to pose relatively high risk to algae. Similar phenomenon was observed in a previous study where algae was the most sensitive species to antibiotics than daphnids and fish in aquatic environment (Wang et al. 2017). It is worth noting that the risk assessment in this study might be overestimated or underestimated without considering the mixture effects of various compounds. As a slight change in the number of algae could affect the balance of ecosystem, and bioaccumulation in invertebrate and fish would happen after long-term exposure to antibiotics (Klaus 2009), further study is required to investigate the chronic and combined risks of antibiotic contamination (Ding et al. 2017).
Antibiotic concentrations in manure, soil, wastewater and groundwater samples collected from various animal farms in Xuzhou City of China were investigated. The total concentration of antibiotics in manures was higher than in soil. The concentration in wastewater was much higher than that in groundwater. Antibiotic in wastewater of livestock farms were higher than in poultry farm. SMT, FLE, CIN and JM accounted for a large proportion of antibiotics in manure and soil samples, while SMP, SME, SMT, FLE, JM and TIL were the main antibiotics detected in surface and ground water samples. The risk assessment of target antibiotics revealed the low risk to aquatic organisms at different trophic levels. JM in wastewater showed relatively high RQs ( > 0.01) for aquatic organisms. To prevent the associated risks to the ecosystem and human health by antibiotics, further investigation needs to be conducted on the characterization of antibiotic-resistant bacteria and genes in this region and precise risk assessment of these antibiotics to the environment. The following control measures should be taken in the later stage to reduce the environmental risk of antibiotics. Establish antibiotic environmental supervision mechanism to control the pollution source of ARGs. Environmental risk assessment guidelines of antibiotics should be formulated to avoid environmental and health risks in the registration process. Moreover, the development of the control standards for antibiotic/ARGs in feces and sewage should be expedited to eliminate contamination from the use end. Strengthen the disposal and management of feces containing antibiotics to limit the environmental overflow of antibiotic/ARGs.
References
Bartelt-Hunt S, Snow DD, Damon-Powell T et al (2011) Occurrence of steroid hormones and antibiotics in shallow groundwater impacted by livestock waste control facilities. J Contam Hydrol 123(3):94–103
Berendsen BJA, Wegh RS, Memelink J et al (2015) The analysis of animal faeces as a tool to monitor antibiotic usage. Talanta 132(132):258–268
Bouki C, Venieri D, Diamadopoulos E (2013) Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: a review. Ecotoxicol Environ Saf 91(4):1–9
Bound JP, Voulvoulis N (2004) Pharmaceuticals in the aquatic environment—a comparison of risk assessment strategies. Chemosphere 56(11):1143–1155
Boxall A, Blackwell P, Cavallo R et al (2002) The sorption and transport of a sulphonamide antibiotic in soil systems. Toxicol Lett 131(1):19–28
Carvalho IT, Santos L (2016) Antibiotics in the aquatic environments: a review of the European scenario. Environ Int 94:736–757
Chen YS, Zhang HB, Luo YM et al (2012) Occurrence and assessment of veterinary antibiotics in swine manures: a case study in East China. Sci Bull 57(6):606–614
Ding H, Wu Y, Zhang W et al (2017) Occurrence, distribution, and risk assessment of antibiotics in the surface water of Poyang Lake, the largest freshwater lake in China. Chemosphere 184:137–147
Erik G, Sha C, Berg OG et al (2011) Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog 7(7):e1002158
Grujić S, Vasiljević T, Laušević M (2009) Determination of multiple pharmaceutical classes in surface and ground waters by liquid chromatography-ion trap-tandem mass spectrometry. J Chromatogr A 1216(25):4989–5000
Hans S, Johnson DJ, Wilson CJ et al (2003) Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol Lett 144(3):383–395
Hu XG, Zhou QX, Luo Y (2010) Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ Pollut 158(9):2992–2998
Jechalke S, Heuer H, Siemens J et al (2014) Fate and effects of veterinary antibiotics in soil. Trends Microbiol 22(9):536–545
Kim KR, Owens G, Kwon SI et al (2012) Occurrence and environmental fate of veterinary antibiotics in the terrestrial environment. Water Air Soil Pollut 223(9):6213–6214
Klaus K (2009) Antibiotics in the aquatic environment: a review—part II. Chemosphere 75(4):417–434
Leung HW, Minh TB, Murphy MB et al (2012) Distribution, fate and risk assessment of antibiotics in sewage treatment plants in Hong Kong, South China. Environ Int 42(1):1–9
Li N, Zhang X, Wu W et al (2014) Occurrence, seasonal variation and risk assessment of antibiotics in the reservoirs in North China. Chemosphere 111:327–335
Li C, Chen J, Wang J et al (2015) Occurrence of antibiotics in soils and manures from greenhouse vegetable production bases of Beijing, China and an associated risk assessment. Sci Total Environ 522(1):101–107
Lin T, Chen YQ, Chen W (2014a) Toxic effect of sulfadiazine on the growth of zebrafish embryos in the water body. J Safety Environ 14(3):324–327
Lin T, Yu S, Chen Y et al (2014b) Integrated biomarker responses in zebrafish exposed to sulfonamides. Environ Toxicol Pharmacol 38(2):444–452
Nie M, Yan C, Dong W et al (2015) Occurrence, distribution and risk assessment of estrogens in surface water, suspended particulate matter, and sediments of the Yangtze Estuary. Chemosphere 127:109–116
Pan X, Qiang Z, Ben W et al (2011) Residual veterinary antibiotics in swine manure from concentrated animal feeding operations in Shandong Province. China Chemosphere 84(5):695–700
Philipp D, Melanie B, Christina S et al (2012) Accumulation of Pharmaceuticals, Enterococcus, and Resistance Genes in Soils Irrigated with Wastewater for Zero to 100 Years in Central Mexico. PLoS ONE 7(12):e45397
Riaz L, Mahmood T, Khalid A et al (2017) Fluoroquinolones (FQs) in the environment: a review on their abundance, sorption and toxicity in soil. Chemosphere 191:704–720
Rosa MB, Elida A, Encarnación M et al (2014) Survey of the occurrence of pharmaceuticals in Spanish finished drinking waters. Environ Sci Pollut Res Int 21(18):10917–10939
Strauss C, Harter T, Radke M (2011) Effects of pH and manure on transport of sulfonamide antibiotics in soil. J Environ Qual 40(5):1652–1660
Sui Q, Cao X, Lu S et al (2015) Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: a review. Emerg Contam 1(1):14–24
Wang N, Noemie N, Hien NN et al (2009) Adverse effects of enrofloxacin when associated with environmental stress in Tra catfish (Pangasianodon hypophthalmus). Chemosphere 77(11):1577–1584
Wang Z, Du Y, Yang C et al (2017) Occurrence and ecological hazard assessment of selected antibiotics in the surface waters in and around Lake Honghu, China. Sci Total Environ 609:1423–1432
Wegst-Uhrich SR, Navarro DA, Zimmerman L et al (2014) Assessing antibiotic sorption in soil: a literature review and new case studies on sulfonamides and macrolides. Chem Cent J 8(1):1–12
Wei R, Ge F, Huang S et al (2011) Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere 82(10):1408–1414
Wei R, He T, Zhang S et al (2019) Occurrence of seventeen veterinary antibiotics and resistant bacterias in manure-fertilized vegetable farm soil in four provinces of China. Chemosphere 215:234–240
Weihai X, Wen Y, Xiangdong L et al (2013) Antibiotics in riverine runoff of the Pearl River Delta and Pearl River Estuary, China: concentrations, mass loading and ecological risks. Environ Pollut 182(6):402–407
Yang J, He M, Wu T et al (2018) Sulfadiazine oxidation by permanganate: Kinetics, mechanistic investigation and toxicity evaluation. Chem Eng J 349:56–65
Zhang H, Zhou Y, Huang Y et al (2016) Residues and risks of veterinary antibiotics in protected vegetable soils following application of different manures. Chemosphere 152:229–237
Acknowledgements
This work was funded by the Postgraduate Research and Innovation Project of Jiangsu Province (Grant No. KYLX16_0569) and the Research on Environmental Safety Monitoring, Assessment and Early Warning Technology of Centralized Drinking Water Source (Grant No. 2018002).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gu, D., Feng, Q., Guo, C. et al. Occurrence and Risk Assessment of Antibiotics in Manure, Soil, Wastewater, Groundwater from Livestock and Poultry Farms in Xuzhou, China. Bull Environ Contam Toxicol 103, 590–596 (2019). https://doi.org/10.1007/s00128-019-02692-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02692-0