Abstract
The antibiotic sulfadiazine (SDZ) is a challenging threat to the health of aquatic organisms, as it frequently occurs in aquatic ecosystems. Tolerance mechanisms and accumulation of SDZ in a floating macrophyte (Eichhornia crassipes) under hydroponic conditions were investigated in this study to provide more insight into the SDZ removal process. Results show that the presence of 1 mg L−1 SDZ decreased the quickest and ranged from 669.45 to 165.34 μg L−1 from days 5 to 25. Exposing E. crassipes to SDZ ( < 1 mg L−1) maintained stable leaf photosynthetic efficiency. The overall increase in superoxide dismutase and peroxidase activities with SDZ treatments indicated that leaves were resistant. SDZ was absorbed by E. crassipes, following the sequence of root > aerial parts under all treatments. These findings suggest that E. crassipes has the ability to phytoremediation SDZ contaminated water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Antibiotics are used worldwide in human and veterinary medicine to treat and cure infections. However, antibiotics are poorly absorbed by animals, and the non-absorbed portion (up to 90%) is excreted via the urine or feces without adequate waste treatment (Sarmah et al. 2006). This process often leads to some portion of antibiotics entering the environment. Aquatic ecosystems are prone to antibiotic pollution, which occurs frequently in the ocean (Du et al. 2017), lakes (Xu et al. 2014), wetlands (Yan et al. 2013), and in drinking water (Stackelberg et al. 2007). Antibiotic concentrations in aquatic ecosystems, including fish and shrimp farms, range from μg L−1 to mg L−1 in water (Kümmerer 2009; Thuy et al. 2011), and μg kg−1 to mg kg−1 in sediments (Thuy et al. 2011; Xu et al. 2014). Sulfadiazine (SDZ) is a sulfonamide, which is widely used as veterinary medicine due to its low cost and broad spectrum of activity against a large number of Gram-positive and Gram-negative bacteria (De Liguoro et al. 2007). Several reports have raised concerns about the potential consequences of the environmental presence of SDZ on plant and aquatic products (Chen et al. 2016; Song et al. 2017).
Due to the increased frequency of detecting antibiotics in water over the past decade, there has been an increased interest in methods to remove these contaminants. Some technologies, including advanced oxidative processes, activated carbon absorption, and membrane filtration, have been successfully applied to remove antibiotics (Elmolla and Chaudhuri 2010; Sharma et al. 2017). However, they are not widely used at a full-scale due to the high cost and potential secondary pollution. Phytoremediation is a low-cost, effective, and eco-friendly technology to remove antibiotics from contaminated water via uptake, transformation, or degradation (Gujarathi et al. 2005; Michelini et al. 2012). Sulfonamide removal percentages of 91.8%–99.5% were reported for Italian ryegrass in a constructed wetland (Xian et al. 2010). Reed exposure to 1000 μg L−1 concentrations of ciprofloxacin, oxytetracycline and sulfamethazine absorbed 13,834, 6901, and 2047 ng g−1, respectively (Liu et al. 2013). In some cases, organic chemical pollutants not only cause stress in plants, but they also inhibit accumulation of pollutants (Susarla et al. 2002). Due to the potential hazard of antibiotics to plants, phytotoxic studies need to be performed to assess the appropriateness of phytoremediation by specific plant species. Effects of antibiotics on plants are reflected in their physiological and biochemical responses.
The floating macrophyte E. crassipes possesses most characteristics required for phytoremediation in its native setting, including rapid proliferation and a spreading root apparatus (Rezania et al. 2015; Xia and Ma 2006). In this study, the possibility of using E. crassipes in SDZ phytoremediation was investigated. The tolerance of E. crassipes to SDZ was determined, via chlorophyll content, chlorophyll fluorescence parameters, and antioxidant enzymes. Additionally, bioaccumulation and translocation of SDZ in E. crassipes were evaluated. These findings will provide a deeper understanding and potential alternative phytoremediation solution for antibiotic contaminated bodies of water.
Materials and Methods
SDZ (98%, purity, CAS No. 68–35-9) was used in this study (Shanghai Macklin Biochemistry Co., Ltd, Shanghai, China). Formic acid and methanol (HPLC grade) were obtained from Tedia Company (Fairfield, OH, USA). All other reagents were analytical reagent grade. Milli-Q water (Millipore Bedford, MA, USA) was utilized in this research.
The experiment was carried out at the Experimental Platform for Ecological Remediation, which contains a large glass greenhouse with abundant light supply at Nanjing Normal University (32°6′27 N, 118°54′19″E), during September 2017. Eichhornia crassipes specimens were transplanted from an uncontaminated pond at the campus of Nanjing Normal University. SDZ was not detected in plants prior to the experiment. Experimental plants were rinsed before being cultivated in half modified Hoagland nutrient solution for one week. Plants were washed thoroughly with tap water followed by deionized water, and approximately 75.17 g of fresh plants with average root length 16.87 cm and leaf width 9.38 cm were planted in each pot. A complete randomized block design was used in triplicate with the following concentrations of SDZ: 0 (control), 0.01, 0.1 and 1 mg L−1, and each concentration was also set without plants for control. Eichhornia crassipes was cultivated in plastic 100 L barrels, containing pH 6.71 water. Each plastic barrel contained three parallel plants and was irrigated with of half Hoagland nutrient solution. The surface of each container was covered with silver paper to prevent photochemical degradation of the antibiotics. During the experiment, water temperatures ranged from 18.3 to 32.1℃ (mean, 25.1℃). Deionized water was added to balance the water volume. Leaf indices were measured, including chlorophyll content, chlorophyll fluorescence parameters, and antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) in leaves at a fixed time. Leaves with strong photosynthetic activity were selected to test. The distribution of SDZ in the plants was separated into aerial parts and roots and was analyzed at the end of the experiment.
Chlorophyll contents (Chl a, b and total Chl) of the plants were measured according to Huang et al. (2004). All plants were weighed to the nearest 0.1 g in each barrel. All leaves were cut into strips and incubated in 80% (v/v) aqueous acetone for 24 h in the dark. Absorbance of the solution was measured with a spectrophotometer (UV -2500 Shimadzu, Tokyo, Japan) at 663 and 645 nm, and total chlorophyll content was calculated as the sum of Chl a and Chl b. Chlorophyll fluorescence parameters were measured by a plant efficiency analyzer (Hansatech Co., King’s Lynn, UK). All parameters, including baseline (Fo), maximum (Fm), photochemical efficiency of PS II (Fv/Fm), as well as potential photochemical efficiency (Fv/Fo) were measured after 20 min of dark adaptation. SOD, POD, and CAT activities were measured according to Xu et al. (2010).
High performance liquid chromatography (HPLC) was used to analyze SDZ concentrations in samples. Water samples were filtered through 0.45 μm filters, and HCl was added to each sample to adjust the pH to 3. Samples were extracted using Waters Oasis HLB extraction cartridges (500 mg, Waters Corp., Milford, MA, USA). Each extraction cartridge was sequentially pre-conditioned with 6.0 ml methanol, 6.0 ml Milli-Q water, and 6.0 mL 10 mM L−1 Na2 EDTA-Mellvaine buffer (pH 3.0). The extraction rate was 5 mL min−1. Subsequently, the cartridge was rinsed with 10 mL Milli-Q water and was eluted with 6.0 mL methanol. Finally, the target fraction was concentrated to dryness under a gentle stream of N2 in a 40 ℃ water bath and dissolved in 40% methanol solution to reach a volume of 1.0 mL.
Plant aerial parts and roots were frozen at − 18 ℃, freeze-dried for 72 h, weighed to the nearest 1.0 g, and finally ground with a sterile pestle. Plant samples were added to centrifuge tubes containing 20 mL Na2EDTA-Mellvaine buffer (pH 3.0). Tubes were shaken on a vortex for 30 s, sonicated for 10 min, and centrifuged at 8000 r min−1 for 10 min. This extraction process was repeated three times for each sample. Samples were fixed in 200 mL Milli-Q water. Target antibiotics were analyzed with an Agilent 1100 series HLPC system (Agilent Technologies Inc., Palo Alto, CA, USA) equipped with diode array detector operated at a wavelength of 270 nm and an Zorbax 300SB-C18 column (4.6 mm × 150 mm, 5 μm). The mobile phase was methanol and 0.1% formic acid solution (20:80, v/v) at a flow rate of 1.0 mL min−1. Column oven temperature was set to 30 ℃, with an injection volume of 20 μL. Quantification of target analyte was based on external calibration curves, and correlation coefficients (R2) of the calibration curves were 0.999. Recovery efficiencies were 74.8%. Limits of quantification (LOQ) of the antibiotics were calculated with signal/noise ratios of 10. LOQ of the samples were 35.5 ng L−1.Bioconcentration factors were calculated as: aerial part bioconcentration factors (ACF) = Ca / Cw, and root bioconcentration factors (RCF) = Cr / Cw, where Ca and Cr were SDZ concentrations in the aerial parts and roots of E. crassipes respectively, Cw is the SDZ concentration in the water.
Data were analyzed with SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA). One-way analysis of variance was used to test for differences among the treatments. Means of the different treatments were compared using the least significant difference (LSD) test. A p-value < 0.05 was considered significant.
Results and Discussion
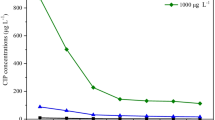
Residue concentrations of SDZ were detected in water (Fig. 1). The concentration of 1 mg L−1 decreased quicker than the 0.01 and 0.1 mg L−1 concentrations and decreased from 669.45 to 165.34 μg L−1 from days 5 to 25. Reduction rates of the 0.01 and 0.1 mg L−1 SDZ concentrations were 74.6% and 63.8%, and the residual concentrations were 7.46 and 63.8 μg L−1 on day 25, respectively. Different initial concentrations of SDZ in the water had different rates of reduction. Different concentrations of SDZ without plants degraded slower than those with plants, especially at 0.01 mg L−1 SDZ concentrations. Initial concentrations were higher, and the final reduction rates were greater, suggesting that a higher SDZ concentration probably resulted in greater absorption by E. crassipes, thus, improving the reduction rate.
Plant chlorophyll content is an important parameter to evaluate photosynthetic activity and can be used as an indicator of pollutant-induced plant stress (Huang et al. 2004). Total chlorophyll contents under SDZ concentrations of 0, 0.01, 0.1 and 1 mg L−1 were 3.37, 3.65, 2.46, and 2.32 mg g−1 on day 7, respectively (Fig. 2). Hormesis caused by the low SDZ concentration was visible. Total chlorophyll content with 0.1 mg L−1 of SDZ was the highest among all concentrations and was 0.28 mg g—1 higher than the control on day 7. Chlorophyll content with SDZ at concentrations > 0.1 mg L−1 was lower than the control over time. These results were similar to Liu et al. (2013) who demonstrated a decrease of reed chlorophyll content after antibiotics exposure. There were no differences of plant chlorophyll contents between the control and higher SDZ. Eichhornia crassipes may show tolerance to higher concentrations of SDZ. Additionally, changes in Chl a and Chl b were similar to the trend that resulted from plant exposure to sulfadimethoxine. The reason for this may be that electron transport flow was blocked****** from photosystem (PS)) by organic pollutants, therefore blinding plant biosynthesis of Chl b (Huang et al. 2004; Michelini et al. 2012).
Chlorophyll fluorescence is a useful biosensor to monitor pollutants (Durrieu et al. 2006; Védrine et al. 2003). At the end of the current experiment period, F0 values for 0, 0.01, 0.1, and 1 mg L−1 SDZ were 187.5, 185.0, 178.5, and 174.1, respectively (Fig. 3). No significant differences were observed between any of the treatments. The Fm values for 0.1 and 1 mg L−1 SDZ were 39.12 and 21.84 lower than the control, respectively. Fv/Fm reflects the potential quantum efficiency of PS-II and is used as a sensitive indicator of plant photosynthetic performance (Johnson et al. 1993). Fv/Fm values are lower when the plant has been exposed to stress. However, the Fv/Fm values were 0.67, 0.73, 0.70, and 0.70 with 0, 0.01, 0.1, and 1 mg L−1 SDZ at the end of the experiment, respectively. No significant differences were observed between the three treatments and the control. Chlorophyll fluorescence was not significantly affected by SDZ. These results differ from the effects of fluoroquinolone antibiotics on plants (Aristilde et al. 2010). The specific SDZ target differed from fluoroquinolone antibiotics, which inhibit activity of the chloroplast-specific enzyme (DNA gyrase). The quinolone ring and secondary amino group in fluoroquinolone antibiotics mediate their action as quinone site inhibitors in PS II (Evans-Roberts et al. 2016; Wall et al. 2004). DNA gyrase is a chloroplast-specific and a bacterial enzyme; thus, fluoroquinolone antibiotics treat it as a specific target in photosynthetic organisms similar to their bacterial target (Wall et al. 2004).
Reactive oxygen species (ROS) are toxic products of aerobic metabolism that act as cell signals in the response to abiotic stress. Plants maintain a steady-state level of ROS by means of an antioxidant enzyme defense system which includes SOD, CAT, and POD (Xu et al. 2010). In this study, changes in antioxidant enzymes in leaves were observed (Fig. 4). SOD is the first line of defense against ROS damage (Liu et al. 2013). It catalyzes the dismutation of superoxide radicals to H2O2 and O2, maintaining the lower levels of superoxide radicals in the cell. The SOD levels in the 0, 0.01, 0.1 and 1 mg L−1 SDZ concentrations at the end of experiment were 214.40, 265.81, 213.53, and 352.02 U g−1, respectively. SOD with 1 mg L−1 SDZ was significantly (137.62 U g−1) higher than the control. ROS content increases with SDZ stress and SOD activity over time (Wim et al. 1996). CAT is an enzyme that decomposes H2O2 into water and oxygen (Montavon et al. 2007). CAT activity increased with the SDZ concentration at the beginning of the experiment, attributed to the plant’s adaptive mechanism. However, a change in CAT activity occurred gradually on day 42. CAT activity in the 0.01, 0.1 and 1 mg L−1 SDZ concentrations decreased by 9.85, 9.46, and 4.71 nmol min−1 g−1 than the control, respectively. POD activity increased with SDZ concentration after day 28. Furthermore, POD activity in the 0.01, 0.1, and 1 mg L−1 SDZ concentrations increased by 202.57, 20.84, and 350.6 μ g−1 on day 42 than those on day 28, respectively. Due to the spatial distribution of POD in the cytosol, vacuole, and extra-cellular plant tissues (Liu et al. 2013), POD activity increased to maintain the balance of H2O2 produced by the action of SOD on superoxide radicals and the generation of OH via the Haber–Weiss reaction through photorespiration (Dordio et al. 2009).
Accumulated levels of SDZ in E. crassipes were closed related to its concentration. The initial elevated external concentrations and high absorption of antibiotics affected E. crassipes. SDZ was not detected in aerial parts or roots at 0.01 mg L−1. The maximum SDZ concentration in aerial parts was 6.65 μg g−1. Root residues were 31.95 and 55.15 μg g−1 for the 0.1 and 1 mg L−1 SDZ. Bioconcentration factors reflect the bioaccumulation of organic compounds in organisms (Azanu et al. 2016). ACF and RCF decreased from the 0.1 to 1 mg L−1 concentrations, and the maximum values of ACF and RCF were 0.029 and 0.31, respectively. Bioconcentration factors in E. crassipes followed the order of RCF > ACF within the three treatments.
The root is the primary plant part contacting SDZ. Thus, SDZ accumulated the most in roots through physicochemical absorption and biological uptake. As SDZ migrated from the root to the aerial parts via the transpiration stream, it was degraded via photolysis in leaves (Babic et al. 2013). There was a higher migration rate when the SDZ concentration was lower. This may have occurred because biological activities decline in plants under stress from higher antibiotic concentrations. In contrast, fluoroquinolone antibiotics accumulate the highest in plant parts (Pan et al. 2014). Ionic compounds (e.g. SDZ) are detected at significantly lower concentrations than non-ionic compounds in plants in most cases (Malchi et al. 2014). However, they were not detected in plants exposed to 0.01 mg L−1 SDZ. Further study is necessary to understand the translocation and degradation mechanisms of antibiotics in E. crassipes.
Final reduction rates were greater when initial SDZ concentrations were higher. Eichhornia crassipes was able to remove 83.47% of the SDZ at 1 mg L−1 after 25 days of exposure. SDZ removal was achieved without any obvious visual symptoms of toxicity, even when plants were subjected to high SDZ concentrations. SDZ pollution in the water did not affect health or survival of E. crassipes, as evidenced by the monitoring of chlorophyll content, chlorophyll fluorescence, and antioxidant enzymes. More antibiotics were absorbed immediately after the initial concentrations were added. Furthermore, the roots absorbed more SDZ than the aerial parts. This study concluded that antibiotics in water may not affect the health of E. crassipes, although E. crassipes may still play an important role in antibiotic contaminated water.
References
Aristilde L, Melis A, Sposito G (2010) Inhibition of photosynthesis by a fluoroquinolone antibiotic. Environ Sci Technol 44:1444–1450
Azanu D, Mortey C, Darko G, Weisser JJ, Styrishave B, Abaidoo RC (2016) Uptake of antibiotics from irrigation water by plants. Chemosphere 157:107–114
Babic S, Perisa M, Skoric I (2013) Photolytic degradation of norfloxacin, enrofloxacin and ciprofloxacin in various aqueous media. Chemosphere 91:1635–1642
Chen J, Xu H, Sun Y, Huang L, Zhang P, Zou C, Yu B, Zhu G, Zhao C (2016) Interspecific differences in growth response and tolerance to the antibiotic sulfadiazine in ten clonal wetland plants in South China. Sci Total Environ 543:197–205
De Liguoro M, Poltronieri C, Capolongo F, Montesissa C (2007) Use of sulfadimethoxine in intensive calf farming: Evaluation of transfer to stable manure and soil. Chemosphere 68(4):671–676
Dordio AV, Duarte C, Barreiros M, Carvalho AJ, Pinto AP, Costa CT (2009) Toxicity and removal efficiency of pharmaceutical metabolite clofibric acid by Typha spp.–potential use for phytoremediation? Bioresour Technol 100:1156–1161
Du J, Zhao H, Liu S, Xie H, Wang Y, Chen J (2017) Antibiotics in the coastal water of the South Yellow Sea in China: occurrence, distribution and ecological risks. Sci Total Environ 595:521–527
Durrieu C, Tran C, Chovelon JM, Barthet L, Chouteau C, Védrine C (2006) Algal biosensors for aquatic ecosystems monitoring. Eur Phys J Appl Phys 36:205–209
Elmolla ES, Chaudhuri M (2010) Comparison of different advanced oxidation processes for treatment of antibiotic aqueous solution. Desalination 256:43–47
Evans-Roberts KM, Mitchenall LA, Wall MK, Leroux J, Mylne JS, Maxwell A (2016) DNA Gyrase is the target for the quinolone drug ciprofloxacin in Arabidopsis thaliana. J Biol Chem 291:3136–3144
Gujarathi NP, Haney BJ, Linden JC (2005) Phytoremediation potential of Myriophyllum aquaticum and Pistia stratiotes to modify antibiotic growth promoters, tetracycline, and oxytetracycline, in aqueous wastewater systems. Int J Phytoremediat 7:99–112
Huang XD, El-Alawi Y, Penrose DM, Glick BR, Greenberg BM (2004) Responses of three grass species to creosote during phytoremediation. Environ Pollut 130:453–463
Johnson GN, Young AJ, Scholes JD, Horton P (1993) The dissipation of excess excitation energy in British plant species. Plant Cell Environ 16:673–679
Kümmerer K (2009) Antibiotics in the aquatic environment – a review – part ii. Chemosphere 75(4):435–441
Liu L, Liu Yh, Liu CX, Wang Z, Dong J, Zhu GF, Huang X (2013) Potential effect and accumulation of veterinary antibiotics in Phragmites australis under hydroponic conditions. Ecol Eng 53:138–143
Malchi T, Maor Y, Tadmor G, Shenker M, Chefetz B (2014) Irrigation of root vegetables with treated wastewater: evaluating uptake of pharmaceuticals and the associated human health risks. Environ Sci Technol 48:9325–9333
Michelini L, Meggio F, La Rocca N, Ferro S, Ghisi R (2012) Accumulation and effects of sulfadimethoxine in Salix fragilis L. plants: a preliminary study to phytoremediation purposes. Int J Phytoremediat 14:388–402
Montavon P, Kukic KR, Bortlik K (2007) A simple method to measure effective catalase activities: optimization, validation, and application in green coffee. Anal Biochem 360:207–215
Pan M, Wong CK, Chu LM (2014) Distribution of antibiotics in wastewater-irrigated soils and their accumulation in vegetable crops in the Pearl River Delta, southern China. J Agric Food Chem 62:11062–11069
Rezania S, Ponraj M, Talaiekhozani A, Mohamad SE, Din MF, Taib SM, Sabbagh F, Sairan FM (2015) Perspectives of phytoremediation using water hyacinth for removal of heavy metals, organic and inorganic pollutants in wastewater. J Environ Manage 163:125–133
Sarmah AK, Meyer MT, Boxall ABA (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65(5):725–759
Sharma V, Vinoth Kumar R, Pakshirajan K, Pugazhenthi G (2017) Integrated adsorption-membrane filtration process for antibiotic removal from aqueous solution. Powder Technol 321:259–269
Song C, Li L, Zhang C, Qiu L, Fan L, Wu W, Meng S, Hu G, Chen J, Liu Y, Mao A (2017) Dietary risk ranking for residual antibiotics in cultured aquatic products around Tai Lake, China. Ecotoxicol Environ Saf 144:252–257
Stackelberg PE, Gibs J, Furlong ET, Meyer MT, Zaugg SD, Lippincott RL (2007) Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci Total Environ 377(2–3):255–272
Susarla S, Medina VF, McCutcheon SC (2002) Phytoremediation: an ecological solution to organicd chemical contamination. Ecol Eng 18:674–658
Thuy HTT, Nga LP, Loan TTC (2011) Antibiotic contaminants in coastal wetlands from Vietnamese shrimp farming. Environ Sci Pollut R 18(6):835–841
Védrine C, Leclerc JC, Durrieu C, Tran-Minh C (2003) Optical whole-cell biosensor using Chlorella vulgaris designed for monitoring herbicides. Biosens Bioelectron 18:457–463
Wall MK, Mitchenall LA, Maxwell A (2004) Arabidopsis thaliana DNA gyrase is targeted to chloroplasts and mitochondria. Proc Natl Acad Sci USA 101:7821–7826
Wim VC, Katelijne C, Marc VM, Dirk I, Luit S (1996) Enhancement of oxidative stress tolerance in transgenic tobacco plants overproducing Fe-superoxide dismutase in chloroplasts. Plant Physiol 112:1703–1714
Xia H, Ma X (2006) Phytoremediation of ethion by water hyacinth (Eichhornia crassipes) from water. Bioresour Technol 97:1050–1054
Xian Q, Hu L, Chen H, Chang Z, Zou H (2010) Removal of nutrients and veterinary antibiotics from swine wastewater by a constructed macrophyte floating bed system. J Environ Manage 91:2657–2661
Xu J, Zhang J, Xie H, Li C, Bao N, Zhang C, Shi Q (2010) Physiological responses of Phragmites australis to wastewater with different chemical oxygen demands. Ecol Eng 36:1341–1347
Xu J, Zhang Y, Zhou C, Guo C, Wang D, Du P, Luo Y, Wan J, Meng W (2014) Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from Taihu Lake, China. Sci Total Environ 497–498:267–273
Yan C, Yang Y, Zhou J, Liu M, Nie M, Shi H (2013) Antibiotics in the surface water of the Yangtze estuary: occurrence, distribution and risk assessment. Environ Pollut 175(8):22–29
Acknowledgments
This research was supported by Major Science and Technology Program for Water Pollution Control and Treatment (Number 2017ZX07203-003).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yan, Y., Chen, Y., Xu, X. et al. Effects and Removal of the Antibiotic Sulfadiazine by Eichhornia crassipes: Potential Use for Phytoremediation. Bull Environ Contam Toxicol 103, 342–347 (2019). https://doi.org/10.1007/s00128-019-02656-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02656-4