Abstract
Heavy metal contamination in agricultural soils has become a serious environmental concern due to their generally high mobility and toxic effects on plants and food security. An incubation study was conducted to assess the effectiveness of biochar (BC), zeolite (ZE) and rock phosphate (RP) stabilizers on the immobilization of cadmium (Cd) in contaminated soils. Various extraction techniques were carried out: a sequential extraction procedure, the European Community Bureau of Reference (BCR), the toxicity characteristics leaching procedure (TCLP) and extraction with ammonium nitrate. In addition, Cd adsorption by these materials was observed using Langmuir and Freundlich isotherms. The results showed that with an increase in soil pH the exchangeable fraction of Cd in soil was significantly reduced by 28%–29.4%, 9%–13% and 4%–14% for BC, ZE, and RP, respectively. According to the Langmuir adsorption isotherm, BC-amended soil showed a higher adsorption capacity (Qm) of Cd from 8.38 to 19.85 mg g−1. Overall, BC offered better results when compared to other amendments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cadmium (Cd) is one of the persistent heavy metals that have distinct toxicological effects on ecosystems and humans due to its high mobility and ready transformation through the soil and into the food-chain (Xu et al. 2013). Accumulation of Cd in soil is due to coal combustion, inadequate battery recycling practices, frequent use of phosphate fertilizers and pigment production. Even at a low concentration (< 1 mg kg−1), Cd in the soil also poses serious threats to living organisms (Adriano 2001). Therefore, the amelioration of Cd-contaminated soil is an important task to reduce its mobility in soils and, thereby, minimize its risks for humans (Bashir et al. 2017). For this purpose, several traditional remediation techniques have been introduced, such as, (phytoextraction, separation, excavation, and land filling) (Xu et al. 2013). In situ immobilization has been receiving much attention worldwide as a cost- effective and less destructive soil remediation option. Heavy metal immobilization can be achieved by the transformation of Cd from a mobile form into to residual form through adsorption, precipitation and complexation (Ahmad et al. 2014). Recently, the use of several organic (compost, plant residues and biochar) and inorganic (clay minerals, phosphate) fertilizers have been shown to be excellent amendments for soil. Biochar (BC) is a carbonaceous, porous and thermally-degraded organically-based material that is produced via pyrolysis. It can be used as a novel remediating agent for organic and inorganic pollutants as well as for enhancing soil fertility (Lehmann et al. 2011). The potential role of biochar in immobilizing heavy metals, particularly Cd from various types of soil is well documented (Bashir et al. 2017). Bashir et al. (2017) suggested that Cd immobilization is increased by the application of sugarcane derived BC in polluted soils. Cd is a transition-heavy metal, having coordination affinity for oxygen- and nitrogen-containing functional groups. Biochar has more adsorption capacity than non-pyrolytic residues due to its porous structure, high alkalinity and C- and N-bearing surface functional groups on the surface of the biochar (Yuan et al. 2011). Adsorption is one of the fastest and efficient methods for treating heavy metal from aqueous solution using organic and inorganic amendments (Bashir et al. 2018b; Xu et al. 2013). For heavy metal removal from soil and solution, biochar operates through various mechanisms such as ion exchange from metal cations towards their anions, electrostatic interactions, precipitation and complex formation with the biochar surface functional groups of biochar (Ahmad et al. 2014).
Natural amendments like zeolite belong to the crystalline hydrous tectosilicate family that has a high sorptive ability for anions and different molecules due to its high cation exchange capacity (Oste et al. 2002). Zeolite has the potential to immobilize heavy metals from the soil. Oste et al. (2002) observed that Cd and Zn were immobilized after the addition of zeolite addition to a polluted soil. Usman et al. (2006) also showed that zeolite application in multi-metal polluted soils significantly reduced the bioavailable fraction of heavy metals. Moreover, they explained that zeolite application to metal polluted soil could efficiently decrease the levels of trace metals especially Cd mobility and its availability to soybean.
In recent years, several studies about heavy metal stabilization by phosphate sources such as di-ammonium phosphate (DAP), rock phosphate (RP) and single super phosphate (SSP) have been used to produce metal phosphate after their addition to metal contaminated soil. In particular, rock phosphate is an alkaline material that is used to reduce metal solubility by increasing soil pH and enhancing sorption of metal on its surface (Chen et al. 2007). Applications of natural rock phosphate to a multi-metal (Cd, Cu, Pb, and Zn) contaminated soil can reduce their solubility and increase sorption from solution (Mignardi et al. 2012). The purpose of this study was to evaluate the comparative effect of amendments on Cd chemical fractionation and observe the behaviour of the Cd adsorption mechanism in biochar, zeolite and rock phosphate amended soils.
Materials and Methods
Soil samples, characterized as red soil were collected from a 0–15 cm depth at Wuhan, Hubei Province, China (30°28′N, 114°21′E). The soil was then transferred to the laboratory, where the samples were air dried and put through a 2 mm plastic sieve before incubation. The basic soil chemical properties pH and EC were measured using a soil/water ratio of 1:2.5 and 1:5 (w/v) using an automated pH and EC meter. Soil textural analyses performed using the pipette method revealed that the soil was silty clay (USDA classification) comprising 54.7% silt, 33.40% clay, and 11.9% clay. Total soil phosphorus and total nitrogen were measured according to Lu (1999) and the cation exchange capacity (CEC) of the soil was quantified by the ammonium acetate method at pH 7.0. Soil Cd was determined with atomic absorption spectrophotometer (AAS-240FS), after soil digestion by a three acids (HCl-HNO3-HClO4) mixture. The physico-chemical properties of the studied soil were as follows: pH 5.2, EC 1.3 mS cm−1, organic matter 25 g kg−1, and CEC 10.1 cmol (+) kg−1.
Rice straw (Oryza sativa L.) samples were collected from a rice field and the cleaned samples were air dried for two weeks and then chopped to pass through a 10 mm mesh sieve. To produce biochar, rice straw was placed in a porcelain crucible, covered with an air tight lid before pyrolysis under limited oxygen conditions at 500°C for 2 h, and then prepared as described previously by Yuan et al. (2011). The basic chemical properties of the biochar applied were as follows: pH 10.1, CEC 12.25 (cmol kg−1), C 49.7% and BET-SA 40 m2g−1. Biochar Fourier-transform spectroscopy (FTIR) analysis was performed using a ratio of 1: 200 mg KBr and biochar samples. The mixtures were ground then dried at 105°C for 24 h for FTIR analysis. The spectra of the samples were measured using a Bruker Vector 22 FTIR spectrometer (OPUS 2.0 software) at wave numbers from 400 to 4000 cm−1. Scanning electron microscope (SEM) images were collected using a JEOL JSM-6400 scanning microscope. Images were obtained at ×200 and ×1000 magnification. Natural rock phosphate was purchased from Zhongxiang, Hubei Province, China. Natural zeolite was used as a clay mineral source for soil amendments. The pH values of rock phosphate and zeolite were measured with a pH meter. The surface area of zeolite was determined using BET method (Autosorb-1, Quantachrome, USA). The basic chemical properties of rock phosphate and zeolite were as follows: pH (11.6, 8.4), CEC (12.2, 36.89 cmol kg−1) and surface area (15.4, 19.7 m2g−1), respectively.
Incubation experiments were conducted in plastic polythene cups with each cup containing 100 g of air-dried soil. The soil was then contaminated with Cd using Cd (NO3)2 to spike 2 mg Cd kg−1 into normal cultivated soil. In accordance with Chinese metals guidelines, the concentration of Cd spiked soil was maintained at a permissible level III under Chinese guideline (GB15618-1995). All soil samples were incubated for 4 months while maintaining 70% (w/v) moisture at 25°C. The contaminated soil was then homogenously amended with biochar (BC), zeolite (ZE) and rock phosphate (RP) at 1.5% and 3% application rate, respectively, on dry soil bases. No amendment was used for the control soil. Each treatment was replicated in triplicate. After the addition of all stabilizers, the soil was thoroughly mixed, and all cups were covered with plastic lids, each of which contained a hole to reduce gaseous exchange and water loss. Over the incubation period the cups were weighed on weekly basis to monitor the moisture loss. Finally, the soil samples were ground to pass through < 2 mm sieve for further chemical analysis.
The European Community Bureau of Reference (BCR) sequential extraction technique was used to measure the metal proportions of the samples (Rauret et al. 1999). Briefly, 0.5 g of ground soil was extracted with four different extractants: 0.11 M acetic acid, 0.1 M hydroxylamine hydrochloride (pH 2), 8.8 M H2O2 and 1 M NH4OAc (pH 2), and HNO3–HF–HClO4 to extract the soluble, reducible, oxidizable and residual portion of soil Cd, respectively.
The solubility product of Cd was estimated from each experimental unit by toxicity characteristic leaching procedure (TCLP) method USEPA 1311 (USEPA 1992). Briefly, 1.0 g of ground soil was extracted with 20 ml of glacial acetic acid solution (pH 2.88 ± 0.02) for 18 h. For the bioavailable Cd analysis, soil samples were extracted with 1 M NH4NO3 to analyze the exchangeable metal fraction as described by Schlichting et al. (1995). After extraction and filtration, the supernatant was analyzed for its Cd concentration through AAS.
For the batch sorption equilibrium study, triplicate soil samples of 1.0 g were weighed into 50 mL polythene bottles. Each bottle also contained 25 ml of a Cd (NO3)2 test solution and with concentrations ranging from 1 to 10 mg L−1. A background electrolyte of 0.01 M NaNO3 was used in the metal solution to retain ionic strength.
The pH of the solution was adjusted to 5.0 by HCl and NaOH. The mixture of soil and metal suspension was shaken on an orbital shaker for 24 h at 120 rpm and centrifuged at 4500 rpm. To determine the capacities of soil to adsorb Cd the Langmuir and Freundlich models were fitted to the data. Soil samples with and without amendments were also used to determine the zeta potential of the soil using Jiang et al. (2008) method.
The results of the soil heavy metal extraction techniques were expressed as standard deviation (SD) and means. One way analysis of variance (ANOVA) and LSD tests (p < 0.05) were used to analyze the data. All statistical analyses were performed using Microsoft Office Excel 2013.
Results and Discussion
The efficiency of stabilizers on soil pH after 120 days incubation is presented in Fig. 1. The soil pH increased significantly (p < 0.05) with the addition of various stabilizers. The soil pH also increased markedly with the increasing application rate of BC, and RP into the Cd-contaminated soil. The soil pH increased from 5.3 to 6.6, 5.6 and 6.8, when soil was incorporated with BC, ZE and RP at the 3% application rate, respectively. According to the findings obtained; BC, ZE and RP significantly increased the soil pH due to their liming effects and high alkalinity (Lu et al. 2014; Zhu et al. 2008; Bashir et al. 2018). During pyrolysis of these organic substances at elevated temperatures, the surface of biochar generates hydroxides, carbonates and a wide variety of functional groups and its high mineral ash contents cause a liming effect which might induce the increase in soil pH (Yuan et al. 2011). Similarly, RP has low surface area but has a high pH and Ca+ concentration due to the presence of higher concentrations of calcium carbonate and phosphate ions in the RP, which could increase the soil pH and promote metal phosphate through precipitation (Zhu et al. 2008).
The distribution of Cd in different fractions varies among all the amendments as shown in Fig. 2. The exchangeable proportion of Cd reduced with the increased addition of BC, RP and ZE stabilizers from the 0% (CK) to 1.5% and 3% application rate. The greater reduction in the acid-soluble portion of soil Cd was 29%, 13% and 14% for BC, ZE and RP incorporation, respectively at the 3% rate, when compared to the control. According to the results, we suggest that the greater surface area, high CEC and pH values and the functional groups on the surface (Fig. 5) of BC, ZE and RP may have the ability to reduce Cd toxicity in contaminated soil as well as increase its stabilization. These results are in accordance with the observations by Bashir et al. (2018a), who suggested that the Cd-soluble form reduced with the increase in soil pH after biochar incorporation. A study by Park et al. (2011) indicated that the application of green-waste derived biochar decreased the acid soluble Cd fraction from 84.2% to 63.1%. Similarly, this reduction might be due to the dissociation of calcium carbonate and phosphate complexes on the RP surface that may take part to increase Cd stabilization through precipitation. It was shown that the addition of RP into acidic soil causes a significant reduction in the acid-soluble portion of Cd, which might be due to the high levels of CaCO3, which may dissociate and induce metal immobilization (Zhu et al. 2008). Chemical immobilization by zeolite is widely understood to reduce exchangeable metal concentration in contaminated soil, due to its adsorption on the lattice of tectosilicate (Oste et al. 2002).
The maximum stabilization of Cd was shifted to the reducible proportion as shown in Fig. 2. The proportion of reducible Cd was greater than that of other three phases under the same situations, which was in accordance with Mohamed et al. (2015) who reported that the application of BC at a 1.5% rate led to a significant decline, 2.91 to 2.38 mg kg−1 in the acid-soluble fraction and enhanced the reducible fraction, which was 1.70–2.33 times lower than 5 mg Cd kg−1. The addition of all soil stabilizers also augmented the residual fraction of Cd in the soil. Our results are in line with Park et al. (2011), Mehmood et al. (2017) and Zhu et al. (2008), who reported that hydrolysis and dissolution of these alkaline substance could increase soil pH and induce precipitation of Cd as CdCO3 and Cd3 (PO4)2, which could increase residual Cd portion in soil. The addition of BC, RP and ZE in Cd-polluted soil followed the following order in enhancing the residual Cd fraction: BC > RP > ZE > control.
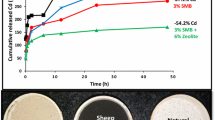
These results showed that the Cd concentration in the TCLP-extract, for treated and untreated soil was lower than the permissible limit (< 1 mg L−1) (USEPA 1992). As shown in Fig. 3 the concentration of Cd in the TCLP extract was significantly (p < 0.05) decreased by 31.69%, 14% and 19.05% with BC, ZE and RP addition, respectively at the 3% application rate. Similarly, the addition of BC, ZE, and RP at 3% application rate played a significant role in decreasing the ammonium nitrate extractable Cd by 78%, 20% and 76%, respectively, as compared to control. In the light of our results, we could suggest that this reduction might occur due to alkaline nature of all amendments. Moreover, the high alkalinity, Si contents, and oxygen-containing functional groups on rice straw-derived biochar might be a strong reason for Cd immobilization in highly polluted soil (Bashir et al. 2018). In present study, addition of RP potentially reduced Cd leachability in TCLP extraction through adsorption and precipitation. These results aligned with those of Chen et al. (2007), who observed that hydroxyapatite and RP amendments resulted in a significant decline in the solubility of heavy metals and enhanced their geochemical stability in polluted soil. Similarly, another previous study, Chen et al. (2000), concluded that zeolite application reduced Cd extractability from soil due to the high CEC and greater surface area.
The Cd adsorption isotherm in non-amended soil and BC-, ZE-, and RP-amended soil were studied, and the results of the adsorption isotherm are shown in Fig. 4a and their equation parameters were given in Table 1. The coefficients of both equations along with R2 and adsorption capacity (Q m ) in Table 1, clearly indicated that Langmuir equation was a better fit (R2 = 0.89–0.99) for our data when compared to the Freundlich equation (R2 = 0.86–0.99) under all amended soils. According to our results, the Cd adsorption capacity increased considerably with the increasing rate of all amendments. Particularly, the maximum Qm of BC-soil was greater (8.38–19.85 mg g−1) as compared to the ZE-soil (1.20–2.42 mg g−1) and the RP-soil (8.36–10.15 mg g−1) and the non-amended soil (1.05 mg g−1).
We propose that Cd (II) adsorption by BC, ZE, and RP-amended soil is greater when compared to the control soil, which might indicate that the amended soil adsorption is chemisorption based. According to our study, there is a higher adsorption capacity in the biochar-amended soil, which might be due to the presence its many functional groups. Biochar has a high CEC, a micro porous structure, and oxygen-containing organic functional groups; and to some extent, it has a lower zeta potential (greater negativity) (Figs. 4b and 6), which plays a key role in the specific adsorption of heavy metals (Jiang et al. 2012; Bashir et al. 2018b). However, based on our FTIR results we suggest that Cd adsorption increases in the soil following the addition of stabilizers. Compared to the control, all amendments performed significantly better than the control but the BC-amended soil showed the greatest adsorption capacity due to the presence of several functional groups on its surface (Figs. 5 and 6).
The effects of three stabilizers (biochar, zeolite and rock phosphate) on Cd immobilization in polluted soil were observed using BCR, TCLP, and NH4NO3 extraction techniques. Among all the amendments applied, RP was the most efficient at increasing the soil pH. Increasing the BC application rates was considered to be a more efficient method to stabilize Cd in soil because BC amendments resulted in a large reduction in the acid-soluble Cd proportion, and induced a significant increase in the more stable residual fraction. Biochar also has potential to decrease the leachability and availability of Cd in soil. Moreover, the maximum Cd sorption capacity observed from BC-amended soil was from 8.38 to 19.08 mg g−1, the highest value among all the amendments. Overall, the order of stabilizers on the stabilization of Cd in polluted soil was therefore; BC > RP > ZE. Based on our results the addition of biochar to Cd-polluted soil could be more effective in enhancing the percentage of Cd removal from polluted soil under acidic conditions. Furthermore, the immobilization of Cd by these applied amendments in naturally Cd-polluted soils needed to be evaluated under field conditions.
References
Adriano DC (2001) Trace elements in terrestrial environments, 2nd edn. Springer, New York
Ahmad M, Lee SS, Lim JE, Lee S, Cho JS, Deok HM, Hashimoto Y, Ok YS (2014) Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere 95:433–441
Bashir S, Hussain Q, Akmal M, Riaz M, Hu HQ, Ijaz SS, Iqbal M, Abro S, Mehmood S, Ahmad M (2017) Sugarcane bagasse-derived biochar reduces the cadmium and chromium bioavailability to mash bean and enhances the microbial activity in contaminated soil. J Soils Sediments. https://doi.org/10.1007/s11368-017-1796-z
Bashir S, Zhu J, Fu Q, Hu HQ (2018) Cadmium mobility, uptake and anti-oxidative response of water spinach (Ipomoea aquatic) under rice straw biochar, zeolite and rock phosphate as amendments. Chemosphere 194:579–587
Chen HM, Zheng CR, Tu C, Shen ZG (2000) Chemical methods and phytoremediation of soil contaminated with heavy metals. Chemosphere 41:229–234
Chen SB, Xu MG, Ma YB, Yang JC (2007) Evaluation of different phosphate amendments on availability of metals in contaminated soil. Ecotoxicol Environ Saf 67:278–285
China Environmental Quality Standard for Soil (GB15618-1995) (1995) China National Standardization Management Committee, China, Beijing
Jiang J, Xu RK, Wang Y, Zhao AZ (2008) The mechanism of chromate sorption by three variable charge soils. Chemosphere 71:1469–1475
Jiang TY, Jiang J, Xu RK, Li Z (2012) Adsorption of Pb(II) on variable charge soils amended with rice-straw derived biochar. Chemosphere 89(3):249–256
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836
Lu RK (1999) Analytical methods for soil agrochemistry. Chinese Agricultural Science and Technology Publishing House, Beijing (Chinese)
Lu K, Yang X, Shen J, Robinson B, Huang H, Liu D, Bolan N, Pei J, Wang H (2014) Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agric Ecosyst Environ 191:124–132
Mehmood S, Rizwan M, Bashir S, Ditta A, Aziz O, Yong LZ, Dai Z, Akmal M, Ahmed W, Adeel M, Imtiaz M, Tu S (2017) Comparative effects of biochar, slag and ferrous–Mn ore on lead and cadmium immobilization in soil. Bull Environ Contam Toxicol. https://doi.org/10.1007/s00128-017-2222-3
Mignardi S, Corami A, Ferrini V (2012) Evaluation of the effectiveness of phosphate treatment for the remediation of mine waste soils contaminated with Cd, Cu, Pb, and Zn. Chemosphere 86:354–360
Mohamed I, Zhang G, Li Z, Liu Y, Chen F, Dai K (2015) Ecological restoration of an acidic Cd contaminated soil using bamboo biochar application. Ecol Eng 84:67–76
Oste LA, Lexmond TM, Van Riemsdijk WH (2002) Metal immobilization in soils using synthetic zeolites. J Environ Qual 31:813–821
Park JH, Choppala GK, Bolan NS, Chung JW, Chuasavathi T (2011) Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348:439–451
Rauret G, Lopez-Sanchez JF, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauviller P (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monitor 1:57–61
Schlichting E, Blume HP, Stahr K (1995) Bodenkundliches Parktikum. Blackwell, Berlin
USEPA (1992) EPA method 1311. TCLP-toxicity characteristic leaching procedure. In: Test methods for evaluating solid waste, 3rd edn. Environmental Protection Agency, Washington
Usman ARA, Kuzyakov Y, Lorenz K, Stahr (2006) Remediation of a soil contaminated with heavy metals by immobilizing compounds. J Plant Nutr Soil Sci 169:205–212
Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B (2013) Removal of Cu, Zn, and Cd fromaqueous solutions by the dairymanure-derived biochar. Environ Sci Pollut Res 20:358–368
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497
Zhu R, Yu R, Yao J, Mao D, Xing C, Wang D (2008) Removal of Cd2+ from aqueous solutions by hydroxyapatite. Catal Today 139:94–99
Acknowledgements
The study was financially supported by National Sci-Tech Support Plan of China (2015BAD05B02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bashir, S., Rizwan, M.S., Salam, A. et al. Cadmium Immobilization Potential of Rice Straw-Derived Biochar, Zeolite and Rock Phosphate: Extraction Techniques and Adsorption Mechanism. Bull Environ Contam Toxicol 100, 727–732 (2018). https://doi.org/10.1007/s00128-018-2310-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-018-2310-z