Abstract
Volatile organic compounds (VOCs) play a central role in atmospheric chemistry. In this work, VOCs in the Botanical Garden of Rio de Janeiro were determined using the TO-15 Method. The park occupies 1,370,000 m2 in the southern area of the city and is next to the Tijuca Forest, which is considered the largest secondary urban forest in the world. The total VOC concentrations ranged from 43.52 to 168.75 µg m−3, depending on the sampling site and dates. In terms of concentration isoprene represented 4 %–14 % of the total VOC masses. The results suggested that the differences in biomass, distance from the street and activities within the park affected the concentrations of VOCs. The ratios of isoprene/aromatic compounds were higher than those determined in other areas of the city, confirming that the atmosphere of this green area has the contribution of other sources. Kinetic and mechanistic reactivities were also evaluated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Volatile organic compounds (VOCs) play a central role in tropospheric chemistry and photochemical air pollution (Atkinson 2000). Urban forests, parks, botanical gardens and nature trees can affect the local and regional air quality by removing atmospheric pollutants, lowering temperatures, changing wind patterns and modifying the boundary layer height (Escovedo and Nowak 2009). Trees absorb gaseous pollutants such as sulfur oxides, nitrogen dioxide and ozone through leaf stomata and can dissolve water-soluble pollutants onto moist leaf surfaces (Nowak 1994). Tree canopies can also intercept particulate matter in the air (Beckett et al. 1998). The uptake and biotransformation of aromatic compounds from the soil and atmosphere have been studied in a number of plants (Nelson and Allard 2012), and there is evidence that aromatic hydrocarbons can be metabolized by plants, but the fate of the metabolites is unknown (Cape 2003). For example, experiments using labeled (14C) aromatic hydrocarbons found that benzene and toluene were assimilated by plants, the aromatic ring of these molecules was cleaved during their metabolic transformation, and their carbon atoms were mainly incorporated into nonvolatile organic acids (Ugrekhelidze et al. 1997). Plants may also have detrimental effects on air quality because of the emission of biogenic volatile organic compounds (BVOCs) (Geng et al. 2011). Many factors influence biogenic compound emissions, such as light and temperature, and the atmospheric concentrations depend on emission factors, meteorology, deposition rates and transportation (Kesselmeier and Staudt 1999).
The Amazon rainforest has been extensively studied over the last 20 years (Bela et al. 2015). Conversely, the Mata Atlantica forest, specifically the Tijuca rainforest, which is considered the largest secondary urban forest in the world, has received little attention (Custodio et al. 2010). The Tijuca Forest divides the city of Rio de Janeiro into northern and southern sections. The Botanical Garden of Rio de Janeiro (Jardim Botânico do Rio de Janeiro, JBRJ) is located in the southern part of the city, at the foot of Corcovado Mountain. The eastern part of the Botanical Garden is near the Tijuca Forest, and 830,000 m2 of the park are occupied by a secondary urban rainforest. The other 540,000 m2 are cultivated and contain more than 9000 plants from 1500 different species from Brazil (Mata Atlantica, Cerrado and Amazonian Forest) and other parts of the world. In 1992, UNESCO designated the park as a biosphere reserve. The southern, northern and western parts of the park are surrounded by the city, Jardim Botanico Avenue, which has heavy car and bus traffic, and Pacheco Leão Street, which has moderate traffic.
In this work, the concentrations of VOCs in the Botanical Garden were determined to get a preliminary description of the main characteristics of this green area. Kinetic and mechanistic reactivities were also evaluated for isoprene, alkanes, alkenes and aromatic compounds.
Materials and Methods
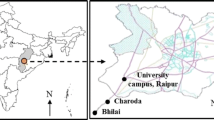
VOCs were sampled and analyzed using Method TO-15 (US EPA 2016). Twenty-four samples were collected at a sampling height of 1.0 m using 6 L stainless steel canisters (Restek Silonite®), with TOV-2™ valves and a flux restrictor to meet a sampling time of 60 min. Samples were collected in the Botanical Garden (22°58′03″S and 43°13′26″W), between 10:00 AM and 2:00 PM on March 28, 2015, May 4, 2015 and May 15, 2015. Of the 24 samples, 20 were collected from Sector 36 (Bromeliad Sector), where 530 species of bromeliads native to South and Central America and species from the subtropical “restinga” were cultivated (some medium-sized trees and shrubs adapted to the drier and nutrient-poor conditions). This sector was approximately 50 m from the service entrance on Pacheco Leão Street, near an internal parking area and near some residences used by the park employees. There also were three greenhouses with bromeliads and orchids and a lake with Victoria lilies. The other 4 samples were collected near the Avenue of Royal Palms (Sector 6, Royal Palms Sector), which was a 750-m line of 134 imperial palms leading from the main entrance of the park to the gardens. The sampling locations were approximately 150 m from the main entrance. A map of the Botanical Garden showing the sampling locations is presented in Fig. 1. Temperature, wind speed and relative humidity data were obtained from a manual instrument (Instrutherm, Model Thal 300, São Paulo, SP, Brazil) at each sampling location. A meteorological station is also installed inside the park.
The canisters were cleaned following the procedures outlined in Method TO-15 (US EPA 2016) using a cleaning system (Teledyne Hasting Instruments, model Omega CN9000A, CA, USA). Briefly, all canisters were evacuated to 500 m Torr at 120°C and maintained in vacuum for 60 min. Then, the canisters were filled with humidified He (50 % relative humidity) to 30 psig. This cycle was completed three more times for a total of four cycles; then, four additional cycles were completed with dry He. Blank samples were generated by pressurizing the clean canisters with He. The canisters were considered clean if less than 0.2 ng of each target compound was detected. Then, the canisters were evacuated below 5 mTorr. A flux restrictor was used to achieve a sampling time of 60 min.
The canister samples were analyzed within 3 days of sampling on a gas chromatograph with a thermal desorption and mass spectrometer detection (GC/MS/TD) system (Agilent, model GC 7890A, MS 5975C, CA, USA and Markes CIA Advanced, OH, USA) according to the TO-15 Method. Typically, 100-500 mL of the air sample was directly extracted from the canister at a flow rate of 20 mL min−1 through a Nafion dryer trap to reduce the water vapor content. The dry samples passed through a cold trap that contained carbon molecular sieves (Markes U-T3ATX-2S) at −10°C (trap hold: 180 s) to retain the VOCs. The VOCs were thermally desorbed (300°C, 60 s, desorbing flow: 50 mL min−1) and transferred to a DB-624 gas chromatographic column for separation (60 m × 0.32 mm × 1.80 μm). He 5.0 was used as the carrier gas at a constant flow of 3.5 mL min−1. The oven temperature program was set as follows: 25°C for 5 min, 25–50°C at 0.8°C min−1, 50–250°C at 5°C min−1 and 250°C for 3 min. The injector temperature was 190°C. Each VOC was identified using selective ion monitoring (SIM) of the most abundant ions. Quantification was performed based on an external analytical curve using a standard reference mixture that covered the entire concentration range of the ambient samples (57 compounds, Restek, 20–60 ppbC, p/n 34445, Bellefonte, PA, USA). The calculated determination coefficients for all of the VOCs were >0.99. All samples were measured in triplicate, and a difference of <25 % was considered acceptable, as stated in the TO-15 Method. The limits of detection (LOD) and quantification (LOQ), which were calculated from the noise standard deviation, were ≥ 0.2 and ≥ 0.6 ng, respectively, for all of the compounds. All sampling and analysis procedures, including the stability of the samples during storage, were verified to ensure that the quality criteria of US EPA Method TO-15 US were fulfilled.
Results and Discussion
Every sampling day was sunny, with temperatures between 24.0 and 32.4°C during sampling times. We also determine wind direction and speed during the sampling. Wind speed was, in general, lower than 1 km h−1 within the park. A total of 52 C4–C12 VOCs were analyzed but only 43 compounds were found at concentrations above the LOQ in all of the samples. The mean concentrations, standard deviations, and number of samples for the main compounds at each location, as well as the temperature and relative humidity at the sampling locations, are presented in Table 1. The selected compounds were the 11 most abundant species, xylenes and ethylbenzene. The mean concentrations of total VOCs and isoprene ranged from 43.52 to 168.75 and 3.16 to 11.27 µg m−3, respectively.
The concentrations in the Royal Palms Sector were lower than those in the Bromeliad Sector. This result may be related to the fact that the Royal Palms Sector is farther from the vehicular circulation area than the Bromeliad Sector is. Additionally, as previously stated, many activities related to research and park maintenance occurred in the Bromeliad Sector. The concentrations determined in the samples collected on May 4 were approximately twice those obtained on March 28 and May 15. On May 4, a Monday, the park was not open to visitors because several maintenance activities were occurring, such as cutting the grass, pruning the trees and shrubs, cleaning the soil and watering. Additionally, some vehicles circulated around the park to transport the machines and tools used for landscape maintenance.
To assess these differences, individual data for all samples were analyzed using non-parametric tests to verify whether the null hypothesis that all populations have identical distribution functions was valid. Using a Kruskal–Wallis non-parametric ANOVA, the calculated p value for the samples collected in the Bromeliad Sector on March 28 and May 15 was 0.221, indicating that there was no reason to conclude that the sample distributions differed. When the samples collected on May 4 were also considered, the p value was 0.068. When the samples collected on March 28 and May 15 were considered to be the same group and were compared to the samples collected on May 4, using a Mann–Whitney U test for two groups of independent non-parametric samples, the p values for all of the variables were <0.05. It is commonly accepted that the null hypothesis can be rejected for p values <0.05, but cutoff values of 0.10 may also be adopted. For the present data, both tests indicated that the data contained moderate evidence against the null hypothesis and suggested that there were some differences between the samples collected in the Bromeliad Sector on May 4 and the other 2 days. When the samples collected at the Royal Palms Sector were included in the analyses, the p value, as calculated using a Kruskal–Wallis non-parametric ANOVA, was 0.023, thus confirming that the null hypothesis was not valid. These results confirmed the previous discussion and suggested that the Bromeliad and the Royal Palms Sectors were different because of the atmospheric composition and that the maintenance activities in the park affected the emissions of anthropogenic and biogenic pollutants.
The values determined for the aromatic compounds in the Botanical Garden were similar to those obtained in 2007–2008 by Godoi et al. (2010). The authors used a passive method and found BTEX (benzene, toluene, xylenes and ethylbenzene) in both an urban park (39,000 m2) and an urban square in the city the Curitiba (Brazil). The authors found that the average benzene concentrations varied from 3.9 to 6.1 µg m−3, with higher values occurring in sparse vegetation areas. For toluene, average concentrations ranged from 6.5 to 7.2 µg m−3. The BTEX concentrations obtained by Custodio et al. (2010), using coconut shell cartridges to collect the samples in the Tijuca Forest, were lower than 2.5 and 1.85 µg m−3 for benzene and toluene, respectively, in all of the samples. The xylene and ethylbenzene concentrations were below the LOD. The authors concluded that the forest acted as a sink for many pollutants.
The toluene/benzene, isoprene/benzene, isoprene/toluene and isoprene/xylenes ratios were also calculated using the mean concentrations (µg m−3) for each sampling location. The values obtained in this work and the literature values are shown in Table 2. The toluene/benzene ratios were lower than 2.7, which was a typical result for an urban area where the main emissions were from a vehicular fleet (Brocco et al. 1997; Godoi et al. 2010). Isoprene/aromatic compound ratios were not determined for the Tijuca Forest and Curitiba. The values obtained in several streets of the northern area of the city of Rio de Janeiro were at least 10 times lower due to the lower concentrations of isoprene and the higher concentrations of BTEX in these areas (Martins et al. 2015). The areas studied by Martins et al. (2015) had high vehicular emissions and scarce vegetation.
The kinetic reactivities were evaluated using the literature values for the rate coefficient (kOH). The target VOC reacted with ·OH radicals, resulting in a 12-h daytime average concentration of the ·OH radical (2.0 × 106 mol cm−3). These values indicated the reaction rate and were calculated as the product of the target VOC concentration, kOH and the ·OH radical concentration. Relative kinetic reactivities were calculated as the ratio of the rate for the target VOCs and the sum of the rate for the studied compounds. The mechanistic reactivity was evaluated in terms of the incremental reactivity (IR), which is defined as the number of ozone molecules that are formed per additional VOC carbon atom to an initial atmospheric reaction mixture of VOCs and NOx. The maximum incremental reactivity (MIR) coefficients that Carter (2016) proposed, in units of grams of formed O3 per gram of added VOC, were used. In this work, the mechanistic reactivity was calculated as the product of the concentration (µg m−3) and the MIR coefficient (adimensional). Relative values were also calculated. Then, the compounds were classified into three groups (alkanes, alkenes and aromatic compounds). Figure 2 shows the mass abundance, kinetic and mechanistic reactivities of isoprene, alkanes, alkenes and aromatic compounds.
The contribution of isoprene to mass abundance and to the atmospheric reactivity was approximately two times higher in the Royal Palms Sector. Table 1 and Fig. 2 suggest that the differences in biomass, distance from the street and activities within the park clearly affect the concentration of isoprene. Because the lifetime of isoprene is approximately 1.5 h, its concentrations depend both on emission factors and on further reactions with ·OH radicals and ozone. The isoprene emission factors and reaction rates depend on temperature and light, which are different within the Botanical Garden, depending on the distribution of the species. The emission factors for isoprene and other BVOCs also depend on the plant genus and species and show great variability. Furthermore, nutrient deficiencies, wounding, and air pollution must be considered potential triggers for both physiological changes and trace gas emissions (Kesselmeier and Staudt 1999). The VOC concentration variability found in this study suggested that the heterogeneous landscape of the Botanical Garden’s arboretum not only reflected the interventions by men to turn the institution into a center of botanic dissemination and conservationism but also had consequential interactions on the park from the urban atmosphere and the potential to reduce the air pollution or, eventually, to form ozone and other secondary pollutants.
The Botanical Garden has significant value for the aesthetic and landscape qualities of the city of Rio de Janeiro. It also allows scientific investigations emphasizing the flora, aiming at conservation and recognizing the importance of biodiversity. Although there is a limited number of results, the results could be considered a starting point for a deeper understanding of urban green areas. More data are needed in order to perform a complete statistical analysis and to get a complete picture of any possible seasonal variations. The results obtained in this study show that an extensive monitoring campaign may clarify the role of the Botanical Garden in the air quality of the city, both as a source of isoprene and other terpenes, which could participate in the formation and removal of ozone, and also as a potential pollutant remover.
References
Atkinson R (2000) Atmospheric chemistry of VOCs and NOx. Atmos Environ 34:2063–2101
Beckett K, Freer-Smith P, Taylor G (1998) Urban woodlands: their role in reducing the effects of particulate pollution. Environ Pollut 99:347–360
Bela MM, Longo KM, Freitas SR, Moreira DS, Beck V, Wofsy SC, Gerbig C, Wiedemann K, Andreae MO, Artaxo P (2015) Ozone production and transport over the Amazon Basin during the dry-to-wet and wet-to-dry transition seasons. Atmos Chem Phys 15:757–782
Brocco D, Fratarcangeli R, Lepore L, Petricca M, Ventrone I (1997) Determination of aromatic hydrocarbons in urban air of Rome. Atmos Environ 31:557–566
Cape JN (2003) Effects of airborne volatile organic compounds on plants. Environ Pollut 122:145–157
Carter WPL (2016) http://www.engr.ucr.edu/~carter/SAPRC/. Accessed in 2016
Custodio D, Guimarães CS, Varandas L, Arbilla G (2010) Pattern of volatile aldehydes and aromatic hydrocarbons in the largest urban rainforest in the Americas. Chemosphere 79:1064–1069
Escovedo FJ, Nowak DJ (2009) Spatial heterogeneity and air pollution removal by an urban forest. Landsc Urban Plan 90:102–110
Geng F, Tie X, Guenther A, Li G, Cao J, Harley P (2011) Effect of isoprene emissions from major forests on ozone formation in the city of Shanghai, China. Atmos Chem Phys 11:10449–10459
Godoi AFL, Godoi RHM, de Azevedo R, Maranho LT (2010) Pollution and density of vegetation: BTEX in some public areas of Curitiba-PR, Brazil. Quim Nova 33:827–833
Kesselmeier J, Staudt M (1999) Biogenic organic compounds (VOC): an overview on emission, physiology and ecology. J Atmos Chem 33:23–88
Martins EM, Nunes ACL, Correa SM (2015) Understanding ozone concentrations during weekdays and weekends in the urban area of the city of Rio de Janeiro. J Braz Chem Soc 26:1967–1975
Nelson AH, Allard AS (2012) Organic chemicals in the environment. Mechanism of degradation and transformation. CRC Press, Boca Raton
Nowak DJ (1994) Air pollution removal by Chicago’s urban forest. In: McPherson EG, Nowak DJ, Rowntree RA (eds) Chicago’s urban forest ecosystem: results of the Chicago Urban forest climate project. USDA Forest Service, Northeastern Forest Experimental Station, Radnor, PA
Ugrekhelidze D, Korte F, Kvesitadze G (1997) Uptake and transformation of benzene and toluene by plant leaves. Ecotoxicol Environ Saf 37:24–29
US EPA (2016) https://www3.epa.gov/ttnamti1/airtox.html. Accessed in 2016
Acknowledgments
The study was funded in part by FAPERJ, CNPq, CAPES and CENPES/Petrobras. The authors acknowledge the collaboration of the employees and researchers of the Botanical Garden, mainly Claudison Rodrigues de Vasconcelos and Thais Moreira Hidalgo de Almeida.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, C.M., Souza, E.C.C.A., da Silva, L.L. et al. Volatile Organic Compounds in the Atmosphere of the Botanical Garden of the City of Rio de Janeiro: A Preliminary Study. Bull Environ Contam Toxicol 97, 653–658 (2016). https://doi.org/10.1007/s00128-016-1887-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1887-3