Abstract
The organochlorine insecticide aldrin is commonly used in intensive agriculture, and demonstrates estrogenic activity. Rotifers such as Brachionus calyciflorus are favored test animals in aquatic toxicology because of their more sensitivity to most toxicants. In the tested concentration range of 0.04–1.28 mg/L, aldrin shortened significantly the durations of embryonic development. Lower concentrations of aldrin had an intriguing effect on the reproduction of the rotifers and are beneficial to their survival. Different endpoints of both development and reproduction had different sensitivity to aldrin. The reproductive endpoint of the rotifers is more sensitive to aldrin than the developmental endpoint.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The organochlorine insecticide aldrin is commonly used to kill soil pests, preserve wood and treat seeds, although it has been banned in technologically advanced countries. Aldrin can persist in the environment, and is susceptible to bio-magnification. Aldrin is readily absorbed into the circulating blood from the gastrointestinal tract, through the skin or by inhalation, and is rapidly converted to dieldrin through a mixed function monooxygenase-dependent pathway (aldrin epoxidase) (Hayes 1982). Chronic exposure of animals and human beings to aldrin or dieldrin has resulted in dose-related hepatomegaly and histological changes (Jager 1970; Edwards and Priestly 1994; Hφyer 1998). Aldrin demonstrates estrogenic activity by a series of assays such as increase in the weights of uteri in immature and ovariectomized mature rats and binding capacity to recombinant human steroid receptors (Chatterjee et al. 1992; Scippo et al. 2004). Like other organochlorine insecticides, aldrin can enter into aquatic environment by direct or indirect routes. Thus, it is important to evaluate the effect of aldrin on aquatic animals.
Zooplankton is frequently used to detect anthropogenic contamination because of their sensitivity to various toxicants and their important role in the ecosystem. Among the zooplanktons, rotifers, especially Brachionus calyciflorus and B. plicatilis, are favored test animals in aquatic toxicology because of their global distribution, small size, simple life cycle, rapid reproduction, short generation time, more sensitivity to most toxicants, simplicity of culture and the commercial availability of resting eggs (Snell and Moffat 1992; Janssen et al. 1993; Snell and Janssen 1995). As test animals, rotifers have been extensively used to monitor the acute and chronic toxicities of heavy metals, pesticides and other pollutants (Snell and Janssen 1995). By chronic toxicity tests, some researchers investigated the effects of toxicants on the survival and reproduction of rotifers, but few researchers dealt with the effects on the development, including embryonic and juvenile developments of rotifers (Xi and Hu 2003; Chu et al. 2005; Xu et al. 2005; Huang et al. 2006; Zha et al. 2007).
The main purpose of the present study was to assess the effects of different concentrations of aldrin on the development, survival and reproduction of freshwater rotifer B. calyciflorus, and detect the relative sensitivity of developmental and reproductive endpoints to aldrin exposure.
Materials and Methods
The rotifer B. calyciflorus used in this experiment was obtained by hatching resting eggs collected from sediments of Lake Jinghu (31°33′N, 118°37′E) and then clonally culturing under controlled laboratory conditions. Stock rotifer cultures had been kept under static-renewal conditions with a 16:8 h light:dark photoperiod at 130 l x at (25 ± 1)°C in an illumination incubator for over 2 months, and with the rotifer culture medium of Gilbert (1963) and the green alga Scenedesmus obliquus as food. Before the experiments commenced, rotifers were cultured in EPA medium (USEPA 1985) and fed on 3.0 × 106 cells/mL of S. obliquus for at least 2 weeks. Algae were grown in a semi-continuous culture using HB-4 medium (Li et al. 1959) renewed daily at 20%. Algae in exponential growth were centrifuged and resuspended in EPA medium.
The pesticide aldrin (standard grade, 99.7%; Supelco company, USA; product number: LB17243) was used as the toxicant. Stock solution of 1,000 mg/L was prepared by dissolving aldrin in 100% acetone, then diluted to the desired concentrations using EPA medium.
In order to choose appropriate toxicant concentrations for life-table experiments, six concentrations of aldrin (0.6, 0.8, 1.0, 1.2, 1.4, and 1.6 mg/L) with a control and a solvent control (containing 0.16% acetone) were used in the acute toxicity test. Each treatment had four replicates. Rotifers with amictic eggs were randomly removed from the stock rotifer cultures (rotifer populations in exponential phases at densities of 50–80 ind./mL and mixis rates of 2%–5%) and placed into a glass dish containing 10 mL of EPA medium with 3.0 × 106 cells/mL of S. obliquus. After 2 h, ten neonates (<2 h old) for each replicate were collected and transferred into a 5-mL glass cup containing 2.5 mL of test solution with 3.0 × 106 cells/mL of S. obliquus. After 24 h, the number of rotifers alive was counted for each cup. The LC50-value was derived following the probit method (Finley 1971).
Based on the LC50-value, we selected seven toxicant concentrations (0.02, 0.04, 0.08, 0.16, 0.32, 0.64, and 1.28 mg/L), a control and a solvent control (containing 0.128% acetone) for the life-table experiments, each treatment consisting of four replicates of ten rotifers. Life-table experiments were conducted in 24-well tissue culture plates and started by introducing one neonate (<2 h old) into each well which contained 0.5 mL test solution with 3.0 × 106 cells/mL of S. obliquus. The rotifers were checked every 3 h during the initial 48 h, and the time of the first egg and neonate produced was recorded. Thereafter, the number of eggs and neonates produced and the number of original test individuals alive were recorded and then neonates were eliminated every 8 h. The original rotifers alive were transferred into freshly prepared test solution every 24 h. The life-table experiments were conducted in darkness at 25 ± 1°C until each individual of every cohort died.
Based on the data collected, the durations of embryonic development, juvenile period, reproductive period and post-reproductive period, and mean lifespan of the rotifers were calculated. Survivorship and fecundity were constructed for each cohort using conventional life-table techniques (Poole 1974), and intrinsic rate of population increase, net reproductive rate, generation time and life expectancy at hatching of the rotifers were calculated according to Krebs (1985) and Lotka (1913).
One-way analysis of variance (ANOVA), with the concentration of aldrin as the independent variable, and each of the durations of principal developmental periods, the mean lifespan and the life-table demographic parameters as the dependent variable, followed by Dunnett’s test was conducted for pair-wise comparisons of each concentration of test chemicals and the solvent control relative to the control (Zar 1999).
Results and Discussion
The durations of principal development stages except post-reproductive stage, and the mean lifespan of the rotifers were significantly influenced by aldrin (one-way ANOVA, p < 0.05). Compared to the controls, acetone at 0.128% did not markedly influenced the duration of embryonic development of rotifers. However, aldrin at 0.04–1.28 mg/L shortened the duration of embryonic development of rotifers by as much as 18.9%–28.7% (Table 1), which was identical to the effects of dieldrin at 0.01, 0.1 and 10.0 μg/L, 17β-estradiol at 0.001–0.1 μg/L and 10.0 μg/L, and chlordecone at 5.0 and 50.0 μg/L (Huang et al. 2006; Zha et al. 2007). Because the algal quality does not affect the duration of embryonic development of the rotifers (King 1967; Xi and Huang 1999), aldrin at 0.04–1.28 mg/L may directly affect the embryonic development of the rotifers. Interestingly, dieldrin, chlordecone and aldrin all have estrogenic activity (Chatterjee et al. 1992; Scippo et al. 2004). However, whether their significant effects on the duration of embryonic development of rotifers are attributed to their endocrine disrupting activity or structure similarity needs further researching, because information on the endocrinology of rotifer reproduction is still scarce.
Aldrin at 0.16–1.28 mg/L prolonged the duration of juvenile period of rotifers by 7.2%–26.6% (Table 1), which was similar to the effects of 17β-estradiol at 0.01, 0.1 and 10.0 μg/L, and chlordecone at 5.0 and 50.0 μg/L (Huang et al. 2006; Zha et al. 2007). Aldrin at 0.16–1.28 mg/L may directly affect the duration of juvenile period of the rotifers, or/and indirectly affect it by altering the algal quality, because the algal quality affects the duration of juvenile period of the rotifers (Xi and Huang 1999; Xi et al. 2001).
Aldrin at 0.04–0.16 mg/L prolonged the duration of reproductive period of rotifers by as much as 21.3%–26.3% (Table 1), which was similar to the effects of dieldrin at 0.001–100.0 μg/L, 17β-estradiol at 0.001, 0.01, 1.0, 100.0 and 1,000.0 μg/L, and chlordecone at 50.0 μg/L (Huang et al. 2006; Zha et al. 2007). However, aldrin at 1.28 mg/L shortened the duration of reproductive period of rotifers by 24.8% (Table 1), identical to the effect of deltamethrin at 2.4 and 3.0 mg/L (Xu et al. 2005). The above stated results indicate that lower concentrations of pollutants with endocrine disrupting activity might have an intriguing effect on the lengthening of reproductive period, but higher concentrations of them might have a toxic effect, or the toxic effect might be higher than the intriguing effect.
Aldrin did not influence the duration of post-reproductive period of the rotifers (Table 1), identical to the effect of glyphosate at 0.1–10.5 mg/L (Chu et al. 2005), but different from the effects of deltamethrin at 1.2–3.6 mg/L, and chlordecone at 0.05 and 5.0 μg/L which shortened the duration of post-reproductive period of the rotifers (Xu et al. 2005; Zha et al. 2007), and those of dieldrin at 0.001 μg/L and 10.0–1,000.0 μg/L, and 17β-estradiol at 100.0 and 1,000.0 μg/L which prolonged the duration of post-reproductive period of the rotifers (Huang et al. 2006). It might be possible that the effects of pollutants on the duration of post-reproductive period of rotifers depend on their species and concentration.
Aldrin at 0.02, 0.04 and 0.16 mg/L prolonged the mean lifespan of rotifers by 18.9%–20.9% (Table 1), which was similar to the effects of glyphosate at 3.0 mg/L, deltamethrin at 1.2 mg/L, dieldrin at 0.001–1,000 μg/L, 17β-estradiol at 0.001–1.0 μg/L, 100.0 and 1,000.0 μg/L, and chlordecone at 50.0 μg/L (Chu et al. 2005; Xu et al. 2005; Huang et al. 2006; Zha et al. 2007). The above stated results indicate that lower concentrations of pollutants with endocrine disrupting activity might have an intriguing effect on the lengthening of not only the reproductive period but also on the lifespan of rotifers.
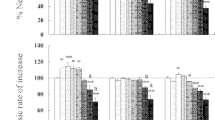
Based on the age-specific survival and fertility of the rotifers exposed to different concentrations of aldrin (Fig. 1), we calculated intrinsic rate of population increase, net reproductive rate, generation time and life expectancy at hatching (Table 2). All the above life-table demographic parameters were significantly influenced by aldrin (one-way ANOVA, p < 0.05). Compared to the controls, acetone at 0.128% did not markedly influence all the life-table demographic parameters of rotifers. However, aldrin at 0.02–0.64 mg/L increased the intrinsic rate of population increase of rotifers by as much as 13.2%–23.7% (Table 2), which was similar to the effects of certain concentrations of pollutants with hormonal activity (Gallardo et al. 1997; Xi and Feng 2004; Huang et al. 2006). Similarly, aldrin at 0.02–0.32 mg/L increased the net reproduction rate of rotifers by as much as 50.6%–79.8% (Table 2), which was identical to the effects of diedrin at 0.001 μg/L, 17β-estradiol at 100.0 and 1,000.0 μg/L, and chlordecone at 0.5–50.0 μg/L (Huang et al. 2006; Zha et al. 2007). The above stated results indicate that lower concentrations of pollutants with endocrine disrupting activity might have an intriguing effect on the reproduction of the rotifers, but the mechanism of their intriguing effects needs further researching.
Aldrin at 0.08 and 0.16 mg/L prolonged significantly the generation time of rotifers by 11.7% and 11.1%, respectively (Table 2), which was similar to the effects of diedrin at 0.001–1,000 μg/L, 17β-estradiol at 0.01, 100.0 and 1,000.0 μg/L, and chlordecone at 0.5 and 50.0 μg/L (Huang et al. 2006; Zha et al. 2007). However, aldrin at 1.28 mg/L shortened the generation time of rotifers (Table 2), which was identical to the effect of thiophanate-methyl at 1.2 and 1.8 mg/L, and deltamethrin at 2.4 and 3.0 mg/L (Xu et al. 2005).
Aldrin at 0.02, 0.08 and 0.16 mg/L increased the life expectancy at hatching of rotifers by 20.1%, 28.1% and 31.7% (Table 2), which was similar to the effects of diedrin at 0.001–1,000.0 μg/L, and 17β-estradiol at 0.01, 1.0, 100.0 and 1,000.0 μg/L (Huang et al. 2006), but different from the effect of chlordecone at 0.0005–50.0 μg/L which did not significantly influence the life expectancy at hatching of the rotifers (Zha et al. 2007). Aldrin at 0.02, 0.08 and 0.16 mg/L increased the life expectancy at hatching of rotifers, which was identical to its effect on the mean lifespan.
Among all the developmental and reproductive parameters, the net reproductive rate was most significantly affected by aldrin, and the intrinsic rate of population growth was affected as much as the durations of all the developmental period. Different endpoints of both development and reproduction of the rotifers had different sensitivity to aldrin. Among the developmental endpoints, embryonic development time was the most sensitive; and among the reproductive endpoints, intrinsic rate of population increase was the most sensitive. Compared with the embryonic development time, the intrinsic rate of population increase is more sensitive.
Snell et al. (1991a) compared the sensitivity of the acute B. plicatilis test with four marine tests for five metals and pentachlorophenol, and found that the rotifer had comparable sensitivity to most compounds, but no single species was consistently the most sensitive to all compounds. A similar comparative study of B. calyciflorus, Daphnia magna and Pimephales promelas (fathead minnow) revealed that the LC50s of the two invertebrates were within one order of magnitude for 9 of 12 compounds (Snell et al. 1991b). Based on the test of 99 environmental samples (effluents, solid wastes and sediments), Persoone et al. (1993) showed that the cyst-based acute test with B. calyciflorus was in 45% of the cases as sensitive and in 17% more sensitive than the conventional D. magna test. A similar comparison between the rotifer test and the bacterial Microtox test made for 250 samples showed that the latter was more sensitive than the former in 68% of the cases and as sensitive in 10% of the cases (Persoone and Janssen 1993). In the present study, LC50 of aldrin to B. calyciflorus was 1.52 mg/L. Compared with all the tested species of freshwater organisms, B. calyciflorus was less sensitive to aldrin than fishes (48–96 h LC50 of 0.9–53 μg/L), nine species of freshwater crustaceans (48–96 h LC50 of 0.1–50 μg/L) and six species of firewater insects (48–96 h LC50 of 1–42 μg/L), but as sensitive as two species of freshwater amphibians (48–96 h LC50 of 68–2,400 μg/L), and more sensitive than one species of freshwater molluscs (48–96 h LC50 of 2,035 μg/L)(US Department of Interior, Fish and Wildlife Service 1980).
In conclusion, aldrin at 0.04–1.28 mg/L, 1.28, 1.28 mg/L shortened significantly the durations of embryonic development and reproductive period, and the generation time of B. calyciflorus, respectively. However, aldrin at 0.16–1.28 mg/L, 0.04–0.16 mg/L, 0.02, 0.04 and 0.16 mg/L, and 0.08 and 0.16 mg/L prolonged the durations of juvenile and reproductive periods, the mean lifespan and the generation time of rotifers, respectively. Aldrin at 0.02–0.64 mg/L, 0.02–0.32 mg/L, and 0.02, 0.08 and 0.16 mg/L increased the intrinsic rate of population increase, the net reproduction rate and the life expectancy at hatching of rotifers, respectively.
References
Chatterjee S, Ray A, Bagchi P, Deb C (1992) Estrogenic effects of aldrin and quinalphos in rats. Bull Environ Contam Toxicol 48:125–130
Chu ZX, Xi YL, Xu XP (2005) Effect of glyphosate on the life history characteristics of freshwater rotifer Brachionus calyciflorus Pallas. Chin J Appl Ecol 16:1142–1145 (in Chinese with English abstract)
Edwards JW, Priestly BG (1994) Effect of occupational exposure to aldrin on urinary d-glucaric acid, plasma dieldrin, and lymphocyte sister chromatid exchange. Int Arch Occup Environ Health 66:229–234
Finley DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, London
Gallardo WG, Hagiwara A, Tomita Y, Soyano K, Snell TW (1997) Effect of some vertebrate and invertebrate hormones on the population growth, mictic female production, and body size of the marine rotifer Brachionus plicatilis Müller. Hydrobiologia 358:113–120
Gibert JJ (1963) Mictic female production in the rotifer Brachionus calyciflorus. J Exp Zool 153:113–124
Hayes WJ Jr (1982) Chlorinated hydrocarbon insecticides. Aldrin and dieldrin. In: Hayes WJ Jr (ed) Pesticides studied in man. Williams and Wilkins, Baltimore
Hφyer AP (1998) Organochlorine exposure and risk of breast cancer. Lancet 352:1816–1820
Huang L, Xi Y-L, Zha C-W, Zhao L-L (2006) Effects of dieldrin and 17β-estradiol on life history characteristics of freshwater rotifer Brachionus calyciflorus Pallas. Bull Environ Contam Toxicol (revised manuscript)
Jager KW (1970) Aldrin, dieldrin, endrin and telodrin: an epidemiological and toxicological study of long-term occupational exposure. Elsevier, Amsterdam
Janssen CR, Ferrando MD, Persoone G (1993) Ecotoxicological studies with the freshwater rotifer Brachionus calyciflorus. I. Conceptual framework and application. Hydrobiologia 255/256:21–32
King CE (1967) Food, age and the dynamics of a laboratory population of rotifers. Ecology 48:111–128
Krebs CJ (1985) Ecology: the experimental analysis of distribution and abundance. Harper & Row, New York, p 800
Li S, Zhu H, Xia Y, Yu M, Liu K, Ye Z, Chen Y (1959) The mass culture of unicellular green algae. Acta Hydrobiol Sin 4:462–472 (in Chinese with English abstract)
Lotka AJ (1913) A natural population norm. J Wash Acad Sci 3:241–248
Persoone G, Janssen CR (1993) Freshwater invertebrate toxicity tests. In: Calow P (ed) Handbook of ecotoxicology. Blackwell, UK
Persoone G, Goyvaerts M, Janssen CR, Coen W, Vangheluwe M (1993) Cost effective acute hazard monitoring of polluted waters and waste dumps with the aid of Toxkits. Final Report EEC, Contract ACE 89/BE 2D3. Commission of the European Communities, Brussels
Poole RW (1974) An introduction to quantitative ecology. McGraw-Hill, New York, p 532
Scippo ML, Argiris C, Weerdt CVA, Muller M, Willemsen P, Martial J, Maghuin-Rogister G (2004) Recombinant human estrogen, androgen and progesterone receptors for detection of potential endocrine disruptors. Anal Bioanal Chem 378:664–669
Snell TW, Janssen CR (1995) Rotifers in ecotoxicology: a review. Hydrobiologia 313/314:231–247
Snell TW, Moffat BD (1992) A 2-d life cycle test with the rotifer Brachionus calyciflorus. Environ Toxicol Chem 11:1249–1257
Snell TW, Moffat BD, Janssen CR, Persoone G (1991a) Acute toxicity tests using rotifers. III. Effects of temperature, strain and exposure time on the sensitivity of Brachionus plicatilis. Ecotoxicol Toxicol Water Qual 6:63–75
Snell TW, Moffat BD, Janssen CR, Persoone G (1991b) Acute toxicity tests using rotifers. IV. Effects of cyst age, temperature, and salinity on the sensitivity of Brachionus calyciflorus. Ecotoxicol Environ Saf 21:308–317
US Department of Interior, Fish and Wildlife Service (1980) Handbook of acute toxicity of chemicals to fish and aquatic invertebrates. Resource publication no. 137. US Government Printing Office, Washington DC
USEPA (1985) Methods for measuring the acute toxicity of effluents to freshwater and marine organisms. In: Peltier WH, Weber CI (eds) EPA/600/4-85/013. US Environment Protect Agency, Washington DC, p 216
Xi Y-L, Feng L-K (2004) Effects of the thiophanate-methyl and glyphosate on asexual and sexual reproduction in the rotifer Brachionus calyciflorus Pallas. Bull Environ Contam Toxicol 73:644–651
Xi Y-L, Hu H-Y (2003) Effect of thiophanate-methyl on the reproduction and survival of the freshwater rotifer Brachionus calyciflorus Pallas. Bull Environ Contam Toxicol 71:722–728
Xi YL, Huang XF (1999) Effect of food supply in both food quality and quantity on the population dynamics of Brachionus urceolaris. Acta Hydrobiol Sin 23:227–234. (in Chinese with English abstract)
Xi YL, Huang XF, Jin HJ (2001) Life history of three types of females in Brachionus calyciflorus Pallas (Rotifera) fed different algae. Hydrobiologia 446/447:95–98
Xu X-P, Xi Y-L, Chu Z-X (2005) The effect of deltamethin on experimental population dynamics of freshwater rotifer Brachionus calyciflorus. Acta Zool Sin 51:251–256 (in Chinese with English abstract)
Zar JH (1999) Biostatistical analysis, vol 4. Prentice Hall, NJ, p 663
Zha C-W, Xi Y-L, Huang L, Zhao L-L (2007) Effect of sublethal exposure to chlordecone on life history characteristics of freshwater rotifer Brachionus calyciflorus Pallas. Bull Environ Contam Toxicol 78:79–83
Acknowledgments
This work was supported by Natural Scientific Foundation of China (30470323), Natural Scientific Foundation of Educational Ministry of China (051286), Excellent Youth Foundation in Anhui Province (04043050), and Natural Scientific Foundation of Educational Committee of Anhui Province, China (2003kj032zd).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, L., Xi, YL., Zha, CW. et al. Effect of Aldrin on Life History Characteristics of Rotifer Brachionus calyciflorus Pallas. Bull Environ Contam Toxicol 79, 524–528 (2007). https://doi.org/10.1007/s00128-007-9271-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-007-9271-y