Abstract
Key message
Mutations in TaCHLI impact chlorophyll levels and yield-related traits in wheat. Natural variations in TaCHLI-7A/B influence plant productivity, offering potential for molecular breeding.
Abstract
Chlorophyll is essential for plant growth and productivity. The CHLI subunit of the magnesium chelatase protein plays a key role inserting magnesium into protoporphyrin IX during chlorophyll biosynthesis. Here, we identify a novel wheat mutant chlorophyll (chl) that exhibits yellow-green leaves, reduced chlorophyll levels, and increased carotenoid content, leading to an overall decline in yield-related traits. Map-based cloning reveals that the chl phenotype is caused by a point mutation (Asp186Asn) in the TaCHLI-7D gene, which encodes subunit I of magnesium chelatase. Furthermore, the three TaCHLI mutants: chl-7b-1 (Pro82Ser), chl-7b-2 (Ala291Thr), and chl-7d-1 (Gly357Glu), also showed significant reductions in chlorophyll content and yield-related traits. However, TaCHLI-7D overexpression in rice significantly decreased thousand kernel weight, yield per plant, and germination. Additionally, natural variations in TaCHLI-7A/B are significantly associated with flag leaf, spike exsertion length, and yield per plant. Notably, the favorable haplotype, TaCHLI-7B-HapII, which displayed higher thousand kernel weight and yield per plant, is positively selected in wheat breeding. Our study provides insights on the regulatory molecular mechanisms underpinning leaf color and chlorophyll biosynthesis, and highlights TaCHLI functions, which provide useful molecular markers and genetic resources for wheat breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat (Triticum aestivum L.) is one of the world's three staple crops, contributing 20% of caloric intake to global populations. Wheat has remarkable adaptability to diverse environmental conditions, making it an integral component of the global food supply (Brenchley et al. 2012). In addressing fluctuating environment, disease and pest stress challenges, and also limited resources, the main aim of current wheat breeding research is to enhance yield per unit area (Song et al. 2023). Photosynthesis is responsible for over 90% of crop biomass production, and improving photosynthetic efficiency at the leaf level may boost overall crop productivity (Makino 2011). Photosynthesis serves as the bedrock of plant development, orchestrating the transformation of light energy into chemical energy, thereby energizing various life-sustaining processes in plants. Investigating photosynthesis more closely is widely recognized as key in achieving significant improvements in crop yields (Singh et al. 2014).
Genetic variation related to photosynthesis in plants has been largely untapped, and always underpins superior variety selection in crop plants (Flood et al. 2011). In the photosynthetic pathway, chlorophyll is a critical pigment, whose degradation via an evolutionarily conserved autophagy pathway is crucial for leaf aging and defenses against pathogens (Dong and Chen 2013). In higher plants, the formation of chloroplast and development is a complicated process that demands a highly coordinated and finely-tuned interplay between gene transcription, translation, protein renewal, and metabolism, whereas defects in any one of these steps can cause chloroplast impairment and leaf pigmentation (Pogson and Albrecht 2011; Zhang et al. 2020). Several studies have investigated chlorophyll biosynthesis mechanisms by examining mutants with altered leaf coloration (Wang et al. 2022; Wu et al. 2022). Such mutants frequently exhibit reduced photosynthetic activity due to a lack of photosynthetic components or altered development due to the accumulation of other pigments (Liu et al. 2016; Ding et al. 2019). These mutants provide valuable tools for studying plant photosynthesis (Wang et al. 2022), photomorphogenesis (Matsumoto et al. 2004), and disease resistance (Shah and Paulsen 2003).
Proto IX is an important substrate in chlorophyll biosynthesis, binding to Fe2+ or Mg2+ and splitting into two pathways to produce heme or chlorophyll, respectively (Hansson et al. 2002). The Mg2+-mediated formation of the MgCh complex facilitates Proto IX chelation to produce Mg-Proto IX, a crucial step in chlorophyll biosynthesis, which ultimately leads to chlorophyll a and b biosynthesis (Brzezowski et al. 2015). The MgCh complex comprises three subunits: CHLI, CHLD, and CHLH, with the latter two belonging to the ATPase-Associated with diverse cellular Activities superfamily. ATPase-Associated with diverse cellular Activities proteins typically form hexamers or heptamers (Vale 2004). Under Mg2+ and ATP catalysis, CHLI and CHLD subunits assemble into two hexameric rings. However, the CHLD subunit possesses an incomplete ATPase domain, necessitating the formation of an CHLI-CHLD complex between CHLI and CHLD for subsequent reactions (Hasson et al., 1999). Changes in CHLI subunits can obstruct chlorophyll biosynthesis, resulting in plant phenotypes characterized by yellowing and depigmentation (Jensen et al. 1996).
In higher plants, CHLI was first studied in barley, where mutants exhibited chlorophyll deficiency along with reduced ATP hydrolysis activity (Hansson et al. 2002). In rice, a frameshift mutation in OsCHLI generated etiolated mutants and disturbed photosynthesis and carbohydrate enrichment pathways (Shim et al. 2023). In foxtail millet, haplotype analysis showed SiCHLI haplotypes associations with metabolic potential (Liang et al. 2023). Many mutants in higher plants exhibit yellow or yellow-green phenotypes (Ding et al. 2019; Ma et al. 2023). Some are also light-sensitive (Ma et al. 2023) and show reduced Mg-chelatase activity (Ding et al. 2019). It is worth noting that some plants have more than two CHLI copies. For example, soybean contains four GmCHLI copies that are differentiated into two interacting types (Li et al. 2016). Pea has two CHLI copies (PsCHLI1 and PsCHLI2) with the latter being more critical. Identification of gene-silenced plants revealed that PsCHLI1 was necessary for chlorophyll biosynthesis but PsCHLI2 was not (Wu et al. 2022). Arabidopsis thaliana has two copies (AtCHLI1 and AtCHLI2) (Rissler et al. 2002). Notably, AtCHLI2 supports some chlorophyll biosynthesis when AtCHLI1 is completely deleted (Huang and Li 2009).
Mechanistic insights into wheat photosynthesis have opened up new avenues in terms of increasing wheat yields, addressing stress tolerance, and other important agronomic traits (Grieco et al. 2020). In recent years, many chlorophyll-related mutants have been identified in wheat (Wu et al. 2018; Ferroni et al. 2020). The Jimai5265yg mutant exhibited a yellow-green phenotype, which was regulated by two genes located on 4AL and 4BL (Wang et al. 2017). Using mapping and cloning analyses, the candidate gene TraesCS2D02G047500 was deemed responsible for the yellow-green phenotype and power mildew resistance in the NAU31 mutant (Zhang et al. 2020). Notably, the TraesCS7A01G480700 gene (also known as TaCHLI-7A) encodes CHLI, and conserved region alterations induce a yellow-green phenotype in wheat (Wang et al. 2020). However, due to wheat genome complexity, the majority of chlorophyll biosynthesis genes have only been mapped and their functions remain to be fully elucidated (Wang et al. 2017; Zhang et al. 2017, 2020).
In this study, we identified a novel yellow-green mutant, which showed reduced chlorophyll and increased carotenoid levels—chlorophyll (chl). Using map-based cloning, we isolated the potentially mutated gene TaCHLI-7D (Asp186Asn), which encoded subunit CHLI. The three TaCHLI wheat mutants chl-7b-1 (Pro82Ser), chl-7b-2 (Ala291Thr), and chl-7d-1 (Gly357Glu) showed significant reductions in thousand kernel weight and yield per plant. However, TaCHLI-7D overexpression in rice exhibited significant reductions in germination, thousand kernel weight, and yield per plant. Additionally, TaCHLI-7A/B haplotype analysis showed that natural TaCHLI-7A/B variations were associated with flag leaf, spike exsertion length, and yield per plant. The favorable haplotype TaCHLI-7B-HapII has also been widely selected in wheat breeding processes in China. Furthermore, we found that chl was resistant to drought, which indicated that TaCHLI has potential roles in wheat drought resistance. Therefore, TaCHLI affected chlorophyll biosynthesis and has potential value in molecular genetic breeding programs.
Materials and methods
Plant materials and growth conditions
Mutant chl was derived from Kenong 9204 (wild type [WT]), which was treated with the chemical mutagen ethyl methane sulfonate. Populations used for genetic analysis and map-based cloning were produced from WT × chl and Yannong 999 × chl crosses. The mutants chl-7b-1, chl-7b-2, and chl-7d-1, which were used to validate TaCHLI gene function, originated from the Kenong 9204 mutant library [http://kn9204.molbreeding.com/]. The genetic transformation of the rice variety Zhonghua 11 was completed by Boyuan Biology (Wuhan, China). The natural population used for haplotype analysis comprised 314 wheat germplasms (Xu et al. 2022), cultivated across eight environments (E1–E8), and categorized by year and location. These included Pulagu (121.41°E, 37.31°N) in 2019–2020 (E1), Shijiazhuang (114.69°E, 37.89°N) in 2019–2020 (E2), Weifang (119.44°E, 36.68°N) in 2019–2020 (E3), Ludong University (121.35°E, 37.51°N) in 2020–2021 (E4), Pulagu in 2020–2021 (E5), Shijiazhuang in 2020–2021 (E6), Muyucun (121.35°E, 37.34°N) in 2021–2022 (E7), and Pulagu in 2021–2022 (E8). Wheat plants were grown in 1.5 m plots with three rows, spaced 30 cm apart, and with 15 manually sown seeds/row.
Kenong 9204 and chl stress treatments were conducted as follows; before treatment, seeds were germinated in Petri dishes for 3 days, selected for uniform growth, and transferred to hydroponic boxes, and finally hydroponically grown for 5 days to the one-leaf stage. Saline treatments using a 125 mM NaHCO3: Na2CO3 solution at a molar ratio of 5:1 (Sigma-Aldrich, Shanghai, China) were conducted for 5 days (Zhang et al. 2023). Drought-stress treatments using a 30% polyethylene glycol (PEG) 6000 solution (Sigma-Aldrich) were conducted for 3 days (Xu et al. 2022). CDC was assessed using the following formula: drought coefficient of plant height/root length/SPAD(DCPH/RL/SPAD) = (PEG stress treatment plant height/root length/SPAD measurements—control group measurements) × 100%, CDC = (DCPH + DCRL + DCSPAD)/3. Seedlings exhibiting new leaf growth were considered survivors. After determining survival rates, above- and below-ground sections of surviving seedlings were measured and weighed to determine fresh and dry weights (after drying at 70 °C for 24 h). Hoagland's nutrient solution (HN) and Hoagland's solution without Ca(NO3)2·4H2O (LN) were used for different nitrogen treatments.
Phenotype evaluations
We recorded 15 agronomic traits, including plant height, spike length, spike exsertion length, awn length, flag leaf length, flag leaf width, number of spikes, number of grains per spike, grain length, grain width, thousand kernel weight, yield per spike, uppermost internode length, second internode length, and third internode length. BLUE values were calculated for each trait using lme4 in the R package. Root phenotypes at the seedling stage, including total root length and total root volume, were scanned using a Microtek ScanMaker (Zhongjing Technology, Shanghai, China) and analyzed in the LA-S plant root phenotyping system (Wseen Testing Technology, Hangzhou, China). Leaf chlorophyll concentrations were estimated using a leaf color meter (SPAD-502, Minolta, Japan) (Markwell et al. 1995).
Photosynthetic pigment content was measured in the first leaf at the seedling stage and the flag leaf at the coleoptile stage in WT and chl. Three biological replicates were tested. Approximately 0.2 g of fresh leaves were soaked in 95% ethanol for 48 h in a dark environment until the leaves turned white. The absorbance from leaf extracts was measured at 470 nm, 649 nm, and 665 nm using a UV-1800 ultraviolet/visible spectrophotometer (TU1810; MAPADA, Beijing, China). Chlorophyll a, chlorophyll b, and carotenoid levels were calculated as follows:
Chlorophyll fluorescence dynamic parameters, including Fo, Fm, Rfd_lss, NPQ_lss, and Fv/Fm, were monitored on a FluorCam portable chlorophyll fluorescence imager (EcoTech, Beijing, China) using the first leaf from the seedling stage. At least three individual plants were used in experiments.
Transmission electron microscopy (TEM)
TEM analyses were performed by Shandong Weiya Biotechnology Co., Ltd (Jinan, Shandong, China). Samples were fixed in a 2.5% glutaraldehyde solution 4 °C overnight and subsequently dehydrated in an ethanol solution series; then stained with 2% uranyl dioxy and lead citrate trihydrate. Observational and photographic data were acquired using transmission electron microscope (JEM-1400plus; JEOL Co., Ltd., Beijing, China).
DNA isolation, primer design, and BSE-seq analysis
The modified cetyltrimethylammonium bromide method was used to extract genomic DNA from young fresh leaves (Sharp et al. 1988), and DNA from 30 lines with green and yellow leaves were separately pooled. Similarly, separate pools were generated for chl. Pools, along with parents (WT and chl), were used for BSE-seq analysis (Beijing Biomarker Technologies Co., Ltd., Beijing, Hubei, China). After bulking pools, 2 μg of genomic DNA from each sample was fragmented into 250 bp using a Bioruptor UCD-200 sonicator (Diagenode, Denville, NJ, USA). To obtain target sized DNA from physically interrupted DNA, totally-fragmented DNA was size-selected using magnetic beads (NucleoMag, Macherey–Nagel, Duren, Germany). For BGI-seq, pre-libraries were constructed using the VAHTS Universal DNA Library Prep Kit (Vazyme Biotech Co., Ltd., Nanjing, China). Selected DNA was then end-repaired with an end-repair enzyme (Vazyme Biotech Co., Ltd., Nanjing, China), and a deoxyadenosine was added to 3’ fragment ends. VAHTS-barcoded DNA and VAHTS-indexed adapters were ligated to sample libraries. Pre-capture amplification steps were performed using the VAHTS protocol over five PCR cycles. Equal product amounts from eight libraries were pooled to generate 2 μg of DNA for hybridization, which was performed at 47 °C for 16 h using the WheatPanExomeV2 /KN9204/AK58 (Tcuni Inc., Chengdu, China) exome capture panel. Hybrids were enriched using capture beads (T1 pure capture bead kit, Thermo Fisher, MA, USA), washed, and amplified using ligation-mediated PCR. Captured library quality was assessed using the Agilent 4200 TapeStation (Agilent Technologies, Santa Clara, CA, USA), quantified by qPCR, and then sequenced using the BGI T7 platform to generate 150 bp paired-end reads.
Clean reads were then aligned to the IWGSC RefSeq v2.1/KN9204/AK58 to obtain bam files using BWA software (default parameters) (Li and Durbin 2009). We use Picard (McKenna et al. 2010) to sort and remove PCR duplications from bam files. Finally, Haplotype Caller in GATK was used to generate raw VCF files. Both read mapping and variant calling used a genome target bed which covered all gene coding sequences (CDS) and 2 kb regions upstream and downstream of the CDS. Bam file filtering parameters were mapping quality (MQ) ≥ 50 & Offset to base alignment quality (BQ) ≥ 20, and VCF file filtering parameters were query quality (QUAL) ≥ 100 || query quality (DP) ≥ 5. Variants were fileted and bulked segregant analysis (BSA) was performed using WheatGmap (Zhang et al. 2021).
Phylogenetic analysis, conserved domains, and gene structures
Using the TaCHLI protein sequence as a query, homologous sequences from the Gramene database (http://ensembl.gramene.org/Tools/Blast) were acquired. Alignments (MEGA11 using ClustalW) (Larkin et al. 2007) were performed and targeted common crops for phylogenetic tree construction using the neighbor-joining method and estimated bootstrap values over 1000 replications. Protein-conserved domains were identified using the National Center for Biotechnology Information (NCBI) Batch Web CD-Search tool (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi). Gene structures were mapped using TBtools (Chen et al. 2020).
qRT-PCR and digitized gene expression
Total RNA was isolated from several tissues using the MiniBEST Plant RNA Extraction Kit (Takara Bio, Beijing, China). First cDNA strand synthesis was facilitated using Trans-Script One-Step gDNA Removal and cDNA Synthesis SuperMix kits (TransGen Biotech, Beijing, China). For qRT-PCR, the CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) and TB Green Premix Ex Taq II (Takara) were used. Assays were conducted over a minimum of three repetitions in each experimental group, ensuring at least three technical replicates. Internal normalization was achieved using rice ubiquitin and wheat glyceric acid phosphate dehydrogenase (GADPH) as reference genes. Primers were designed in NCBI (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) and primer server (BETA) in Wheatomics (http://wheatomics.sdau.edu.cn/PrimerServer/). OsCHLI expression pattern data were obtained from ePlant (https://bar.utoronto.ca/eplant_rice/).
Subcellular localization
The TaCHLI-7D coding region sequence was cloned by homologous cloning technology, fused to the 35S-GFP fluorescent protein, transformed into wheat protoplasts via genetic transformation (Yoo et al. 2007), and incubated at 24 °C for 18–24 h. Target protein subcellular localization was determined by observing cellular green fluorescence under laser confocal microscopy (Carl Zeiss LAM510, Germany).
Transcriptome sequencing and analysis
For transcriptome sequencing, WT and chl samples, constituting the first leaf at the seedling stage and the flag leaf at the jointing stage, where phenotypic disparities were most pronounced, were selected. Flag leaves from ten plants per variety were pooled, and three independent biological replicates conducted. Transcriptome sequencing and analyses were performed by Bgi Co., Ltd. (Guangdong, Shenzhen, China). Fragments Per Kilobase of exon model per million mapped fragments (FPKM) values were extracted from transcripts, and DESeq2 (version 1.6.3) was used to analyze differentially expressed genes (DEGs). Genes with a false discovery rate < 0.05 and absolute log2 Fold Change of ≥ 1 values were identified as DEGs.
Vector construction and rice transformation
The overexpression vector pUbi::TaCHLI-7D was generated by amplifying the full-length TaCHLI-7D open reading frame by PCR and followed by insertion into the modified vector pCAMBIA1301 using homologous recombination (Wu et al. 2016). Agrobacterium-mediated transformation was then used to introduce this vector into ZH11 plant lines. Phenotypic assessments of transgenic plants were then conducted using three independent transgenic lines (comprising 15 positive lines) Grain length, grain width, and thousand kernel weight were measured using the Intelligent Test Analysis System (TOP Cloud-Agri Technology, Zhejiang, China).
Statistical analysis
Two-tailed Student’s t-tests and Duncan’s multiple range tests were performed in IBM SPSS Statistics v26 (IBM Corp., Armonk, NY, USA). Significance was accepted at *P < 0.05 and **P < 0.01.
Results
chl phenotypic characterization
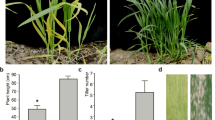
We developed an ethyl methane sulfonate mutant library using the wheat cultivar Kenong 9204 (wild type, WT) and identified a mutant with significant leaf color changes, accompanied by changes in chlorophyll (Fig. 1E–F)—chlorophyll (chl). The mutant exhibited a yellow-green leaf phenotype at the seedling stage (Fig. 1A). In comparison to the WT, chl showed significant reductions in chlorophyll a (− 18%), chlorophyll b (− 72%), and Soil and Plant Analyzer Development (SPAD) levels (− 28%), while carotenoid levels were significantly increased (+ 53%) (Fig. 1E–H). Chlorophyll content changes have the potential to affect photosynthetic efficiency, while chlorophyll fluorescence parameters can be used to evaluate photosynthetic efficiency in plants from multiple perspectives (Wang et al. 2022). Specifically, chl exhibited significant reductions in minimum initial fluorescence (Fo) (− 26%), maximum fluorescence (Fm) (− 17%), steady-state fluorescence decay rates (Rfd_lss) (− 17%), and non-photochemical quenching under steady-state light (NPQ_lss) (− 55%) when compared to WT. In contrast, a slight difference in the maximum photosynthetic efficiency of photosystem II (Fv/Fm) (+ 3.19%) was observed (Fig. 1B–D, I–M). These results indicated that chl-mediated reductions in photosynthetic pigments decreased chlorophyll fluorescence and plant vitality while having a relatively smaller impact on maximum energy conversion efficiency in the Photosystem II (PSII) reaction center.
Comparison of photosynthetic comparisons between WT and chl. A Comparison of plant grown for 7 days between WT and chl. Yellow boxes indicated subsequent chlorophyll fluorescence parameters and sites from where sections were sampled. Scale bar = 1 cm. B-D Images showing chlorophyll fluorescence parameters between WT and chl plants, including Fo (B), Fm (C), and Fv/Fm (D). Scale bar = 1 cm. E–H Comparisons of chlorophyll a (chl a) (E), chlorophyll b (chl b) (F), and carotenoid (G) between WT and chl plants. H Comparison of SPAD between WT and chl. I-M Comparison of chlorophyll fluorescence parameter comparisons between WT and chl, including Fo (I), Fm (J), Fv/Fm (K) Rfd_Lss (L), and NPQ_Lss (M). Error bars indicate the standard deviation (n = 24). N, O Transmission electron microscopic images showing leaves of WT and chl at the seedling stage and the jointing stage. The top four images are at 5000 × . Scale bar = 5 μm. The bottom left two images are at 50,000 × . Scale bar = 500 nm. The bottom right two images are at 30,000 × . Scale bar = 1 μm. P Comparison of multi-period plastoglobulus number. Q Comparison of multi-period stroma number between WT and chl. Error bars indicated the standard deviation (n = 6). Two-tailed Student’s t-tests were performed between WT and chl. NS, not significant; **P < 0.01
To investigate the potential impact of the mutation on chl chloroplasts, we used transmission electron microscopy (TEM), which showed that chl chloroplasts exhibited significant deformations (Fig. 1N, O). Specifically, a notable reduction in the number of plastoglobulus during the seedling (− 71%) and the jointing stage (− 25%) was observed when compared to WT (Fig. 1P). Similarly, a substantial reduction in stroma numbers was observed at both the seedling (− 58%) and the jointing stage (− 58%) (Fig. 1Q).
chl displayed a yellow-green leaf phenotype throughout the entire growth period (Fig. 2A, B). To unravel the implications of this on yield-related traits, a more detailed investigation was conducted. Under field conditions, chl exhibited a significant reduction in various agronomic traits when compared to WT (Table S2). Specifically, tiller number (− 61%), plant height (− 36%), spike length (− 18%), spikelet number per spike (− 22%), awn length (− 11%), flag leaf length (− 21%), and flag leaf width (− 12%) were all reduced (Fig. 2C, F–I, Table S2). Notably, a reduction in the second internode length (− 45%) was more pronounced than the third (− 25%) (Table S2). Eventually, the decline in photosynthesis levels significantly declined the grain number per spike (-18%), grain length (− 12%), grain width (− 27%), thousand kernel weight (− 36%), and yield per plant (− 61%) (Fig. 1D, E, K–O).
Comparison of phenotypic characterization between WT and chl. A, B Comparison of kernel between WT and chl at the jointing stage and the mature stage. Scale bar = 5 cm. C Comparison of spike between WT and chl. Scale bar = 2 cm. D Comparison of kernel between WT and chl. Scale bar = 4 mm. E Comparison of yield per plant of WT and chl. Scale bar = 1 cm. F–O Agronomic trait differences between WT and chl. Error bars indicate the standard deviation (n = 30). Two-tailed Student’s t-tests were performed between WT and chl. NS, not significant; **P < 0.01
Molecular cloning of the candidate gene TaCHLI-7D
To analyze the genetic factors responsible for chl phenotypic changes, we backcrossed chl with the WT and the wheat variety Yanong 999. Genetic analyses indicated that chl was a mutant controlled by a nuclear recessive gene (Table S3). For the two F3 segregating populations with extreme phenotypes (yellow or green), 30 plants were selected for bulked segregant exome sequencing (BSE-Seq). We observed a dense distribution of high-quality single nucleotide polymorphisms (SNPs) in the 575–595 Mb region of chromosome 7D (based on the Chinese Spring RefSeq v2.1 sequence) (Fig. 3A). From these findings, we used map-based cloning and designed 10 polymorphic insertion–deletion (InDel) markers based on InDel sites between 580–590 Mb. Genotyping of 497 yellow plants in the segregating population narrowed the target interval to a 3.59 Mb region in 582,781,459–586,373,343 bp (Fig. 3B). We have identified 37 genes and 2 SNPs in this interval, with only SNP 1 located in the gene region (Table S4-S5). The gene, TraesCS7D03G1109500, is functionally annotated as the chloroplast magnesium chelatase subunit ChlI. SNP 1 resulted in a missense mutation in the mutant TraesCS7D03G1109500, leading to a change in the protein from Asp to Asn at position 187. Domain analysis showed that this gene belongs to the CHLI superfamily, and therefore it was named TaCHLI-7D (Fig. 3C). Based on these findings, we hypothesized that TraesCS7D03G1109500 is a candidate gene for further analysis.
Map-based cloning of TaCHLI-7D. A SNP numbers in the 1 Mb window size from BSE-Seq. B Fine mapping of chl, dark-green rectangles represent WT genotypes, yellow rectangles represent chl genotypes, and light green rectangles represent the interval in the chromosome where crossover took place. C TaCHLI-7D gene and protein structure map. The light green rectangle indicates the TaCHLI-7D gene (TraesCS7D03G1109500). The cytochrome CHLI superfamily domain was predicted in the CHLI protein. Solid lines show the gene mutation sites and the position of the amino acid transition
Phylogenetic and expression analysis of TaCHLI
To explore TaCHLI-7D functions in wheat, we performed a BLASTP analysis using its protein sequence against the international wheat genome sequencing consortium (IWGSC) RefSeq v2.1 database (Zhu et al. 2021). TaCHLI-7A (TraesCS7A03G1163900) and TaCHLI-7B (TraesCS7B03G1030900), which were highly homologous to TaCHLI-7D, were identified. TaCHLI-7A, TaCHLI-7B, and TaCHLI-7D protein lengths were 421 amino acids (aa), 420 aa, and 420 aa, respectively (Table S6), and all belonged to the CHLI superfamily, suggesting similar functions in wheat. Phylogenetic analysis revealed that TaCHLI homologs were divided into two groups in cereals, and that they were most closely related to barley (HORVU. MOREX. r3. 7HG0738240) and rice (Os03g0563300) (Fig. 4A).
Phylogenetic relationships between CHLI proteins and TaCHLI gene expression. A Phylogenetic analysis of CHLI protein homologs; from left to right: gene structures and domain families. B Relative TaCHLI-7A/7B/7D expression in WT and chl. Wheat GADPH was used as an internal control. Error bars indicated the standard deviation (n = 3). C Subcellular TaCHLI-7D-GFP fusion protein localization in wheat protoplasts. An empty GFP vector was used as a negative control. Images were scanned using confocal microscopy. Scale bar = 10 μm
To clarify TaCHLI expression profiles, we analyzed the expression levels of WT and chl across different tissues at the mature stage using qRT-PCR specific primers. The TaCHLI-7A/7B/7D were expressed in all examined tissues, exhibiting higher expression in major photosynthesis tissues, such as spike and leaf, and lower expression in root and grain (Fig. 4B–D). Moreover, TaCHLI-7A/B/D expression in chl was higher in root and stem, and lower in leaf, spike, and grain, compared with WT (Fig. 4B–D). Subcellular localization demonstrated that TaCHLI-7D was located in chloroplasts, consistent with TEM observations in chloroplasts (Figs. 4E, 1N, O). These suggested TaCHLI-7D could play an important function on the chloroplast development.
TaCHLI-7D functional validation in wheat mutants and rice overexpression lines
To investigate TaCHLI function in wheat, we screened an EMS mutant library of the WT. We identified three mutants in TaCHLI: chl-7b-1 (Pro82Ser), chl-7b-2 (Ala297Thr), and chl-7d-1 (Gly357Glu) (Fig. S1A, B), which exhibited yellowing phenotypes similar to chl during the seedling stage. These mutants displayed varying degrees of leaf chlorosis. In the later growth stage, chl-7b-1 and chl-7d-1 demonstrated whole-plant or leaf senescence, while chl-7b-2 exhibited a yellowing phenotype similar to chl (Fig. 4C).
Compared with WT, chlorophyll a (− 46%, − 61%, and − 29%), chlorophyll b (− 42%, − 13%, and − 23%), and SPAD (− 10%, − 16%, and − 28%) levels in chl-7b-1, chl-7b-2, and chl-7d-1 mutants all decreased, respectively. The carotenoid levels in chl-7b-1 and chl-7d-1 mutants were significantly decreased and increased by 45% and 28%, respectively, while the chl-7b-2 was unchanged (Fig. 5D-G).
Morphological characters of TaCHLI wheat mutants. A, B Genomic structures of TaCHLI-7B (A) and TaCHLI-7D (B). The deep yellow line is the variant position in chl-7b-1, chl-7b-2, and chl-7d-1. C WT, chl-7b-1, chl-7b-2, and chl-7d-1 plants at the seedling stage. Scale bar = 10 cm. D-G Differences in photosynthetic pigments, including chlorophyll a (chl a) (D), chlorophyll b (chl b) (E), carotenoid (F), and SPAD (G) between WT and chl-7b-1, chl-7b-2, and chl-7d-1 plants at the seedling stage. H Whole plants of WT, chl-7b-1, chl-7b-2, and chl-7d-1 at the heading stage. Scale bar = 10 cm. I Flag leaves of WT, chl-7b-1, chl-7b-2, and chl-7d-1 at the heading stage. Scale bar = 3 cm. J Grain length and width of WT, chl-7b-1, chl-7b-2, and chl-7d-1 at the mature stage. Scale bar = 2 cm. K Comparisons of yield per plant between WT, chl-7b-1, chl-7b-2, and chl-7d-1 at the mature stages. Scale bar = 5 cm. L-S Comparison of agronomic traits between WT, chl-7b-1, chl-7b-2, and chl-7d-1 plants, including plant height (L), spike length (M), flag leaf length (N), flag leaf width (O), grain length (P), grain width (Q), thousand kernel weight (R), and yield per plant (S). Error bars indicate the standard deviation (n = 6). Two-tailed Student’s t-tests were performed between WT and chl-7b-1, chl-7b-2, and chl-7d-1. NS, not significant; *P < 0.05, **P < 0.01
In the later growth stage, chl-7b-1 and chl-7d-1 demonstrated whole-plant or leaf senescence, while chl-7b-2 exhibited a yellowing phenotype similar to chl (Fig. 5H). Additionally, the agronomic traits of chl-7b-1, chl-7b-2, and chl-7d-1, such as plant height (− 33%, − 10%, and − 30%), spike length (− 26%, − 14%, and − 37%), flag leaf length (− 18%, − 26%, and + 1%), and flag leaf width (− 60%, − 42%, and − 22%) were all changed, respectively (Fig. 5I, L–O). Also, most yield traits such as grain length (− 24%, − 5%, and − 9%), grain width (− 33%, − 4%, and − 20%), thousand kernel weight (− 78%, − 33%, and − 36%), and yield per plant (− 95%, − 79%, and − 67%) were significantly reduced, respectively (Fig. 5J, K, P–S). chl-7b-1 experienced the most severe decline due to whole-plant senescence, resulting in smaller grains. Therefore, TaCHLI-7B/D mutations impacted on photosynthesis, leading to chlorotic or prematurely senescent phenotypes similar to chl and ultimately affecting yield traits.
To further explore TaCHLI-7D biological functions, we cloned full-length TaCHLI-7D into an overexpression transgenic vector pUbi::TaCHLI-7D (ZH11-OE) and introduced it into the japonica cultivar Zhonghua 11 (ZH11). Two different transgenic lines (ZH11-OE#1 and ZH11-OE#2) were selected for observation. Compared to ZH11, transgenic ZH11-OE plants exhibited significant increases in transcript levels, ranging from 7.78 to 14.53 times higher (Fig. S1E). In contrast to ZH11, the overall growth vigor of ZH11-OE plants was poorer (Fig. S1A), with shorter flag leaves (− 29% and − 5%, respectively), low levels of photosynthetic pigments, including chlorophyll a (− 8% and − 9%, respectively), chlorophyll b (− 32% and − 30%, respectively), carotenoids (− 11% and − 7%, respectively) and lower SPAD (− 7% and − 18%, respectively) in both lines (Fig. S1 F-I, K). Plant height (− 5% and − 2%, respectively), grain length (− 4% and − 5%, respectively), and grain width (− 4% and − 4%, respectively) did not change significantly (Fig. S1G–I). Eventually, this caused severe yield reductions, with thousand kernel weight (− 42% and − 32%, respectively) and yield per plant (− 50% and − 46%, respectively) decreased (Fig. S1C, N, O). Furthermore, germination rates in both the lines decreased by 47% (Fig. S1D, P). Therefore, TaCHLI-7D overexpression severely affected germination and photosynthesis, leading to reduce the yields. From these observations, both wheat mutants and TaCHLI overexpression in rice indicated that the mutation affected chlorophyll biosynthesis, which severely affected SPAD values and yields, and was potentially valuable in improving photosynthetic traits in plants.
Chl transcriptome sequence analysis
In an in-depth analysis of yellow phenotype formation in chl, we sampled first leaves and flag leaves at seedling stage and the jointing stage to extract RNA for RNA-Seq, with clean reads aligned with the Chinese Spring RefSeq v2.1 genome. Differentially expressed genes (DEGs) were identified based on |log2 (fold change) |≥ 1 and P < 0.05 values. The seedling stage generated 6135 DEGs, with 4032 down- and 2103 upregulated genes (Fig. 6A). In contrast, the jointing stage generated 8419 DEGs, with 4108 down- and 4311 upregulated genes (Fig. 6B). Importantly, 1134 DEGs were shared between the seedling stage and the jointing stage (Fig. 5C).
Transcriptome analysis of WT and chl at the seedling stage and the jointing stage. A Volcano plots showing up- or downregulated WT and chl genes at the seedling stage. B Volcano plots showing up- or down-regulated WT and chl genes at the jointing stage. C Venn diagram showing differentially expressed genes (DEGs) between WT and chl at the seedling stage and the jointing stage. D GO enrichment analysis of DEGs in WT and chl plants at the seedling and the jointing stage, and a combined DEGs analysis from 2 stages. E Comparisons between fragments per kilobase of exon model per million mapped fragments (FPKM) and relative expression levels for six genes. Gray represents FPKM values and green represents relative expression levels. Error bars indicate the standard deviation (n = 3)
DEG enrichment analyses were conducted across the seedling and the jointing stage, and DEGs common to both were analyzed using Gene Ontology (GO) terms. GO annotations segregated DEGs into three categories: biological processes, molecular functions, and cellular components, with notable enrichment in the former two (Table S7). At the seedling stage, GO terms were predominant for the reductive pentose phosphate cycle, jasmonic acid signaling regulation, and ribulose bisphosphate carboxylase activity, along with pathways related to photorespiration (Fig. 6D). It was noteworthy that pathways related to cellular responses to heat and water stress were enriched, suggested that TaCHLI-7D had potential in drought stress in wheat.
At the jointing stage, chloroplast and photosynthesis-related pathways, including chloroplast organization and PSII, were predominantly enriched (Fig. 6D). This indicated that photosynthesis-related genes had crucial roles at this stage. Moreover, a combined DEGs analysis from both the seedling stage and the jointing stage revealed significant enrichment in photosynthetic electron transport in PSII, the chloroplast thylakoid lumen, and the magnesium-dependent protein serine phosphatase activity pathway, which were all related to photosynthesis and suggested that TaCHLI-7D was closely related to photosynthesis.
We further validated the RNA-Seq results by randomly selecting six genes from GO pathways significantly enriched in the intersection DEGs, including the magnesium-dependent protein serine phosphatase activity pathway and the protoporphyrinogen IX biosynthetic process pathway. These genes were subjected to qRT-PCR analysis, which confirmed that the trends observed in the qRT-PCR results were consistent with those from the RNA-Seq data (Fig. 6E).
TaCHLI haplotypes analysis
To investigate natural variations in TaCHLI toward agronomic traits, coding and 2 kb promoter regions in TaCHLI-7A/B/D in 42 wheat accessions (with high genetic diversity) were analyzed for sequence polymorphisms (Table S7). Six co-segregated SNPs were identified for TaCHLI-7A. SNPs 1–5 were located in the promoter region, while SNP 6 was situated in the gene region. These SNPs were divided into two haplotypes. Additionally, for TaCHLI-7B, we identified two InDels and one SNP, InDel 1 and SNP are located in the promoter region, and InDel 2 is located in the gene region, from which TaCHLI-7B was divided into four haplotypes. Based on the previously identified InDel 1 and InDel 2, two InDel markers (TaCHLI-7B-1 and TaCHLI-7B-4) were developed and detected using polyacrylamide gels (Fig. 7I). Primers are listed (Table S1). However, no nucleotide variations were identified in TaCHLI-7D (Fig. 7O).
Haplotype analysis of TaCHLI associations with yield-related traits in wheat. A Nucleotide variation in TaCHLI-7A. B Frequency changes in TaCHLI-7A haplotypes across the history of wheat breeding in China. C-H Comparison of TaCHLI-7A haplotypes agronomic traits, including plant height (C), flag leaf length (D), flag leaf width (E), spike exsertion length (F), thousand kernel weight (G), and yield per plant (H). Two-tailed Student’s t-tests were performed between HapI and HapII. NS, not significant; **P < 0.01. NS, not significant. I Nucleotide variation in TaCHLI-7B. J Frequency changes in TaCHLI-7A haplotypes across the history of wheat breeding. K-N Comparisons TaCHLI-7B haplotypes agronomic traits, including plant height (K), spike exsertion length (L), thousand kernel weight (M), and yield per plant (N). Error bars indicated the standard deviation. Different letters indicate significant differences in Duncan’s multiple range tests (P < 0.05). O Nucleotide variation in TaCHLI-7D
Next, we calculated the best linear unbiased estimate (BLUE) values for six agronomic traits across 314 wheat accessions in eight environments between 2020 and 2023. When combined with TaCHLI haplotypes and phenotypic data from multiple environments and BLUE values from 314 wheat accessions, TaCHLI haplotype effects were clarified. When compared with TaCHLI-7A-HapI, TaCHLI-7A-HapII reduced flag leaf length (− 7%), flag leaf width (− 5%), and spike exsertion length (− 16.57%), and increased thousand kernel weight (+ 3%) and yield per plant (+ 5%), but plant height showed no significant differences (Fig. 7C–H). For TaCHLI-7B, when compared to the other three haplotypes, TaCHLI-7B-HapII showed a significant decrease in plant height (− 8%, − 10%, and − 11%) and spike exsertion length (− 17%, − 24%, and − 30%), respectively, while increased thousand kernel weight (+ 3%, + 3%, and + 4%), and yield per plant (+ 4%, + 3%, and + 4%), respectively, were identified (Fig. 7K–N). These findings suggested TaCHLI-7A/B haplotypes could provide effective genetic resources and molecular markers for high-yield cultivar development.
To determine TaCHLI haplotype selection characteristics in wheat breeding, we assessed 200 Chinese wheat accessions from different breeding years (Xu et al. 2022) and used them to analyze frequency in different TaCHLI-7A/B haplotypes. TaCHLI-7B-HapII frequency was raised from landraces (%) to modern cultivars (%) (Fig. 7J), while the frequency of the two TaCHLI-7A haplotypes remained essentially unchanged (Fig. 7B). Moreover, we investigated the geographical distribution characteristics of TaCHLI haplotypes in 314 wheat accessions from six continents (Xu et al. 2022). For TaCHLI-7A, the TaCHLI-7A-HapII distribution frequency accounted for a larger ratio than TaCHLI-7A-HapI in six continents, which suggested that TaCHLI-7A-HapII varieties could widely adapt to complex conditions (Fig. S2A). For TaCHLI-7B, TaCHLI-7B-HapI/III/IV were widely distributed across all continents, while TaCHLI-7B-HapII was only found in Asia (20%) and North America (14%) (Fig. S2B). Thus, TaCHLI-7B-HapII was positively selected in wheat breeding process and had great potential for global wheat breeding selection.
Drought-resistance potential of chl
We serendipitously observed that chl possessed a degree of resistance to droughty environments. To corroborate this drought-resistance potential, both WT and chl were grown in a PEG6000-simulated arid environment. After cultivation for 7 days, chl displayed notable reductions in shoot height (− 17%) and root length (− 21%) compared to WT (Fig. S3A-C). After PEG6000 treatment, chl survival rates were significantly higher than WT (+ 9%) in three experimental groups, after which the comprehensive drought-resistance coefficient (CDC) for WT and chl was calculated (-12%) (Fig. S3D-E). The saline-alkali resistance of chl was evaluated with 125 mM saline-alkali treatment solution. And chl displayed notable reductions in shoot height (− 20%) and root length (− 12%), compared to WT (Fig. S4A-C). After recovered, the survival rate of the chl was significantly lower than WT (-53%) in three experimental groups (Fig. S3D).
In order to explore the stress resistance of WT and chl, the same concentration of treatment solution was used to evaluate the resistance of WT and chl. In the control group, plant height (− 17%), root length (− 14%), and fresh weight (− 31%) of chl were significantly reduced, while dry weight had no changed (Fig. S5A). After 7 days of salt-alkali treatment, plant height (− 43%), root length (− 31%), and fresh weight (− 35%) of chl decreased greatly, and dry weight have no changed (Fig. S5B). After 7 days of PEG6000 treatment, plant height (+ 62%) and root length (+ 44%) of chl increased compared with WT, and have no significant change in fresh weight and dry weight (Fig. S5C). These results showed that chl had certain drought resistance from the bud stage to the seedling stage, but no saline-alkali resistance.
Discussion
In recent years, leaf color mutants have been widely used to study photosynthesis (Ma et al. 2023; Shim et al. 2023). Previous studies have shown that many leaf color mutants have been examined, but only a few leaf color-related genes have been cloned (Zhang et al. 2017, 2020; Wang et al. 2020). In our study, a recessive gene controlling the yellow-green mutant chl was identified from WT (Fig. 1, 2). We then localized (Fig. 3A, B) and cloned (Fig. S1) the candidate gene TaCHLI-7D which was determined by mapping and belonged to the CHLI family (Fig. 3C). CHLI is a highly conserved protein in plants (Fig. S9), suggesting similar functions. There have been many reports on CHLI such as rice (Ma et al. 2017), pea (Wu et al. 2022), and strawberry (Ma et al. 2023) in recent years. CHLI is implicated in the chlorophyll biosynthesis of magnesium ion chelation which affects chlorophyll content. Previously, our strawberry (Ma et al. 2023) and pea (Wu et al. 2022) studies indicated that mutations in this protein decreased chlorophyll a and chlorophyll b levels (Fig. 1E-F). However, it is noteworthy that carotenoids were elevated in chl (Fig. 1G), which was not previously reported in other leaf color mutants. Additionally, in broomcorn millet, electron microscopic observations of yellow-green mutants showed decreased and deformed chloroplast and decreased chloroplast stroma and these results were similar to our results (Fig. 1N, O) (Wang et al. 2022), but chlorophyll fluorescence parameter analyses showed no Fv/Fm decreases (Fig. 1K). This indicated that the effects of leaf yellowing on PSII in wheat were smaller when compared to other plants. In wheat, TaCHL-7A is a homologous gene of TaCHLI-7D (Wang et al. 2020), and its overexpression in A. thaliana appeared to impair seed germination. Furthermore, TaCHLI-7D overexpression in rice also reduced germination rates and severely impaired growth (Fig. S1A, D, M). presumably due to the following reasons: OsCHLI has high homology with TaCHLI-7D and high background levels in leaves, and the relative expression of OsCHLI increased tenfold in the rice overexpression lines, which may have disrupted the chlorophyll biosynthesis pathway resulting in the reduction of germination rate and yield (Table S6, Fig. S6). In addition, although the yield of chl, chl-7b-1, chl-7b-2, chl-7d-1, and rice OE lines decreased, they did not affect the heading stage, so the effect of TaCHLI-7D on the yield may be more complicated (Fig. 5, Fig. S1).
Haplotype analysis is an efficient germplasm resource screening method (Wang et al. 2023). Previous SiCHLI haplotype studies showed that the haplotype was significantly correlated with photosynthetic metabolic potential at seedling and the mature stage (Liang et al. 2023). After haplotyping TaCHLI-7A/B/D, TaCHLI-7A haplotypes exerted significant regulatory effects on flag leaf length and width, and TaCHLI-7A-HapII, with shorter flag leaves and better yields, was widely selected in wheat breeding processes in China (Fig. 7A–H). Also, TaCHLI-7B haplotypes affected plant height, spike exsertion length, and yield per plant (Fig. 7K, L, N), with TaCHLI-7B-HapII positively selected in wheat breeding process (Fig. S2B). Since the natural variation in TaCHLI-7A/B was mainly in the promoter and variation in the gene region does not result in amino acid changes, we hypothesize that there are variations in gene expression across haplotypes (Fig. 7 A, I). The genetic backgrounds of natural populations are different, and superior haplotypes affect agronomic traits at different times, and our next step will be to investigate the differences in promoter expression between haplotypes by using the Dual Luciferase Reporter Assay System (DLR).
Recent leaf color mutant studies reported that genes associated with autophagy mechanisms were involved in plant stress responses, and were significantly related to plant aging (Wang et al. 2018). Wheat and rice studies reported that leaf color mutants demonstrated resistance to powdery mildew or drought (Ramkumar et al. 2019; Zhang et al. 2020), indicating potential relationships between photosynthesis-related mutations and plant resistance to stress and disease. We used PEG6000 to simulate drought treatment of WT and chl at the first leaf stage (Fig. S3). Before treatment, chl plants were small and weak, but after treatment and recovery, chl showed a higher survival rate and CDC (Fig. S4 D-E). This observation suggested that chl enhanced drought tolerance, although the precise mechanisms warrant further investigation. Salinity treatment data showed that chl was not salinity resistant compared to WT (Fig. S4). We used the same PEG concentrations and salt-alkali treatment solutions to treat WT and chl seeds for 7 days when compared with plants cultured for 7 days (Fig. S5A–C). We observed that chl was resistant to drought both at the seedling and one-leaf stage (Fig. S5C). Additionally, elevated chl photooxidation resistance, possibly due to increased carotenoid levels, may also have enhanced drought resistance (Fig. 1G).
Respiration has important influences on drought stress resistance (Shah and Paulsen 2003), drought-tolerant crops in previous studies generally have stronger respiration, and stomata are generally more efficient and numerous (Hadi et al. 2016). Microscope observation of stomata at the one-leaf stage showed that the stomata of chl showed deformation, but the total number of stomata was not significantly different compared to WT (Fig. S7). The reason for the drought resistance of chl was speculated to be related to the higher nitrogen efficiency of low chlorophyll plants mentioned in previous reports (Leister 2023). To verify this, we treated plants with different nitrogen concentrations and showed that SPAD differences became smaller (Fig. S9). In metabolomic strawberry studies, a yellow-green leaf mutant promoted nitrogen assimilation and inhibited carbon assimilation (Ma et al. 2023). These studies proved that an appropriate reduction of chlorophyll content was significant in developing drought-resistant crops, and may provide new directions for drought-resistant crops in the future.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Transcriptome data (project number: PRJNA1087981) are available from the National Genomics Data Center (https://ngdc.cncb.ac.cn/).
References
Brenchley R, Spannagl M, Pfeifer M, Barker GLA, D’Amore R, Allen AM, McKenzie N, Kramer M, Kerhornou A, Bolser D, Kay S, Waite D, Trick M, Bancroft I, Gu Y, Huo N, Luo M-C, Sehgal S, Gill B, Kianian S, Anderson O, Kersey P, Dvorak J, McCombie WR, Hall A, Mayer KFX, Edwards KJ, Bevan MW, Hall N (2012) Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature 491:705–710
Brzezowski P, Richter AS, Grimm B (2015) Regulation and function of tetrapyrrole biosynthesis in plants and algae. BBA-Bioenergetics 1847:968–985
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R (2020) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13:1194–1202
Ding Y, Yang W, Su C, Ma H, Pan Y, Zhang X, Li J (2019) Tandem 13-lipoxygenase genes in a cluster confers yellow-green leaf in cucumber. Int J Mol Sci 20:3102
Dong J, Chen W (2013) The role of autophagy in chloroplast degradation and chlorophagy in immune defenses during Pst DC3000 (AvrRps4) infection. PLoS ONE 8:e73091
Ferroni L, Živčak M, Sytar O, Kovár M, Watanabe N, Pancaldi S, Baldisserotto C, Brestič M (2020) Chlorophyll-depleted wheat mutants are disturbed in photosynthetic electron flow regulation but can retain an acclimation ability to a fluctuating light regime. Environ Exp Bot 178:104156
Flood PJ, Harbinson J, Aarts MGM (2011) Natural genetic variation in plant photosynthesis. Trends in Plant Sci 16:327–335
Grieco M, Roustan V, Dermendjiev G, Rantala S, Jain A, Leonardelli M, Neumann K, Berger V, Engelmeier D, Bachmann G, Ebersberger I, Aro EM, Weckwerth W, Teige M (2020) Adjustment of photosynthetic activity to drought and fluctuating light in wheat. Plant Cell Environ 43:1484–1500
Hadi PA, Armin SM, Hassan P, Mohammad P (2016) Stomatal responses to drought stress. Water Stress and Crop Plants, pp 24–40
Hansson A, Kannangara CG, Wettstein D, Hasson M (1999) Molecular basis for semidominance of missense mutations in the XANTHA-H (42-kDa) subunit of magnesium chelatase. P Natl Acad Sci USA 96:1744–1749
Hansson A, Willows RD, Roberts TH, Hansson M (2002) Three semidominant barley mutants with single amino acid substitutions in the smallest magnesium chelatase subunit form defective AAA+ hexamers. P Natl Acad Sci USA 99:13944–13949
Huang Y-S, Li H-m (2009) Arabidopsis CHLI2 can substitute for CHLI1. Plant Physiol 150:636–645
Jensen PE, Gibson LCD, Henningsen KW, Hunter CN (1996) Expression of the chlI, chlD, and chlH genes from the Cyanobacterium synechocystis PCC6803 in Escherichia coli and demonstration that the three cognate proteins are required for magnesium-protoporphyrin chelatase activity. J Biol Chem 271:16662–16667
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Leister D (2023) Enhancing the light reactions of photosynthesis: Strategies, controversies, and perspectives. Mol Plant 16:4–22
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25(14):1754–1760
Li Q, Fang C, Duan Z, Liu Y, Qin H, Zhang J, Sun P, Li W, Wang G, Tian Z (2016) Functional conservation and divergence of GmCHLI genes in polyploid soybean. Plant J 88:584–596
Liang H, He Q, Zhang H, Zhi H, Tang S, Wang H, Meng Q, Jia G, Chang J, Diao X (2023) Identification and haplotype analysis ofSiCHLI: a gene for yellow–green seedling as morphological marker to accelerate foxtail millet (Setaria italica) hybrid breeding. Theor Appl Genet 136:24
Liu X, Yang C, Han F, Fang Z, Yang L, Zhuang M, Lv H, Liu Y, Li Z, Zhang Y (2016) Genetics and fine mapping of a yellow-green leaf gene (ygl-1) in cabbage (Brassica oleracea var. capitata L.). Mol Breeding 36:82
Ma X, Sun X, Li C, Huan R, Sun C, Wang Y, Xiao F, Wang Q, Chen P, Ma F, Zhang K, Wang P, Deng X (2017) Map-based cloning and characterization of the novel yellow-green leaf gene ys83 in rice (Oryza sativa). Plant Physiol Bioch 111:1–9
Ma Y-Y, Shi J, Wang D, Liang X, Wei F, Gong C-M, Qiu L, Zhou H, Folta KM, Wen Y, Feng J (2023) A point mutation in the gene encoding magnesium chelatase I subunit influences strawberry leaf color and metabolism. Plant Physiol 192:2737–2755
Makino A (2011) Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiol 155:125–129
Markwell J, Osterman JC, Mitchell JL (1995) Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Technical Commun-Stc 46:467–472
Matsumoto F, Obayashi T, Sasaki-Sekimoto Y, Ohta H, Takamiya K-i, Masuda T (2004) Gene expression profiling of the tetrapyrrole metabolic pathway in Arabidopsis with a mini-array system. Plant Physiol 135:2379–2391
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303
Pogson BJ, Albrecht V (2011) Genetic dissection of chloroplast biogenesis and development: an overview. Plant Physiol 155:1545–1551
Ramkumar MK, Senthil Kumar S, Gaikwad K, Pandey R, Chinnusamy V, Singh NK, Singh AK, Mohapatra T, Sevanthi AM (2019) A novel stay-green mutant of rice with delayed leaf senescence and better harvest index confers drought tolerance. Plants 8:375
Rissler HM, Collakova E, DellaPenna D, Whelan J, Pogson BJ (2002) Chlorophyll biosynthesis. Expression of a second Chl I gene of magnesium chelatase in Arabidopsis supports only limited chlorophyll synthesis. Plant Physiol 128:770–779
Shah NH, Paulsen GM (2003) Interaction of drought and high temperature on photosynthesis and grain-filling of wheat. Plant Soil 257:219–226
Sharp PJ, Kreis M, Shewry PR (1988) Location of β-amylase sequences in wheat and its relatives. Theor Appl Genet 75:286–290
Shim K-C, Kang Y, Song J, Kim YJ, Kim JK, Kim C, Tai TH, Park I, Ahn S (2023) A frameshift mutation in the Mg-chelatase I subunit gene OsCHLI is associated with a lethal chlorophyll-deficient, yellow seedling phenotype in rice. Plants 12:2831
Singh J, Pandey P, James D, Chandrasekhar K, Achary VMM, Kaul T, Tripathy BC, Reddy MK (2014) Enhancing C3 photosynthesis: an outlook on feasible interventions for crop improvement. Plant Biotechnol J 12:1217–1230
Song L, Liu J, Cao B, Liu B, Zhang X, Chen Z, Dong C, Liu X, Zhang Z, Wang W, Chai L, Liu J, Zhu J, Cui S, He F, Peng H, Hu Z, Su Z, Guo W, Xin M, Yao Y, Yan Y, Song Y, Bai G, Sun Q, Ni Z (2023) Reducing brassinosteroid signalling enhances grain yield in semi-dwarf wheat. Nature 617:118–124
Vale RD (2004) AAA Proteins. Lords of the ring. J Cell Biol 150:F13-19
Wang Y, Zheng W, Zheng W, Zhu J, Liu Z, Qin J, Li H (2017) Physiological and transcriptomic analyses of a yellow-green mutant with high photosynthetic efficiency in wheat (Triticum aestivum L.). Funct Integr Genomic 18:175–194
Wang P, Mugume Y, Bassham DC (2018) New advances in autophagy in plants: Regulation, selectivity and function. Semin Cell Dev Biol 80:113–122
Wang C, Zhang L, Li Y, Ali Buttar Z, Wang N, Xie Y (2020) Single nucleotide mutagenesis of the TaCHLI gene suppressed chlorophyll and fatty acid biosynthesis in common wheat seedlings. Front Plant Sci 11:97
Wang Y, Wang J, Chen L, Meng X, Zhen X, Liang Y, Han Y, Li H, Zhang B (2022) Identification and function analysis of yellow-leaf mutant (YX-yl) of broomcorn millet. BMC Plant Biol 22:463
Wang Y, Wang X, Zhai L, Zafar S, Shen C, Zhu S, Chen K, Wang Y, Xu J (2023) A novel effective panicle number per plant 4 haplotype enhances grain yield by coordinating panicle number and grain number in rice. Crop J 12:202–212
Wu Y, Fu Y, Zhao S, Gu P, Zhu Z, Sun C, Tan L (2016) CLUSTERED PRIMARY BRANCH 1, a new allele of DWARF11, controls panicle architecture and seed size in rice. Plant Biotechnol J 14:377–386
Wu H, Shi N, An X, Liu C, Fu H, Cao L, Feng Y, Sun D, Zhang L (2018) Candidate genes for yellow leaf color in common wheat (Triticum aestivum L.) and major related metabolic pathways according to transcriptome Profiling. Int J Mol Sci 19:1594
Wu C, Wang J, Zhu J, Ren J, Yang Y, Luo T, Xu L, Zhou Q, Xiao X, Zhou Y, Luo S (2022) Molecular characterization of Mg-chelatase CHLI subunit in Pea (Pisum sativum L). Front Plant Sci 13:821683
Xu H, Sun H, Dong J, Ma C, Li J, Li Z, Wang Y, Ji J, Hu X, Wu M, Zhao C, Qin R, Wu J, Ni F, Cui F, Wu Y (2022) The brassinosteroid biosynthesis gene TaD11-2A controls grain size and its elite haplotype improves wheat grain yields. Theor Appl Genet 135:2907–2923
Yoo S, Cho Y, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2:1565–1572
Zhang L, Liu C, An X, Wu H, Feng Y, Wang H, Sun D (2017) Identification and genetic mapping of a novel incompletely dominant yellow leaf color gene, Y1718, on chromosome 2BS in wheat. Euphytica 213:1–11
Zhang H, Chen Y, Niu Y, Zhang X, Zhao J, Sun L, Wang H, Xiao J, Wang X (2020) Characterization and fine mapping of a leaf yellowing mutant in common wheat. Plant Growth Regul 92:233–247
Zhang L, Dong C, Chen Z, Gui L, Chen C, Li D, Xie Z, Zhang Q, Zhang X, Xia C, Liu X, Kong X, Wang J (2021) WheatGmap: a comprehensive platform for wheat gene mapping and genomic studies. Mol Plant 14:187–190
Zhang H, Yu F, Xie P, Sun S, Qiao X, Tang S, Chen C, Yang S, Mei C, Yang D, Wu Y, Xia R, Li X, Lu J, Liu Y, Xie X, Ma D, Xu X, Liang Z, Feng Z, Huang X, Yu H, Liu G, Wang Y, Li J, Zhang Q, Chen C, Ouyang Y, Xie Q (2023) A Gγ protein regulates alkaline sensitivity in crops. Science 379:eade8416
Zhu T, Wang L, Rimbert H, Rodriguez JC, Deal KR, De Oliveira R, Choulet F, Keeble-Gagnère G, Tibbits J, Rogers J, Eversole K, Appels R, Gu YQ, Mascher M, Dvorak J, Luo MC (2021) Optical maps refine the bread wheat Triticum aestivum cv. Chin Spring Genome Assembly Plant J 107:303–314
Funding
This work was supported by Shandong Provincial Fund for Excellent Young Scholars (Grant No. ZR2022YQ19), the National Natural Science Foundation of China (Grant Nos. 32072051, 32272119 and 32101726), the Agricultural Variety Improvement Project of Shandong Province (Grant No. 2021LZGC013), the Natural Science Foundation of Shandong Province, China (grant no. ZR2022MC119), and the Taishan scholar young expert (grant no. 20230119).
Author information
Authors and Affiliations
Contributions
YW designed the experiments and managed the project. ZW, HX, FW, LS, XM, ZL, CX, HJ, GD, XH, and YG performed experiments. FC, HS, RQ, and CZ supervised the project and provided technical assistance. YW, ZW, and HX analyzed data and wrote the manuscript. All authors have read and agreed to the final published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Communicated by Philomin Juliana.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Xu, H., Wang, F. et al. EMS-induced missense mutation in TaCHLI-7D affects leaf color and yield-related traits in wheat. Theor Appl Genet 137, 223 (2024). https://doi.org/10.1007/s00122-024-04740-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-024-04740-8