Abstract
Dioscorea alata is a polyploid species with several ploidy levels and its basic chromosome number has been considered by most authors to be x = 10. Standard chromosome counting and flow cytometry analysis were used to determine the chromosome number of 110 D. alata accessions of the CIRAD germplasm collection. The results revealed that 76% of accessions have 2n = 40 chromosomes, 7% have 2n = 60 chromosomes and 17% have 2n = 80 chromosomes. Progenies were produced from 2n = 40 types of D. alata and the segregation patterns of six microsatellite markers in four different progenies were analysed. The Bayesian method was used to test for diploid versus tetraploid (allo- and autotetraploid) modes of inheritance. The results provided the genetic evidence to establish the diploidy of plants with 2n = 40 chromosomes and to support the hypothesis that plants with 2n = 40, 60 and 80 chromosomes are diploids, triploids and tetraploids, respectively, and that the basic chromosome number of D. alata is x = 20. The findings obtained in the present study are significant for effective breeding programs, genetic diversity analysis and elucidation of the phylogeny and the species origin of D. alata.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yams belonging to the genus Dioscorea, family Dioscoreaceae, constitute an important staple food in tropical and subtropical regions (Ayensu and Coursey 1972). D. alata and D. rotundata are the two major cultivated species with the greatest economic importance and are widely distributed throughout the world. D. alata is native to the Asia-Pacific and D. rotundata to Africa, and both belong to the same botanical section, Enantiophyllum. The importance of yams in terms of food security has given impetus to the establishment of several genetic improvement programmes of D. alata throughout the tropical regions, in order to develop high-yielding varieties with resistance to diseases and tuber characteristics adapted to farmers’ requirements. However, little is known about the origin, diversity and genetics of this species, limiting the effectiveness of genetic improvement programs.
The genus Dioscorea includes over 600 species. From the published cytological studies on 72 Dioscorea species, it was concluded that the basic chromosome number of Dioscorea species is x = 10 or 9 (Essad 1984). The basic chromosome number of x = 10 is reported in all the Asian species. The African and American species are considered to have a chromosome number of x = 10 or 9. However, recent segregation studies of microsatellite markers have revealed that the so called tetraploid species D. rotundata (2n = 40) is a diploid with the basic chromosome number of x = 20 (Scarcelli et al. 2005). Another recent report, also based on SSR marker segregation studies, has revealed that the American yam D. trifida (2n = 80), belonging to the section Macrogynodium, which was considered to be octoploid, is actually a tetraploid with the basic chromosome number of x = 20 (Bousalem et al. 2006).
The species D. alata, in contrast to the two mentioned above, consists of accessions with different chromosome numbers. Several studies have been conducted to determine the chromosome number of D. alata accessions using chromosome counts and flow cytometry analysis. Sharma and De (1956) and Sharma and Sharma (1957) indicated the existence of clones with 2n = 20, 30, 40, 50 and 70 chromosomes. Three levels of ploidy (2n = 40, 60 and 80) were described by Sundara Raghavan (1958) and Ramachandran (1968). The extensive cytological screening of Indian accessions by Abraham and Nair (1991), Caribbean accessions by Gamiette et al. (1999) and Pacific accessions by Malapa et al. (2005) also revealed only three ploidy levels of 2n = 40, 60, and 80 chromosomes. These authors concluded that plants with 2n = 40, 60 and 80 chromosomes are tetraploids, hexaploids and octoploids (2n = 4x, 6x, 8x), respectively, on the basis of a basic number x = 10.
Meiotic studies of D. alata were carried out by Abraham and Nair (1991) and Abraham (1998) on 2n = 40 and 60 varieties. The authors observed bivalent formation in 2n = 40 types and trivalent formation in 2n = 60 types and concluded that they were allotetraploids and auto-allohexaploids, respectively.
A genetic linkage map was constructed on 2n = 40 D. alata genotypes based on AFLP markers (Mignouna et al. 2002). The authors observed that markers segregated like a diploid and suggested that 2n = 40 types are allotetraploids.
The aim of the present study was to re-examine the ploidy status of D. alata, and to arrive at a reliable conclusion on its basic chromosome number, which is fundamental information required for effective breeding programs and genetic diversity studies. We studied the segregation patterns of six microsatellite markers in four different progenies derived from crosses between genotypes with the chromosome number of 2n = 40, and we used the Bayesian method to test different modes of inheritance (diploidy, allotetraploidy and autotetraploidy) and to determine the most probable segregation hypothesis. We also determined the number of chromosomes in our genetic resource collections using standard chromosome counting and flow cytometry analysis.
Materials and methods
Plant materials for segregation analysis
Four progenies derived from crossing four male clones (24M, 17M, 31M and Pyramide) with two distinct female parents (27F and St. Vincent) were analyzed. St. Vincent and Pyramide are varieties cultivated in the West Indies. The other four genotypes are breeding lines originating from the D. alata selection program at CIRAD, Guadeloupe. All the clones have 2n = 40 chromosomes. Female plants were grown in isolated plots and surrounded by the male clones. Seed production varied from one population/plot to the next. Seeds were germinated in a greenhouse and transplanted to the field. The number of plants for the four populations were 65 (24M × 27F), 25 (17M × St. Vincent), 20 (31M × 27F) and 15 (Pyramide × 27F) individuals, respectively.
Cytometry screening and chromosome counting
One hundred and ten accessions of the CIRAD D. alata germplasm collection were analyzed using flow cytometry (Table 1).
Mitotic chromosome counts were carried out on root tips of four representative accessions (639a, 613a, 760a and 754a) known to have a different chromosome number according to previous flux cytometry analysis (Malapa et al. 2005).
Methods
DNA extraction
DNA was extracted from 100 mg of leaves using the DNeasy Plant Mini Kit (Qiagen).
Screening of SSR markers
Six SSR markers were used in the study. Three were developed from D. alata species (Da2F10, Da1F8, Da3G04) and three from the wild yam D. abyssinica (Dab2E07, Dab2D11, Dab2D08) (Tostain et al. 2006). These markers were selected from a set of 40 SSR markers developed from five different yam species, viz., D. alata, D. abyssinica, D praehensilis (Tostain et al. 2006), D. japonica (Misuki et al. 2005) and D. trifida (Hochu et al. 2006), for their capacity to reveal a high polymorphism on a maximum 133 D. alata accessions of the CIRAD germplasm collection.
Microsatellite amplification
Amplification reactions were performed in a final volume of 20 μL in the presence of 5 ng of template DNA, 4 pmol of the reverse primer, 1 pmol of the forward primer, 0.2 mM of each deoxynucleotide, 2 mM MgCl2 and 1 U Taq polymerase. The forward primer was 5′-labelled with one of the three fluorophores (FAM, TET or HEX). PCR was carried out using a PTC100 thermocycler (MJ Research). After 5 min at 94°C, 30 cycles were performed as follows: 30 s at 94°C, 30 s at annealing temperature and 30 s at 72°C, followed by a final extension step of 5 min at 72°C. Amplified products were detected on an ABI PRISM™ 3100 automatic sequencer (Applied Biosystems), and microsatellite alleles were scored using GENESCAN 3.1 software (Applied Biosystems).
Microsatellite segregation analysis
The Bayesian method was adopted to discriminate between the different inheritance hypotheses (diploidy, autotetraploidy or allotetraploidy). This approach is relevant to compare many assumptions and can be used even when observed genotypes are unexpected under a scenario, or sample sizes are small, compared to χ2 statistics (Olson 1997; Posada and Buckley 2004; Beaumont and Rannala 2004; Bousalem et al. 2006; Catalán et al. 2006).
Assignment of parental genotypes
Under the diploid model, the parental genotypes could be directly assigned from the scored SSR profiles, since a maximum of two alleles were observed for each parent genotype for all loci analyzed. In this case, individuals with a single amplified product are expected to be homozygous (aa) if null alleles are absent, and individuals with two amplified products be heterozygous (ab).
Under the autotetraploid model, individuals with a single amplified product are expected to be homozygous (having four copies or the same allele), if null allele are absent, and individuals with two amplified products represent more complex cases since different genotype configurations are possible, depending on whether the alleles are in equal dosages (aabb) or in triple dosages (aaab/abbb). All possible genotypic configurations were tested.
Under the allotetraploid model, the observed amplification products of an individual may correspond to two different genomic complements. Two different hypotheses were tested: first, we considered that two independently segregating loci may present alleles of the same sizes (homoplasy, hypothesis A) and, second, we considered that alleles of the same size may not be shared between the two loci (hypothesis B). Under the homoplasy hypothesis, individuals with two amplified products (ab) can correspond at seven different genotypic configuration levels: aa/bb, bb/aa, ab/ab, aa/ab, ab/aa, ab/bb, and bb/ab, and all were included in the analysis.
Computation of Bayesian probabilities
The probabilities of observing the progeny genotypes under each hypothesis or likelihoods were computed using the multinomial distribution described by Olson (1997):
where n is the total number of progeny, n i is the observed number of progeny of genotype i, and P i (H j ) is the expected proportion of genotype i under hypothesis j. To take the observed, but unexpected genotypes under a given scenario into account, a P error value of 10−3 was assigned to this genotypic class (Bousalem et al. 2006). For the tetraploid inheritance hypotheses, the expected proportions of each genotype in progeny and likelihoods were computed using a PERL program designed by J. David (Bousalem et al. 2006).
Computation of Bayes factors
A Bayes factor in favor of diploidy over tetraploidy was computed for each microsatellite marker in each population as follows:
where Hd i is the ith diploidy hypothesis (there is only one in this case), and Ht i is the ith tetraploidy hypothesis.
A Bayes factor <1 indicates that the 4x hypothesis is more likely than the 2x hypothesis, whereas a Bayes factor value >1 indicates that the 2x hypothesis is more likely than the 4x hypothesis. A Bayes factor value of 20 indicates that diploidy is 20 times more likely than tetraploidy, and provides positive evidence to support the diploid hypothesis. A Bayes factor >200 indicates that the 2x hypothesis is 200 times more likely than the 4x hypothesis, and provides very strong evidence to support the diploid hypothesis (Kass and Raftery 1995; Bousalem et al. 2006).
Testing independence of SSR loci
The independence of microsatellite loci was tested with the independence LOD Score test of JoinMap 4 software (Van Ooijen 2006). Since low LOD scores may reveal light links (Scarcelli et al. 2005), three different LOD score values were tested (4, 3 and 2).
Chromosome counting
Only mitotic chromosome counts were made. Roots were harvested from plants grown in pots. They were treated with 0.04% 8-hydroxyquinoline for 4 h, fixed for 48 h in 3:1 ethanol:acetic acid and stored in 70% ethanol at 4°C. The fixed roots were rinsed twice in H2O for 10 min each, treated in 0.25 N HCl for 10 min, rinsed for 10 min in H2O and placed in a digestion buffer (0.01 M citrate buffer, pH 4.5, 0.075 M KCl) for 10 min. The root tips were cut and placed in an enzyme solution (5% Onozuka R-10 cellulase, 1% Y-23 pectolyase in a digestion buffer) in a microtube at 37°C for 60–90 min (the time varying with the size of the roots). Root tips were then rinsed in H2O and spread on a slide with a drop of 3:1 ethanol:acetic acid. Slides were stored at −70°C. The chromosomes were counterstained with DAPI (4′, 6-diamidino-2-phenylindole). The slides were mounted in vectashield antifade solution (Vector Lab), observed under an OLYMPUS DP50 microscope and photographed with analySIS (R) Soft-Imaging-System software. A minimum of three different metaphases were inspected per genotype.
Ploidy screening by flow cytometry
Isolation and staining of nuclei
To isolate and stain nuclei, 0.5 cm2 of leaves were chopped up with the same amount of leaves of an internal standard with a double-edged razor blade in 500 μl of extraction buffer (0.14 M NaCl, 0.003 M KCl, 0.012 M NaH2PO4, 0.002 M KH2PO4, 0.25% triton X-100 and 100 μl of β-mercaptoethanol, pH 7.4). The suspension was filtered through a 15-μm pore filter and mixed with 150 μl of extraction buffer supplemented with propidium iodide (200 μg/ml). The suspensions were then incubated for approximately 5 min at room temperature. After incubation, each sample was run on a flow cytometer.
Flow cytometric analysis
Measurements were performed on a FACScalibur Laser flow cytometer (Beckton Dickinson, USA). Intensity histograms were evaluated with WinMDI software Ver. 2.8.
Data analysis and estimation of nuclear genome size
The nuclear DNA contents of the different accessions were calculated by comparison of the relative positions of the G 0–1 peaks of the clone ‘760a’ used as an internal reference, which has 2n = 60 chromosomes.
Measurements of the genome size of D. alata yam were made by comparison with a control “Lisbon” lemon, whose absolute genome size had been previously measured at 0.786 pg/2C (Ollitrault and Jacquemond 1994). Calculations were made according to the formula:
where Q = yam clone ‘760a’ DNA content (pg/2C), R = control 2C DNA content (0.786 pg), E = yam G 0–1 peak mean, S = control G 0–1 peak mean.
Results
Flow cytometry and chromosome counts
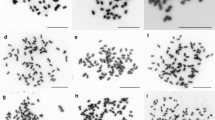
Mitotic chromosome counts indicated that clones 639a and 613a had 40 chromosomes (39 ± 1 and 38 ± 1.5), the clone 760a had 60 chromosomes (59 ± 1.5) and the clone 754a had 80 chromosomes (79 ± 1.5). Figure 1 presents a cell at metaphasic stage of the clone ‘760a’ used as an internal reference. Chromosome counting in yam is difficult because of the small size of chromosomes, their tendency to stick together and their satellites that are sometimes as large as the chromosomes themselves (Essad 1984; Gamiette et al. 1999; Bousalem et al. 2006).
The use of flow cytometry made it possible to classify D. alata accessions into three clusters that correspond to the following 2C DNA amounts: 0.93 ± 0.08 pg, 1.43 pg, 1.98 ± 0.05 pg. Results revealed that 84 genotypes had 2n = 40 chromosomes (cluster I), eight clones had the same chromosome number as the internal reference (2n = 60, cluster II) and 18 clones had 2n = 80 chromosomes (cluster III). Figure 2 shows the histograms obtained for one clone having 2n = 40 chromosomes and one clone having 2n = 80 chromosomes, using the clone 760a (2n = 60) as an internal reference.
Microsatellite marker inheritance
Population screening for contaminated genotypes
We first checked if some individuals could have a different male parent that expected or could result from parthenocarpy. Five contamined genotypes that did not have the alleles of the expected male parent were identified in the 24M × 27F cross, two in the 17M × St. Vincent cross, three in the 31M × 27F cross and five in the Pyramide × 27F cross. Finally, the four segregating populations totaled 60, 23, 17 and 10 individuals, respectively. To exemplify the computation of the Bayes factor, we first illustrated (Table 2), the results for the Da2F10 loci on the 24M × 27F population. The male has two alleles, 114 and 116 bp (phenotype ab), and the female 27F has two alleles, 116 and 132 bp (phenotype bc).
Tetraploid inheritance likelihoods
Since the two parents have only two alleles, it would mean that one of the alleles is in triple dosage or that the two alleles are in equal dosages. Under polysomic inheritance 4x, three allelic configurations are possible for each parental genotype (abbb, aaab, aabb and bccc, bbbc, bbcc), yielding nine segregation hypotheses. Under disomic inheritance 2x, seven allelic configurations are possible for the male parent (aa/bb, bb/aa, ab/ab, aa/ab, ab/aa, ab/bb and bb/ab) and seven for the female parent (bb/cc, cc/bb, bc/bc, bb/bc, bc/bb, bc/cc and cc/bc), yielding 48 different scenarios.
Likelihoods of the tetraploid hypotheses varied from 10−22 to 10−120 (Table 2).
Diploidy versus tetraploidy
Comparing the likelihoods of the tetraploid segregations (10−22 to 10−120) and the diploid pattern (10−6), diploidy appeared to be the most likely hypothesis for Da2F10 locus. The Bayes factors testing diploidy versus tetraploidy clearly indicate that diploid segregation is much more likely than tetraploid segregation. Under the homoplasy hypothesis (two independently segregating loci may present alleles of the same sizes), diploidy is 6 × 1016 times more likely than tetraploidy. Under the most restrictive conditions (non-shared allelic size), the Bayes factor value was higher (BF = 5 × 1018). This locus showed a significant deviation from the expected Mendelian segregation ratio, which is common in plants and can arise either from differences in gametophytic or sporophytic viability or from differences in seed viability (Zamir and Tadmor 1986; Lyttle 1991; Taylor and Ingvarsson 2003).
The Bayes factors under the other loci and the four segregating populations are given in Table 3. The six microsatellites do not reveal polymorphism for all of the progeny studied, and the microsatellites shown are those that are polymorphous for each of the progenies. Results showed that the diploid inheritance appeared much more likely (FB > 2 × 102) than the tetraploid inheritance for three loci in the population 17M × St. Vincent (Da2F10, FB = 7 × 1013; Da3GO4, FB = 4 × 109; Dab2D11, FB = 3 × 102), two loci in the population Pyramide × 27F (Da2F10, FB = 6 × 102; Dab2D11, FB = 1 × 103) and one locus (Da2F10) in the populations 24M × 27F (BF = 6 × 1016) and 31M × 27F (FB = 4 × 102). Under the most restrictive conditions (non-shared allelic size), the Bayes factors were significantly higher for all the loci except for one (population Pyramide × 27F, locus Da2F10). For the other loci, no statistical difference clearly appeared between the likelihood of different scenarios.
Significant effects of population size and the number of segregating alleles were observed. The Bayes factor values for the loci Da2F10 were significantly lower in the small populations, Pyramide × 27F (N = 10, FB = 6 × 102) and 31M × 27F (N = 17, FB = 4 × 102), than for the large population, 24M × 27F (N = 60, FB = 6 × 1016), but they could differentiate between the diploid and tetraploid distributions. The most discriminating loci are these with more than two segregating alleles (three and four alleles). This suggests that diploid inheritance could be confused with tetraploid inheritance when the number of segregating alleles in the population is too low.
We carried out analyses to see if the three microsatellite loci (Da2F10, Da3G04 and Dab2D11) that gave the most robust results (FB > 2 × 102) were independent. These microsatellites were found to be independent for all LOD scores tested (4, 3 and 2).
Discussion
The results in the present study provide genetic evidence supporting a diploid segregation of D. alata accessions with 2n = 40 chromosomes, based on segregation patterns of microsatellite markers in four different crosses. The Bayesian approach made it possible for us to compare a high number of segregation hypotheses corresponding to the three possible segregation models (diploidy, autotetraploidy or allotetraploidy). The possibility of differentiating between the diploid and tetraploid segregations clearly depended on the number of alleles segregating in the populations. Under the tetraploid model, the number of genotypic classes is higher than under diploidy, and the observed genotypes have more of a chance to fit into one of the expected classes when the number of segregating alleles is low. For loci with two segregating alleles (ab), the diploid inheritance appeared slightly more likely than the tetraploid scenarios, or no statistical differences were observed. On the other hand, the segregation pattern of loci with three (abc) and four (abcd) alleles clearly indicated that diploid segregation is much more likely than tetraploid segregation. In such cases, we should observe some individuals with all the observed alleles (abc/abcd) in the progenies. However, this pattern has never been observed in any of the segregating populations for any locus under study, making tetrasomy very unlikely. These results support the hypothesis that D. alata accessions with 2n = 40 chromosomes would be diploid and not tetraploid as usually assumed.
Only one other complex hypothesis could explain why the observed segregations could reflect tetraploidy. This is the allotetraploid model with two null alleles in one genome. However, this sounds very unlikely with the results obtained for the loci Da2F10, as it would mean that all six parental varieties have two null alleles. Another explanation would be that the diploid pattern is associated with the non-detection of microsatellite polymorphism in one of the two genomes. This also seems unlikely in view of the results obtained in our microsatellite marker screening. Forty different SSRs were tested that have been developed from five different yam species: D. alata, D. abyssinica, D. praehensilis, D. japonica and D. trifida. We observed that plants with 2n = 40, 60 and 80 chromosomes had no more than two, three and four alleles per locus, respectively, for all 40 loci analyzed (data not shown), corresponding to patterns of diploid, triploid and tetraploid plants, respectively. Moreover, we tested two SSR primers derived from D. trifida species belonging to the botanical section Macrogynodium from which D. alata (Enantiophyllum section) is very distant. Their capacity to amplify on this distant species makes it unlikely that they were not able to amplify D. alata’s putative homologous genomes.
There have been conflicting results on the ploidy levels of D. alata reported in both older and more recent studies. Five levels of ploidy (2n = 20, 30, 40, 50 and 70) were described by Sharma and De (1956) and Sharma and Sharma (1957). Sundara Raghavan (1958) and Ramachandran (1968) indicated the existence of clones with 2n = 40, 60 and 80 chromosomes. Hamon et al. (1992) indicated the existence of six ploidy levels, 3x, 3.5x, 4.5x, 6x, 7x and 8.5x, by flow cytometry analysis. The most reliable studies are those of Abraham and Nair (1991), Gamiette et al. (1999) and Malapa et al. (2005) who observed clones with 2n = 40, 60 and 80 chromosomes and concluded that three ploidy levels, 2n = 4x, 6x and 8x, existed on the basis of a basic number x = 10. Our results are in agreement with those of the latter three authors, but since the tetraploidy of clones with 2n = 40 chromosomes is very unlikely and diploidy suggested, we propose that the ploidy levels are actually 2x, 3x and 4x, and that the basic number is x = 20. Our finding on the basic chromosome number of x = 20 for D. alata is in agreement with the recent results obtained for D. rotundata, which is in the same botanical section Enantiophyllum as D. alata (Scarcelli et al. 2005) and also for D. trifida that belongs to the more distant Macrogynodium section (Bousalem et al. 2006). More research is needed to re-examine the basic chromosome numbers of other species of Dioscoreaceae.
The 2n = 60 plants are now considered as triploids (previously considered as hexaploids). The males are found to be sterile with irregular meiosis (Abraham 1998) and the females on pollination with fertile pollen do not set viable seeds (Abraham and Nair 1991).
Our results show that diploid D. alata clones exist, whereas this had not been previously proven (Essad 1984; Gamiette et al. 1999; Mignouna et al. 2002). The origin of D. alata is subject to debate. One of the hypotheses is that D. alata is an interspecific hybrid between the wild species D. persimilis and D. hamiltonii (Prain and Burkill 1939; Degras 1993; Mignouna et al. 2002). The fact that the clones with 2n = 40 chromosomes are diploids and not allotetraploids as previously supposed (Abraham and Nair 1991; Gamiette et al. 1999; Mignouna et al. 2002), invalidates this assumption.
Conclusion and future prospects
In conclusion, our results in the present study indicate that D. alata accessions with 2n = 40, 60 and 80 chromosomes are diploid, triploid and tetraploid (2n = 2x, 3x, 4x), respectively and not tetraploid, hexaploid and octoploid (2n = 4x, 6x, 8x) as usually assumed. This knowledge is essential for improvement programs and will make it possible to optimise breeding methods to obtain improved varieties. We now need to determine the type of segregation of 2n = 80 tetraploid clones. Studies based on the observation of meioses and the characterisation of allele segregation is in progress and will allow us to determine whether they are auto or allotetraploids.
References
Abraham K (1998) Occurrence of hexaploid males in Dioscorea alata L. Euphytica 99:5–7
Abraham K, Nair PG (1991) Polyploidy and sterility in relation to sex in Dioscorea alata L. (Dioscoreaceae). Genetica 83:93–97
Ayensu ES, Coursey DG (1972) Guinea yams. The botany, ethnobotany, use and possible future of yams in West Africa. Econ bot 26:301–318
Beaumont MA, Rannala B (2004) The Bayesian revolution in genetics. Nat Rev Genet 5:251–261
Bousalem M, Arnau G, Hochu I, Arnolin R, Viader V, Santoni S, David J (2006) Microsatellite segregation analysis and cytogenetic evidence for tetrasomic inheritance in the American yam Dioscorea trifida and a new basic chromosome number in the Dioscoreae. Theor Appl Genet 113:439–451
Catalán P, Segarra-Moragues JG, Palop-Esteban M, Moreno C, Gonzalez-Candelas F (2006) A Bayesian approach for discriminating among alternative inheritance hypotheses in plant polyploids: the allotetraploid origin of genus Borderea (Dioscoreaceae). Genetics 172:1939–1953
Degras L (1993) The yam. A tropical root crop. In: Coste T (ed) MacMillan/ CTA, London and Basingstoke, 408 pp
Essad S (1984) Variation géographique des nombres chromosomiques de base et polyploïdie dans le genre Dioscorea à propos du dénombrement des espèces transversa Brown, pilosiuscula Bert et trifida. Agron 4:611–617
Gamiette F, Bakry F, Ano G (1999) Ploidy determination of some yam species (Dioscorea spp.) by flow cytometry and conventional chromosomes counting. Genet Resour Crop Evol 46:19–27
Hamon P, Brizard JP, Zoundjihekpon J, Duperray C, Borgel A (1992) Etude des index d’ADN de huit espèces d’ignames (Dioscorea sp.) par cytométrie en flux. Can J Bot 70:996–1000
Hochu I, Santoni S, Bousalem M (2006) Isolation, characterization and cross-species amplification of microsatellite DNA loci in the tropical American yam Dioscorea trifida. Mol Ecol Notes 6:137–140
Kass R, Raftery A (1995) Bayes factors. J Am Stat Assoc 90:773–795
Lyttle TW (1991) Segregation distortion. Annu Rev Genet 25:511–557
Malapa R, Arnau G, Noyer JL, Lebot V (2005) Genetic diversity of the greater yam (Dioscorea alata L.) and relatedness to D. nummularia Lam. and D. transversa Br. as revealed with AFLP markers. Genet Resour Crop Evol 52:919–929
Mignouna HD, Mank RA, Ellis THN, Van den Bosch N, Asiedu R, Abang MM, Peleman J (2002) A genetic linkage map of water yam (Dioscorea alata L) based on AFLP markers and QTL analysis for anthracnose resistance. Theor Appl Genet 105:726–735
Misuki I, Tani N, Ishida K, Tsumura Y (2005) Development and characterization of microsatellite markers in a clonal plant, Dioscorea japonica Thunb. Mol Ecol Notes 5:721–723
Ollitrault P, Jacquemond C (1994) Facultative apomixis, spontaneous polyploidization and inbreeding in Citrus volkameriana seedlings. Fruits 49:398–400
Olson MS (1997) Bayesian procedures for discriminating among hypotheses with discrete distributions: inheritance in the tetraploid Astilbe biternub. Genetics 147:1933–1942
Posada D, Buckley TR (2004) Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol 53:793–808
Prain D, Burkill IH (1939) An account of the genus Dioscorea. 1. Species which turn to the right. Ann Rev Bot Gard 14:211–528
Ramachandran K (1968) Cytological studies in Dioscorea. Cytologia 33:401–410
Scarcelli N, Daïnou O, Agbangla C, Tostain S, Pham JL (2005) Segregation patterns of isozyme loci and microsatellite markers show the diploidy of African yam Dioscorea rotundata (2n = 40). Theor Appl Genet 111:226–232
Sharma AK, De DN (1956) Polyploidy in Dioscorea. Genetica 28:112–120
Sharma AK, Sharma A (1957) Investigations leading to a new theory of differentiation in plant cells. Genet Iber 9:143–162
Sundara Raghavan R (1958) A chromosome survey of Indian Dioscoreas. Proc Indian Acad Sci Sect B 48:59–63
Taylor DR, Ingvarsson PK (2003) Common features of segregation distortion in plants and animals. Genetica 117:27–35
Tostain S, Scarcelli N, Brottier P, Marchand JL, Pham JL, Noyer JL (2006) Development of DNA microsatellite markers in tropical yam (Dioscorea sp.). Mol Ecol Notes 6:173–175
Van Ooijen JW (2006) JoinMap 4, software for the calculation of genetic linkage maps in experimental populations. Kyazma BV, Wageningen
Zamir D, Tadmor Y (1986) Unequal segregation of nuclear genes in plants. Bot Gaz 147(3):335–358
Acknowledgments
The authors express their sincere gratitude to IFCPAR (Indo-French Centre for the Promotion of Advanced Research) for supporting this work. We thank Dr. J. David who provide us with the programme to calculate the Bayesian probabilities, and Dr A. D’Hont, D. Triaire and M. Goud who provided support for the chromosome counting.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Bradshaw.
Rights and permissions
About this article
Cite this article
Arnau, G., Nemorin, A., Maledon, E. et al. Revision of ploidy status of Dioscorea alata L. (Dioscoreaceae) by cytogenetic and microsatellite segregation analysis. Theor Appl Genet 118, 1239–1249 (2009). https://doi.org/10.1007/s00122-009-0977-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-009-0977-6