Abstract
Wide-compatibility varieties (WCVs) are a special class of rice germplasm that is able to produce fertile hybrids when crossed to both indica and japonica subspecies. Previous studies determined ‘Dular’ and 02428 as two WCVs and identified a number of QTLs as having large effects on fertility of inter-subspecific hybrids. In this study, we developed five near-isogenic lines (NILs) for three of the QTLs, f5, f6 and S5, by backcrossing and marker-assisted selection, using ‘Dular’ and 02428 as the donors and ‘Zhenshan 97’ as the recipient. Three of the NILs each carried one introgressed allele, and two NILs each carried two introgressed alleles in combinations. The NILs were testcrossed to an indica tester ‘Nanjing 11’ and a japonica tester ‘Balilla’. The results showed that the f5 allele from ‘Dular’ (f5-Du) is a neutral allele conferring wide compatibility, with a large effect on both pollen and spikelet fertility, and the f6 allele from ‘Dular’ (f6-Du) is a neutral allele for spikelet fertility with smaller effect. The S5 allele from 02428 (S5-08) was confirmed to be a neutral allele for spikelet fertility. It is likely that f6 and S5 are the same locus as deduced by their genomic locations and effects. The results also showed that even in combination, two neutral alleles of different loci were not able to produce normal fertility hybrids in typical indica–japonica crosses. The implications of the findings in rice breeding programs are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The strong hybrid vigor in the F1s between indica and japonica subspecies of Asian cultivated rice (Oryza sativa L.) has attracted a large amount of research interest, with the hope for developing hybrid rice by making use of such heterosis (Yang et al. 1962; Chu et al. 1964; Yuan 1987). However, hybrid sterility frequently occurs in such inter-subspecific crosses; the fertility of indica–japonica hybrids varies widely from fully fertile to almost completely sterile, with the majority of such hybrids showing significantly reduced fertility (Kato et al. 1928; Oka 1988; Liu et al. 1996; Zhang et al. 1997).
Wide-compatibility varieties (WCVs) are a special class of rice germplasm that is able to produce fertile hybrids when crossed to both indica and japonica varieties (Ikehashi and Araki 1984). The discovery of WCVs brought hope for breaking the fertility barrier between indica and japonica subspecies and provided a possibility for exploiting the very strong heterosis demonstrated in crosses between the two subspecies. Consequently, there has been considerable interest in understanding the mechanisms underlying wide compatibility and hybrid sterility. Ikehashi and Araki (1986), among others, proposed an allelic interaction model for explaining the mechanism of hybrid sterility and wide compatibility. According to this model, there are three alleles at the S5 locus: a neutral allele (S n5 ), an indica allele (S i5 ), and a japonica allele (S j5 ). A zygote formed of the S n5 allele with either of the other two alleles, e.g., S n5 S i5 or S n5 S j5 , would be fully fertile, whereas a zygote of S i5 S j5 would be partly sterile. Ikehashi and Araki (1986) also located the S5 locus on chromosome 6. Utilization of the wide-compatibility gene (S n5 ) for development of inter-subspecific hybrids has been a practice in many rice breeding programs (Araki et al. 1988; Ikehashi 1991).
However, it was soon realized that the mechanisms underlying hybrid sterility and wide compatibility are rather complex, which cannot be explained by the S5 locus alone. In addition to S5, a number loci causing female gamete abortion by allelic interaction (Yanagihara et al. 1992; Wan et al. 1993, 1996) and QTLs for defective female gametophyte development were also identified in an inter-subspecific cross (Liu et al. 2001). Male gamete abortion was also considered to play a key role in indica–japonica hybrid sterility. Zhang and Lu (1989, 1993) and Zhang et al. (1994) identified six loci for F1 pollen sterility and proposed that hybrid sterility in indica–japonica hybrids was mainly caused by allelic interactions at the F1 pollen sterility loci. Moreover, there were also gametophyte genes that were reported as causing male gamete abortion or selective fertilization and distorted segregation in inter-subspecific hybrids (Lin et al. 1992, 1993; Kinoshita 1995; Lu et al. 2000).
Two WCVs, ‘Dular’ and 02428, have been widely used in indica–japonica hybrid rice breeding programs in China and other parts of the world. Liu et al. (1997), using a three-way cross (02428/‘Nanjing 11’//‘Balilla’) population and a molecular marker linkage map, identified a major locus for wide compatibility that corresponded well to the S5 locus reported previously (Ikehashi and Araki 1986; Liu et al. 1992; Zheng et al. 1992; Yanagihara et al. 1995) and two minor loci located on chromosomes 2 and 12, respectively. Wang et al. (1998), also using a three-way cross (‘Balilla’/‘Dular’//‘Nanjing 11’) population, resolved five QTLs as conferring significant effects on hybrid fertility, with the one on chromosome 5 (f5) showing the largest effect, followed by f6, also seeming to correspond to the S5 region.
However, several issues remain to be resolved. The most important issue concerns whether the alleles from WCVs identified above are neutral alleles conferring wide compatibility or indica–japonica alleles for hybrid sterility. Compatibility tests demonstrated that WCVs vary greatly in both compatible spectra and levels of compatibility (Pan et al. 1990; Liu et al. 1996; Zhang et al. 1997), indicating that the WCVs differ in their alleles for wide compatibility or hybrid sterility. Previous results (Liu et al. 1992; Liu et al. 1997) established beyond doubt that the S5 allele from 02428 is a neutral allele. However, based on the results of the three-way cross by Wang et al. (1998), it could only be inferred that the identified QTL alleles had high compatibility with the indica tester, despite the observation that ‘Dular’ has both a wide spectrum and high level of compatibility (Ikehashi and Araki 1988; Gu et al. 1993; Liu et al. 1996). Thus, it is necessary to test the ‘Dular’ alleles for their compatibility with japonica testers.
Second, it can be inferred that f6 and S5 are located in similar chromosomal regions based on molecular marker linkage maps (Liu et al. 1997; Wang et al. 1998). However further assessment is needed to ascertain whether they are indeed the same locus. Moreover, the allele from ‘Dular’ at this locus seemed to have a much smaller effect on hybrid fertility than the one from 02428, based on the data from the two populations (Liu et al. 1997; Wang et al. 1998), which again needs to be assessed under a common genetic background.
The study reported in this paper was undertaken to determine whether the f5 and f6 alleles from ‘Dular’ are neutral alleles conferring wide compatibility and to characterize the three loci (f5, f6, and S5) with respect to their effects on wide compatibility, using a set of near-isogenic lines (NILs) developed in this work.
Materials and methods
Plant materials
According to the results of previous studies, ‘Dular’, an indica WCV from India conferring both a wide spectrum and high level of wide compatibility when crossed to a range of indica and japonica varieties (Liu et al. 1996; Zhang et al. 1997), exhibited large effects on indica–japonica hybrid fertility at the f5 locus on chromosome 5 and the f6 locus on chromosome 6 (Wang et al. 1998). These two alleles were referred to as f5-Du and f6-Du, respectively. 02428, a japonica WCV widely used in inter-subspecific hybrid rice breeding programs in China, carried an allele for wide compatibility at the S5 locus (Liu et al. 1992; Liu et al. 1997) that was referred to as S5-08. ‘Zhenshan 97’, a typical indica cultivar and the parent for a number of widely cultivated elite hybrids, did not have wide compatibility and accordingly, the ‘Zhenshan 97’ alleles at these loci were referred to as f5-ZS, f6-ZS, and S5-ZS, respectively.
In this study, ‘Zhenshan 97’ was used as the recurrent parent, ‘Dular’ as the donor parent for the f5-Du and f6-Du alleles and 02428 as the donor parent for the S5-08 allele. In addition, ‘Balilla’, a typical temperate japonica variety introduced from Italy, was used as tester for compatibility to japonica and ‘Nanjing 11’, a typical indica variety developed by Jiangsu Academy of Agricultural Sciences, China, was used as tester for compatibility to indica. Both ‘Balilla’ and ‘Nanjing 11’ have been designated as testers for screening WCVs in Chinese rice breeding programs (Gu et al. 1991).
Development of NILs
The f5-Du, f6-Du, and S5-08 alleles were introgressed from the respective parental lines into ‘Zhenshan 97’ by successive backcrossing, combined with selection using molecular markers. In this scheme, the progeny of each backcross was selected for the presence of the target allele, using two or more flanking markers tightly linked to the target locus. Five simple sequence repeat (SSR),—RM122, MRG0200 (forward: 5′-CTTGCCTAACCCGTCTTGAC-3′; reverse: 5′-TCGATGTGTTGTCTTGTCCC-3′), MRG0259 (forward: 5′-TGGTCTTTCAAGAATGGGACA-3′; reverse: 5′-TGGACTAGCTTCCCTTGAGC-3′), RM413, and RM267—and two restriction fragment length polymorphic (RFLP) markers—R830 and R3166, flanking the f5 locus (Wang et al. 1998; Temnykh et al. 2000, 2001; McCouch et al. 2002)—were used for selecting the presence of the f5-Du allele. Four SSR markers, RM225, RM314, RM253, and RM276 (Liu et al. 1997; Wang et al. 1998; Temnykh et al. 2000, 2001), were used for selecting the presence of both f6-Du and S5-08 alleles. Positive individuals were then used for the next backcrossing.
In the BC3F1, RFLP and SSR markers (Table 1) were used to “clean up” the introgressed chromosomal segments from WCVs for other hybrid sterility loci reported in previous studies (Liu et al. 1997; Wang et al. 1998; Song et al. 2005).
In the BC5F1 or BC6F1, the selected individuals were assayed using molecular markers for recovering the genetic background of ‘Zhenshan 97’. A total of 117 SSR markers, polymorphic between ‘Dular’ and ‘Zhenshan 97’ and distributed evenly on the 12 chromosomes, were used in the ‘Zhenshan 97’ × ‘Dular’ cross. Similarly, a total of 118 SSR markers were used in the ‘Zhenshan 97’ × 02428 cross. Individuals with the highest proportion of the recurrent parent genotypes were kept as NILs of ‘Zhenshan 97’. Further, the neutral alleles of different loci were combined by inter-crossing the NILs.
Compatibility tests and field planting
The resulting NILs with heterozygous genotypes at the target loci were testcrossed to ‘Balilla’ for compatibility to japonica. The NILs homozygous for the introgressed alleles and ‘Zhenshan 97’ (as a control) were testcrossed to ‘Nanjing 11’ for compatibility to indica.
The progenies of compatibility tests and all the other indica–japonica hybrids were planted in the summer rice growing season at the experimental farm of Huazhong Agricultural University, Wuhan, China. The planting time was 19 May 2003 and 16 May 2004. Thus, the planting placed the temperature sensitive stage for fertility in late July and early August, at which time the average daily temperature was 30.6°C in 2003 and 29.7°C in 2004, favorable for the fertility of the inter-subspecific hybrids (Li et al. 1996; Lu et al. 2002).
Seedlings of 25 days were transplanted, and the planting density was 16.5 cm between plants in a row, 26.4 cm between rows, with 12 plants per row. Field management followed essentially the normal agricultural practices. Irrigation of the field was maintained to avoid drought stress.
Trait measurements
For pollen fertility, one or two panicles per plant were sampled at the time of heading and fixed in 70% (v/v) ethanol. Six florets per panicle were taken from the upper, middle, and lower portions of the panicle. One anther per floret was collected, and the six anthers from the same panicle were mixed and spread on a microscope slide. Pollens were stained with an I2–KI solution containing 0.1% (w/v) iodine and 1% (w/v) iodine potassium. More than 500 pollen grains from each individual were observed with a microscope for estimating percentage of fertile stainable pollen. Spikelet fertility of each plant was scored as seed setting rate on the basis of four to five panicles.
Molecular marker assay
The experiment procedures for RFLP assay, including DNA isolation, digestion, electrophoresis, and Southern blot hybridization, were essentially as described previously (Liu et al. 1997). The SSR primers of the RM series were designed according to Temnykh et al. (2000, 2001) and those of the MRG series were according to the rice genome sequences of Monsanto Company that were made available by McCouch et al. (2002). SSR analysis was carried out essentially according to the procedures described by Wu and Tanksley (1993).
Data processing and statistical analysis
Local linkage maps for the f5, f6, and S5 genomic regions were constructed using MAPMAKER/EXP 3.0 (Lincoln et al. 1992a) with a LOD threshold of 3.0, which was also used for searching loci governing pollen fertility and spikelet fertility, using MAPMAKER/QTL 1.0 at a LOD threshold of 3.0 (Lincoln et al. 1992b).
Results
The NILs
Five individuals were selected. The selected individuals were heterozygous at the target genomic regions and homozygous for the ‘Zhenshan 97’ alleles in the rest of the genome with exceptions only in a few regions. In particular, these individuals were homozygous for the ‘Zhenshan 97’ alleles for markers representing all the regions known to harbor loci (or QTLs) for indica–japonica hybrid sterility (Table 1), as reported in previous studies (Liu et al. 1997; Wang et al. 1998; Song et al. 2005). According to the alleles and combinations they carried, these individuals were referred to as NILs designated ZS(f5-Du/f5-ZS), ZS(f6-Du/f6-ZS), ZS(S5-08/S5-ZS), ZS(f5-Du/f5-ZS, f6-Du/f6-ZS), and ZS(f5-Du/f5-ZS, S5-08/S5-ZS), respectively. The recovery rates of the recurrent parent genome were 98.75, 98.75, 97.92, 96.67, and 96.67% for these NILs, as determined by molecular marker assays.
Compatibility tests to the indica variety
Ten hybrid plants per genotype identified using tightly linked molecular markers from the testcrossed populations of the NILs homozygous for the introgressed alleles to ‘Nanjing 11’ were selected for trait measurements for compatibility to indica. The pollen and spikelet fertility data (Table 2) showed that all the crosses produced normal fertile hybrids, when compared to the control cross, ‘Zhenshan 97’ × ‘Nanjing 11’.
Forty-eight plants per NIL were genotyped from the self-pollinated progenies of the NILs heterozygous for the target loci (Table 3), from which the fertility data were taken. All the three genotypes of the self-pollinated progenies from each of the NILs also showed normal fertility. In addition, no distorted segregation of the three genotypes was observed (Table 3). Thus, all three alleles that were transferred to ‘Zhenshan 97’ from the WCVs had complete compatibility with the indica alleles.
Compatibility tests to the japonica variety
Fertility distributions in the segregating populations
Molecular marker assay of the plants in the field planting of 2003 using tightly linked markers identified a total of 131 hybrid plants from the cross ZS(f5-Du/f5-ZS, f6-Du/f6-ZS) × ‘Balilla’, 130 hybrid plants from ZS(f5-Du/f5-ZS, S5-08/S5-ZS) × ‘Balilla’, 65 hybrid plants from ZS(f5-Du/f5-ZS)בBalilla’, 59 hybrid plants from ZS(f6-Du/f6-ZS) × ‘Balilla’, and 55 hybrid plants from ZS(S5-08/S5-ZS) × ‘Balilla’. The two two-locus testcross populations were planted again in 2004. After assaying of the plants using molecular markers, 12 plants for each of the four genotypes in the two segregating populations were evaluated for pollen and spikelet fertility.
In the progenies from ZS(f5-Du/f5-ZS, f6-Du/f6-ZS) × ‘Balilla’ and ZS(f5-Du/f5-ZS, S5-08/S5-ZS) × ‘Balilla’, the distributions of pollen fertility segregation were clearly bimodal, with an apparent valleys at approximately 50–65% of fertile pollen (Fig. 1a, c), indicating a simple genetic control in each case most likely by a single locus. However, the distributions of spikelet fertility seemed to be more complex, with three apparent peaks in both populations (Fig. 1b, d).
In the segregating population of ZS(f5-Du/f5-ZS) × ‘Balilla’, distributions of both pollen and spikelet fertility appeared to be bimodal (Fig. 1e, f), again indicating a likely single-locus control. However, no significant segregation was observed in the progenies from the crosses of ZS(f6-Du/f6-ZS) × ‘Balilla’ and ZS(S5-08/S5-ZS) × ‘Balilla’, with the majority of pollen fertility in the range of 4–45% and spikelet fertility between 1% and 17% (data not shown).
The effects of f5, f6, and S5
The results of compatibility tests of the NILs are presented in Tables 4 and 5. Comparison between the two genotypes for each of the three loci (Table 4) showed that the two genotypes at the f5 locus had the largest differences in both pollen and spikelet fertility; the genotype having the f5-Du allele produced much higher fertility than the other genotype, whereas differences between the two genotypes at the other two loci were not significant.
Similar to the results of single-locus analysis, testcrosses of two-locus genotypes (Table 5) also showed that f5 was the only locus causing statistically significant difference in pollen fertility. All the two-locus genotypes involving the f5-Du allele had much higher pollen fertility than those having the f5-ZS allele.
Testcrosses of the two-locus genotypes also detected the effects of the other two loci (f6 and S5) on spikelet fertility, in addition to the large effects of the f5 locus. It is clear from Table 5 that the effects of these two loci were dependent both on the genotypes of the f5 locus and on the environmental conditions. In 2003, the effects of f6 and S5 on spikelet fertility were significant only in the presence of the f5-Du allele, while in 2004, both f6 and S5 had significant effects on spikelet fertility in both the presence and absence of the f5-Du allele. However, the amounts of effects of the two loci were much larger in the presence of the f5-Du allele than otherwise.
Comparison of the data provided in Table 5 also indicated that the magnitudes of differences in spikelet fertility caused by allelic substitutions at f6 and S5 were similar within each year, suggesting that the magnitudes of gene effects at the two loci were similar.
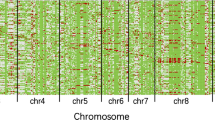
To evaluate further the relative effect of each locus on pollen and spikelet fertility, the data obtained in 2003 were assessed using MAPMAKER/QTL 1.0. The analysis resolved one major QTL with very large effect on both pollen and spikelet fertility in all three segregating populations located in the interval between MRG0200 and MRG0259 on chromosome 5 corresponding to the f5 locus (Table 6; Fig. 2). The analysis also detected significant effects of f6 and S5 on spikelet fertility in the ZS(f5-Du/f5-ZS, f6-Du/f6-ZS) × ‘Balilla’ and ZS(f5-Du/f5-ZS, S5-08/S5-ZS) × ‘Balilla’ populations, respectively (Table 6; Fig. 2). In both cases, the alleles from WCVs contributed to the increase of spikelet fertility. Table 6 also revealed that the effect of S5 seemed to be larger than f6, using f5 as the reference. The locations of the f5, f6, and S5 loci on the molecular linkage maps are shown in Fig. 2.
The locations of f5, f6, and S5 loci in the molecular marker linkage maps. The arrow on each chromosomal region indicates the position of the LOD peak. a Map location of f5 locus, determined using the populations of ZS(f5-Du/f5-ZS, f6-Du/f6-ZS) × ‘Balilla’, ZS(f5-Du/f5-ZS, S5-08/S5-ZS) × ‘Balilla’, and ZS(f5-Du/f5-ZS) × ‘Balilla’. b Map location of f6 locus, determined using the population of ZS(f5-Du/f5-ZS, f6-Du/f6-ZS) × ‘Balilla’. c Map location of S5 locus determined using the population of (f5-Du/f5-ZS, S5-08/S5-ZS) × ‘Balilla’
Discussion
Cytological investigation of the inter-subspecific sterility revealed that both male and female gamete abortions and reduced affinity between the uniting gametes, as well as anther indehiscence and non-synchronization of male and female gamete development in the same spikelet, all contribute to hybrid sterility (Yokoo 1984; Li 1988; Ling et al. 1991; Maeka et al. 1991; Wang et al. 1991, 1992; Li and Ouyang 1992; Liu et al. 1993, 1997, 2004; He et al. 1994; Teng et al. 1996; Zhu et al. 1996). Recently, Song et al. (2005) identified a major QTL for male gamete abortion and a major QTL for female gamete abortion in a segregating population. Their results determined that the S5 locus on chromosome 6, previously identified as a locus for wide compatibility by spikelet fertility analysis, was a major locus for embryo sac fertility, and a QTL on chromosome 5 had a major effect on pollen fertility. Both of the loci had large effects on spikelet fertility.
The most important finding of the present study is the determination of f5 as a locus for indica–japonica hybrid sterility and identification of f5-Du from ‘Dular’ as a neutral allele that is able to increase the fertility of the hybrids when crossed to both indica and japonica varieties. In particular, the results clearly demonstrated that the f5-Du allele exerted a large effect on hybrid fertility by specifically increasing pollen fertility, thus proving to be a neutral allele for pollen fertility. In the indica genetic background provided by ‘Zhenshan 97’, this allele can produce normal fertile hybrids when testcrossed to the indica tester. When testcrossed to the japonica tester, this allele could increase the pollen fertility (I2–KI stainability) by more than 50% and the spikelet fertility by over 20%, although the fertility of the testcross progenies with the japonica tester was still far from normal, presumably because of allelic differences in numerous other loci. Interestingly, the f5 locus appeared to be located in the same genomic region as the pf5 (spf5) locus identified by Song et al. (2005). In addition, Zhuang et al. (2002) also mapped a locus for pollen fertility on chromosome 5 located in the same vicinity. Thus, quite likely, the f5 locus identified using ‘Dular’ and pf5 (spf5) using 02428 are the same locus.
Another important finding is the confirmation of f6 as a locus for indica–japonica hybrid sterility, at which the f6-Du allele from ‘Dular’ is a neutral allele conferring wide compatibility. The f6 and S5 loci appeared to have high similarities, as demonstrated by the f6-Du and S5-08 alleles: both alleles had effects only on spikelet fertility, not on pollen fertility; the magnitudes of effects caused by substitutions of the two alleles were also similar, both of which were much smaller than the f5-Du allele; the two loci are located in the same genomic interval on chromosome 6. Thus, it is reasonable to assume that f6 is also a locus for embryo sac fertility and hence, f6 and S5 are most likely to be the same locus.
It should be also noted that previous studies showed that cool temperature could significantly reduce the pollen fertility and spikelet fertility of indica–japonica hybrids, even in the presence of wide compatibility genes (Li et al. 1996, 1997; Lu et al. 2002). To avoid the influence of temperature on hybrid sterility in the present study, the planting dates for all the experimental materials were arranged such that the entire reproductive development stage was under high-temperature conditions. We also planted the F1s of ‘Dular’ × ‘Balilla’ and 02428 × ‘Nanjing 11’ in the same field in 2003 and 2004 as controls, both of which showed normal pollen fertility and spikelet fertility in both years, indicating that the observed fertility segregations in all the populations were not affected by temperature.
A notable feature observed from the results of this study and the study of Song et al. (2005) is the large influence of the male fertility on spikelet fertility. Theoretically, a few normal pollen grains would be enough for a flower to set seed. Thus the large effect of the f5 locus on pollen fertility strongly indicates the severe scarcity of fertile pollen in the indica–japonica hybrids, suggesting that the actual fertility (or germinability) of the pollen produced by the indica–japonica hybrids is much lower than observed with I2–KI staining. Lin et al. (1992) found that in indica–japonica hybrids, the germinability of the pollen was less than 10% in the hybrids, and a large portion of the pollen was not functional, although 45–55% of the pollen grains appeared to be morphologically normal. In addition, anther indehiscence may also be a cause for spikelet sterility, as was found in the F1 hybrid between rice varieties ‘Silewah’ and ‘Hayakogane’ (Maeka et al. 1991). In any case, shortage in the supply of fertile pollen may diminish the effects of other components of spikelet fertility such as embryo sac fertility. This may especially be the case in a cross between a typical indica variety like ‘Zhenshan 97’ and a typical japonica variety like ‘Balilla’, in which the hybrid may be heterozygous at the majority, if not all, of the loci for indica–japonica hybrid sterility and adding one neutral allele for embryo-sac fertility to the hybrid may not increase fertility much because of the unavailability of fertile pollen. This may provide an explanation for the large dependence of the effects of S5 and f6 on f5, such that the effects of S5 and f6 were significant only in the presence of the f5-Du allele.
The results also have significant implications for indica–japonica hybrid rice breeding programs. Most of the current breeding programs only use the S5 locus to manipulate the fertility of the hybrids, due largely to the limited information from previous studies. The results of Song et al. (2005) and this study clearly indicate that alleles at S5 locus alone is far from sufficient to overcome hybrid sterility of indica–japonica crosses, because the f5 locus plays a more important role in determining spikelet fertility by exerting a major effect on pollen fertility. Moreover, the results further showed that, even in combination, the alleles of the two loci f5 and S5 (f6) are still not sufficient to produce normal fertility hybrids in a typical indica–japonica cross like ‘Zhenshan 97’/‘Balilla’. Hence, to achieve normal spikelet fertility, more neutral alleles conferring wide compatibility should be introduced. In this connection, it should be noted that it has been repeatedly reported that ‘Dular’ has both a wide-compatible spectrum and a high level of compatibility, as it produces highly fertile hybrids when crossed to a wide range of indica and japonica varieties (Pan et al. 1990; Liu et al. 1996; Zhang et al. 1997). So far, ‘Dular’ has been identified to carry neutral alleles at a number of loci, including f5, f6 (S5), S7, S8, S9, ga11, and ga14 (Wan et al. 1996; Lu et al. 2000) and confirmed to be a very useful WCV. However, this variety is agronomically undesirable because of tall and thin culm and poor yielding. Thus, it may be a better strategy to transfer these identified neutral alleles into elite indica or japonica varieties by marker-assisted selection and the resulting introgression lines can then be selectively used in inter-subspecific hybrid rice breeding programs.
References
Araki H, Toya K, Ikehashi H (1988) Role of wide-compatibility genes in hybrid rice breeding. In: Hybrid rice. IRRI, Manila, pp 79–83
Chu LH, Chou YC, Tau C (1964) Breeding studies on hybridization between O sativa L subsp hsien and O sativa L subsp keng in cultivated rice (in Chinese with English abstract). Acta Agron Sin 3:69–84
Gu MH, Pan XB, Chen ZX, Yang WC (1991) A study on compatibility of standard wide compatibility testers of rice in China (in Chinese with English abstract). Sci Agric Sin 24:27–32
Gu M, You A, Pan X (1993) Analysis on allelic relationship of wide compatibility genes among several WCVs (Oryza sativa L.). Sci Agric Sin 26:13–21
He GH, Zheng JK, Yin GD, Yang ZL (1994) Gamete fertility of F1 between Indica and Japonica (in Chinese with English abstract). Chin J Rice Sci 8:177–180
Ikehashi H (1991) Genetics of hybrid sterility in wide hybridization in rice. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 14. Rice. Springer, Berlin Heidelberg New York, pp 113–127
Ikehashi H, Araki H (1984) Variety screening of compatibility types revealed in F1 fertility of distant cross in rice. Jpn J Breed 34:304–313
Ikehashi H, Araki H (1986) Genetics of F1 sterility in remote crosses of rice. In: IRRI (ed) Rice genetics. IRRI, Manila, pp 119–130
Ikehashi H, Araki H (1988) Multiple alleles controlling F1 sterility in remote crosses of rice (Oryza sativa). Jpn J Breed 38:283–291
Kato S, Kosaka H, Hara S (1928) On the affinity of rice varieties as shown by fertility of hybrid plants. Bull Sci Fac Agric Kyushu Univ 3:132–147
Kinoshita T (1995) Report of the committee on gene symbolization. Rice Genet Newsl 12:94–125
Li XQ (1988) Improving the fertility of indica–japonica hybrids by using the wide-compatibility gene (in Chinese). Hybrid Rice 31–33
Li BJ, Ouyang XZ (1992) Cytological study on the abortion of spikelets of F1 from indica and japonica. In: Yuan LP (ed) Current status of two line hybrid rice research (in Chinese with English abstract). Agricultural, Beijing, pp 286–289
Li HB, Zhang Q, Liu AM, Zou JS, Chen ZM (1996) A genetic analysis of low-temperature-sensitive sterility in indica–japonica hybrids. Plant Breed 115:305–309
Li HB, Wang J, Liu AM, Liu KD, Zhang Q, Zou JS (1997) Genetic basis of low-temperature-sensitive sterility in indica–japonica hybrids of rice as determined by RFLP analysis. Theor Appl Genet 95:1092–1097
Lin SY, Ikehashi H (1993) A gamete abortion locus detected by segregation distortion of isozyme locus EST-9 in wide crosses (Oryza sativa L.). Euphytica 67:35–40
Lin SY, Ikehashi H, Yanagihara S, Kawashima A (1992) Segregation distortion via male gamete in hybrids between Indica and Japonica or wide-compatibility varieties of rice (Oryza sativa L.). Theor Appl Genet 84:812–818
Lincoln S, Daly M, Lander E (1992a) Constructing genetic maps with MAPMAKER/EXP 3.0. Whitehead Institute Technical Report, 2nd edn. Whitehead Institute, Cambridge
Lincoln S, Daly M, Lander E (1992b) Mapping genes controlling quantitative traits with MAPMAKER/QTL 1.1. Whitehead Institute Technical report, 2nd edn. Whitehead Institute, Cambridge
Ling DH, Ma ZR, Chen MF, Liang CY, He BS (1991) Types of male sterile mutants in somaclones from somatic cell culture of indica rice (in Chinese with English abstract). Acta Genet Sin 18:132–139
Liu A, Zhang Q, Li H (1992) Location of a gene for wide-compatibility in the RFLP linkage map. Rice Genet Newsl 9:134–136
Liu YS, Zhou KD, Yin GD, Luo WZ (1993) Preliminary cytological observations on female sterility of hybrids between indica and japonica rice (in Chinese with English abstract). Acta Biol Exp Sin 26:95–99
Liu KD, Zhou ZQ, Xu CG, Zhang Q, Saghai Maroof MA (1996) An analysis of hybrid sterility in rice using a diallel cross of 21 parents involving indica, japonica and wide compatibility varieties. Euphytica 90:275–280
Liu YS, Sun JS, Zhou KD (1997) Cytological basis causing spikelet sterility of intersubspecific hybrid in Oryza sativa (in Chinese with English abstract). Acta Biol Exp Sin 30:335–341
Liu KD, Wang J, Li HB, Xu CG, Liu AM, Li XH, Zhang Q (1997) A genome-wide analysis of wide compatibility in rice and the precise location of the S5 locus in the molecular map. Theor Appl Genet 95:809–814
Liu YS, Zhu LH, Sun JS, Chen Y (2001) Mapping QTLs for defective female gametophyte development in an inter-subspecific cross in Oryza sativa L. Theor Appl Genet 102:1243–1251
Liu HY, Xu CG, Zhang Q (2004) Male and female gamete abortions, and reduced affinity between the uniting gametes as the causes for sterility in an indica/japonica hybrid in rice. Sex Plant Reprod 17:55–62
Lu CG, Takabatake K, Ikehashi H (2000) Identification of segregation-distortion-neutral alleles to improve pollen fertility of indica–japonica hybrids in rice (Oryza sativa L.). Euphytica 113:101–107
Lu CG, Wang CL, Zong SY, Zhao L, Zou JS (2002) Effects of temperature on fertility and seed set in intersubspecific hybrid rice (in Chinese with English abstract). Acta Agron Sin 28:499–504
Maeka M, Inuka T, Shinbashi N (1991) Spikelet sterility in F1 hybrids between rice varieties Silewah and Hayakogane. Jpn J Breed 41:359–363
McCouch SR, Teytelman L, Xu Y, Lobos KB, Clare K, Walton M, Fu B, Maghirang R, Li Z, Xing Y, Zhang Q, Kono I, Yano M, Fjellstrom R, DeClerck G, Schneider D, Cartinhour S, Ware D, Stein L (2002) Development and mapping of 2,240 new SSR markers for rice (Oryza sativa L.). DNA Res 9:199–207
Oka HI (1988) Origin of cultivated rice. Scientific Societies, Tokyo, pp 181–209
Pan XB, Gu MH, Chen ZX, Hu XY (1990) A comparative study on major wide compatibility varieties of rice. In: Yuan LP (ed) Current status of two line hybrid rice research. Agricultural, Beijing, pp 236–245
Song X, Qiu SQ, Xu CG, Li XH, Zhang Q (2005) Genetic dissection of embryo sac fertility, pollen fertility, and their contributions to spikelet fertility of intersubspecific hybrids in rice. Theor Appl Genet 110:205–211
Tanksley SD (1993) Mapping polygenes. Annu Rev Genet 27:205–233
Temnykh S, Park WD, Ayres N, Cartihour S, Hauck N, Lipovich L, Cho YG, Ishii T, McCouch SR (2000) Mapping and genome organization of microsatellite sequences in rice (Oryza sativa L.). Theor Appl Genet 100:697–712
Temnykh S, Declerck G, Luashova A, Lipovich L, Cartinhour S, McCouch S (2001) Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon associations, and genetic marker potential. Genome Res 11:1441–1452
Teng JL, Xue QZ, Wang YX (1996) Ultrastructural observations of pollen and anther wall developments between subspecies in rice (Oryza sativa L.) (in Chinese with English abstract). J Zhejiang Agric Univ 22:467–473
Wan J, Yanagihara S, Kato H, Ikehashi H (1993) Multiple alleles at a new locus causing hybrid sterility between a Korean indica variety and a Javanica variety in rice (Oryza sativa L.). Jpn J Breed 43:507–516
Wan J, Yamaguchi Y, Kato H, Ikehashi H (1996) Two new loci for hybrid sterility in cultivated rice (Oryza sativa L.). Theor Appl Genet 92:183–190
Wang YX, Yan JQ, Xue QZ, Shen SQ (1991) Cytological studies on partial male sterility of F1 hybrids between subspecies in rice (in Chinese with English abstract). Acta Agric Univ Zhejiangensis 17:417–422
Wang CL, Zhang ZL, Tang SZ, Shi JD (1992) Exploitation of heterosis between Indica and Japonica by three-line method. I. Differentiation between indica–japonica sterility and cytoplasmic male sterility (in Chinese with English abstract). Jiangsu J Agric Sci 8:1–7
Wang J, Liu KD, Xu CC, Li XH, Zhang Q (1998) The high level of wide-compatibility of ‘Dular’ has a complex genetic basis Theor Appl Genet 97:407–412
Wu KS, Tanksley SD (1993) Abundance, polymorphism and genetic mapping of microsatellites in rice. Mol Gen Genet 241:225–235
Yanagihara S, Kato H, Ikehashi H (1992) A new locus for multiple alleles causing hybrid sterility between an AUS variety and Javanica varieties in rice (Oryza sativa L.). Jpn J Breed 42:793–801
Yanagihara S, McCouch SR, Ishikawa K, Ogi Y, Maruyama K, Ikehashi H (1995) Molecular analysis of the inheritance of the S-it 5 locus, conferring wide compatibility in indica/japonica hybrids of rice (O. sativa L.). Theor Appl Genet 90:182–188
Yang SR, Shen XY, Gu VYL, Cao DJ (1962) The report of rice breeding by indica–japonica crosses (in Chinese). Acta Agron Sin 1:97–102
Yokoo M (1984) Female sterility in an indica–japonica cross of rice. Jpn J Breed 34:219–227
Yuan LP (1987) Strategies of hybrid rice breeding (in Chinese). Hybrid Rice 1–3
Zhang GQ, Lu YG (1989) Genetic studies on the hybrid sterility in cultivated rice (Oryza sativa L.) I Diallel analysis of the hybrid sterility among isogenic F1 sterility lines (in Chinese with English abstract). Chin J Rice Sci 3:97–101
Zhang GQ, Lu YG (1993) Genetic studies on the hybrid sterility in cultivated rice (Oryza sativa L.) II A genic model for F1 pollen sterility (in Chinese with English abstract). Acta Genet Sin 20:222–228
Zhang GQ, Lu YG, Zhang H, Yang JC, Liu GF (1994) Genetic studies on the hybrid sterility in cultivated rice (Oryza sativa L.) IV Genotypes for F1 pollen sterility (in Chinese with English abstract). Acta Genet Sin 21:34–41
Zhang Q, Liu KD, Yang GP, Saghai Maroof MA, Xu CG, Zhou ZQ (1997) Molecular marker diversity and hybrid sterility in indica–japonica rice crosses. Theor Appl Genet 95:112–118
Zheng K, Shen P, Qian H, Wang J (1992) Tagging genes for wide compatibility in rice via linkage to RFLP markers. Chin J Rice Sci 6:145–150
Zhu XH, Cao XZ, Zhu QS (1996) Cytological studies on spikelet sterility of indica–japonica hybrids in rice (in Chinese with English abstract). Chin J Rice Sci 10:71–78
Zhuang CX, Mei MT, Zhang GQ, Lu YG (2002) Chromosome mapping of the S-b locus for F1 pollen sterility in cultivated rice (Oryza sativa L.) with RAPD markers (in Chinese with English abstract). Acta Genet Sin 29:700–705
Acknowledgements
This work was supported by grants from the National Program on the Development of Basic Research, the National Special Key Project of Functional Genomics and Biochips and the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Möllers
Rights and permissions
About this article
Cite this article
Wang, G.W., He, Y.Q., Xu, C.G. et al. Identification and confirmation of three neutral alleles conferring wide compatibility in inter-subspecific hybrids of rice (Oryza sativa L.) using near-isogenic lines. Theor Appl Genet 111, 702–710 (2005). https://doi.org/10.1007/s00122-005-2055-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-005-2055-z