Abstract
Megaloptera larvae are important bioindicator species and potential resource insects. To further cultivate their economic role, their living environment must be examined in more detail. In this study, we analyzed the physiological and biochemical effects of a sublethal dose of imidacloprid, a widely used neonicotinoid insecticide, on the larvae of Protohermes xanthodes. After treatment with imidacloprid, P. xanthodes larvae exhibited clear symptoms of poisoning, including the head curling up toward the ventral surface. Additionally, the activity of acetylcholinesterase was significantly inhibited following exposure. The activities of glutathione S-transferases initially continuously increased but showed a slight decrease after 8 days. Catalase activity initially increased and then decreased following imidacloprid treatment; superoxide dismutase activity fluctuated over time, and peroxidase activity continuously increased. The expression levels of HSP70s genes were evaluated using qRT-PCR. These results indicate that P. xanthodes larvae exhibit a toxic response to imidacloprid exposure, manifested as oxidative stress, as observed through behavioral and physiological indicators.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Megaloptera larvae are predatory insects living in freshwater environments. Species of the Corydalidae family may display a limited distribution and a high sensitivity to water pollution from human activities (Docile et al. 2016), making them relevant bioindicator species for water quality and conservation (Rico-Sánchez et al. 2020; Contreras-Ramos et al. 2008, Rivera-Gasperín et al. 2019). Besides, Megaloptera larvae are used in some regions as a protein and polysaccharide-rich food resource, with antidiuretic effects (Shi et al. 2019).
Globally, neonicotinoids are the most widely used class of insecticides (Jeschke and Nauen 2008; Lexmond et al. 2015). Among these, imidacloprid is one of the most extensively used neonicotinoid pesticides (Bass et al. 2015). Concurrently, the widespread use of imidacloprid has brought about numerous environmental issues (Pestana et al. 2009; Cloyd and Bethke 2011). Many studies indicate that neonicotinoids persist in the environment for extended periods, with concentrations measured in ng/g in various farm fields (Li et al. 2017; Thompson et al. 2020). Imidacloprid is selectively toxic to insects, particularly aquatic larvae, due to their higher density of nicotinic acetylcholine receptors and greater affinity for imidacloprid compared to adults (Matsuda et al. 2001; Maloney et al. 2021). Besides affecting target organisms, imidacloprid can also disperse among non-target organisms (Cloyd and Bethke 2011; Sharma et al. 2019; Singh and Leppanen 2020). Numerous researchers have shown that imidacloprid tends to leach into the soil and then into aquatic environments, and its continuous exposure can lead to accumulation in aquatic organisms (Flores-Céspedes et al. 2012; Adak et al. 2012; Frew et al. 2018).
Previous studies have shown that imidacloprid causes changes in the physiological indices in some non-target species (Vohra et al. 2014; Siregar et al. 2021; Seifert and Stollberg 2005). When female albino rats were orally administered 10 and 20 mg/kg of imidacloprid per day, there was a significant reduction in average feed intake in the high-dose group, as well as a significant decrease in the relative weight of the heart and spleen (Vohra et al. 2014). Ge and collaborators found that zebrafish induce oxidative stress and DNA damage when exposed to imidacloprid (Ge et al. 2015). It has been observed that topical exposure to imidacloprid (24 or 48 h) was more toxic to the stingless bee Scaptotrigona postica than a 24-h dietary exposure. This observation may be due to the presence of pesticides in the midgut following dietary exposure, which interact with various enzymes involved in neonicotinoid metabolism (Soares et al. 2015). Roessink and collaborators discovered that prolonged exposure to imidacloprid in water exhibits significant chronic toxicity to various aquatic insects (Roessink et al. 2013). Sub-chronic exposure to imidacloprid has been investigated for its capacity to modify an organism’s physiological responses (Wang et al. 2016). The effects of imidacloprid on aquatic invertebrates were primarily on their nervous system, feeding behavior, and locomotion. For example, the feeding behavior of the shrimp Gammarus pulex was severely affected after experiencing exposure to imidacloprid at concentrations above 30 µg/L and could not be restored even 3 days after being transferred to clean water. However, at lower concentrations (0.81 ~ 9.0 µg/L), although no significant changes in feeding were observed, an increase in feeding was observed at the end of the exposure (Agatz et al. 2014). It was shown that imidacloprid inhibits cholinergic synaptic excitability within the central nervous system of snail Lymnaea stagnalis, further revealing its potential effects on the nervous system of aquatic invertebrates (Vehovszky et al. 2015).
Furthermore, oxidative damage biomarkers and antioxidant enzymes have been extensively employed for evaluating the ecotoxicity of contaminants, including oxygen species (ROS), malondialdehyde (MDA), antioxidant enzymes, and stress proteins, as assessment tools for the toxicity of numerous xenobiotics. In response to the stress induced by imidacloprid, acetylcholinesterase within the synapse fails to metabolize imidacloprid within the postsynaptic nAChRs, leading to persistent nerve impulses that trigger oxidative stress (Martelli et al. 2020). Excessive accumulation of reactive ROS in organisms leads to oxidative stress and lipid peroxidation, resulting in the production of MDA. Consequently, MDA concentration reflects the ROS abundance to some extent (Chen et al. 2015). The concentration of ROS is regulated by various defense mechanisms, which encompass several antioxidant and detoxifying enzymes, including superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and polyphenol oxidase (PPO) (Cheung et al. 2001; Maity et al. 2008; Chen et al. 2018). Therefore, the assessment of MDA levels and enzyme activities can illustrate the deleterious effects of imidacloprid on the aquatic biological community.
The synthesis of heat shock proteins (HSPs) represents an adaptive response of organisms to external environmental stressors and is ubiquitous throughout biological communities, with significant surges in heat shock protein synthesis occurring in cellular systems when organisms are exposed to environmental stressors, pathological conditions, or physiological challenges (Sorger 1991; Roberts et al. 2010).
Environmental pollutants are agents that trigger the upregulation of HSP expression in aquatic organisms. Elevated levels of various HSPs can be detected following exposure to environmental contaminants, including pesticides, heavy metals, organic compounds, and others (Rhee et al. 2009; Joseph and Raj 2011; Lee et al. 2006). The accumulation of HSPs is related to the severity of stress, making HSPs suitable biomarkers for evaluating the response of biological communities to environmental and physiological stressors (Hightower 1991; Sanders 1993). In fish, HSPs are regarded as potential biological markers for assessing pesticide toxicity (Joseph and Raj 2011).
It has been observed that when the Protohermes costalis (Megaloptera: Corydalidae) is exposed to cadmium (Cd), six HSPs belonging to the HSP70 family are synthesized, with PcosHSP68 showing upregulated expression in both exposed to 150 mg/L (PL) and 1000 mg/L (PH) CdCl2 treatment. This finding highlights the involvement of HSP genes in the larvae of the Megaloptera group (Wen et al. 2022). Research on gene expression related to pesticides in Megaloptera larvae has exhibited a basis. Hence, the effects of toxic substances on organisms can also be inferred through the expression patterns of HSP genes. Here, we studied the toxic impacts of imidacloprid on Protohermes xanthodes (Megaloptera: Corydalidae) larvae and its effects on survival, detoxification, and antioxidant response to understand their physiological responses to a common insecticide.
Methods and materials
Chemicals and insects
Imidacloprid (70% purity) was obtained from Bayer CropScience China Co. Ltd. The experimental kits employed in this study were of analytical grade and were procured from the Nanjing Jiancheng Bioengineering Institute (Jiangsu, China). The progenitors of all P. xanthodes larvae were gathered from the Emei River (Sichuan, China). These larvae were subsequently reared in a laboratory until they matured into adults, at which point they were conclusively identified as P. xanthodes (Liu et al. 2006). The final instar larvae, derived from the parents, were obtained from the breeding facility of Chongsheng Biotechnology Co. Ltd. for testing purposes. These final instar P. xanthodes larvae (referred to as “larvae” hereafter) were subjected to an acclimatization period of 14 days in tanks within insect-rearing chambers, where environmental conditions included a water temperature of 24 ± 1 °C, a 12-h light and 12-h dark cycle, and continuous oxygen. During this period, they were fed live shrimp Caridina sp. Following acclimatization, a selection of larvae was transferred to transparent tanks measuring 44 cm × 30 cm × 25 cm, each containing 4 L of water. Each transparent tank housed twenty larvae, and a constant oxygen supply was maintained in the water.
Determination of sublethal concentration
Imidacloprid was initially dissolved in water and diluted to various concentrations (1, 2, 4, 8, and 16 mg/L). The larvae were then placed into 4 L of imidacloprid solution at different concentrations to determine the sublethal concentration of imidacloprid for larvae. Three replicates, each consisting of 20 larvae, were employed for each treatment group. An equivalent volume of water devoid of imidacloprid was used as a control group. Mortality rates were recorded at intervals of 0, 1, 2, 4, 8, and 16 days. Toxicity regression equations and LC50 values were calculated based on corrected mortality data using SPSS software. Toxicity regression equations and LC50 values were calculated based on mortality data.
Experimental design
Based on the results indicating the sublethal concentration, a concentration of 13.29 mg/L was selected for the experiment. The larvae were raised in water tanks containing 4 L of imidacloprid solution. Each larva was individually housed in a separate, water-permeable container, allowing light penetration and preventing any conflicts among them. An equivalent volume of water devoid of imidacloprid was used as a control group. Both the pesticide-treated and control groups underwent three replications, each with 40 larvae. The experiments were conducted under controlled laboratory conditions, maintaining a temperature of 24 ± 1 °C and a 12-h light and 12-h dark cycle for 16 days. During the experiment, the imidacloprid solution was refreshed with a new one every 2 days.
Recording of poisoning symptoms
Larvae were collected at various times. Symptoms were immediately recorded following the collection of larvae. In previous observations of their behavior, it was found that larvae exposed to external physical stimuli outwardly show marked curling self-defense behavior. When larvae are exposed to imidacloprid, it may cause a blockage of normal nerve conduction, leading to persistent stimulation, which results in persistent curling behavior. During our observations, we recorded the larvae’s physical condition after stimulation, assessed the degree of curling, and recorded their behavioral responses.
Assays of physiological indicators
After the recording of poisoning symptoms, collected larvae were frozen at − 80 °C. After sample collection, each replicate was weighed and then pulverized into a powdered state using a mortar under ice water bath conditions. Following this, phosphate-buffered saline (PBS) was added to the tube at a ratio of nine times (mass-to-volume, g/ml), and the mixture was thoroughly mixed. The resulting homogenate was centrifuged at 2500 r/min for 10 min at 4 °C, and the supernatant was used to measure MDA content, enzyme activity, and protein concentration. All physiological indicators were measured using kits from Nanjing Jiancheng Biotechnology Research Institute Co., Ltd. (Jiangsu, China). The kits numbers used were A024-1–1, A003-1–2, A001-3–2, A007-1–1, A084-1–1, and A004-1–1. Measurements were performed according to the steps shown in the instructions. The numerical units represent the specific activity of the enzyme, with U/mg being the number of units of enzyme activity per milligram of protein, and nmol/mg is the amount of the substance per milligram of protein. The results were taken as the mean of three replicates with three larvae per replicate.
Expression pattern analysis of HSP70s
Larvae were collected at specified time points, namely 0, 1, and 3 days post-imidacloprid treatment, and subsequently frozen at − 80 °C. At each time point, three replicates were sampled for testing with three larvae in each replicate.
Primers (Table 1) were designed as in the study of Wen et al. (2022) on Protohermes costalis and validated by Personal Biotechnology Co., Ltd (Shanghai, China).
Reverse transcription was performed by mixing 1 µg of total RNA with 1 µL of Oligo(dT), 1 µL of 10 mM dNTPs, and RNase-free dH2O was added to a volume of 10 µL. The mixture was incubated at 65 °C for 5 min, and then the following reagents were added: 4 µL of fivefold one-chain reaction buffer, 0.5 µL RNase inhibitor (40 U/µL), 1 µL MMLV RT (200 U/µL), and RNase-free dH2O up to 20 µL. This mixture was incubated at 42 °C for 30–60 min. Finally, the reaction was heated at 95 °C for 5 min to end the reaction and placed on ice for subsequent experiments (Wen et al. 2022).
RT-qPCR was conducted utilizing a PikoReal 96-well PCR system (Applied Biosystems, USA) with a final reaction volume of 10 µL, comprising 5 µL of 2 × SYBR real-time PCR premix (Personalbio, China), 0.4 µL of each primer (10 µM), 1 µL of sample cDNA, and RNase-free dH2O added to reach a total volume of 10 µL. The cycling parameters were as follows: initial denaturation at 95 °C for 5 min, followed by 40 cycles, each consisting of 15 s of denaturation at 95 °C and 30 s of annealing at 60 °C. α-tubulin and β-tubulin were utilized as reference genes as in P. costalis (Bustin et al. 2009). Three biological replicates were conducted for each RT-qPCR reaction. A negative control reaction, lacking a template, was performed using DEPC water. The relative quantification of gene expression was performed using the 2−ΔΔCt method (Livak and Schmittgen 2001).
Statistical analysis
All statistical analyses were conducted using the SPSS software. Prior to parametric tests, a variance chi-square test was performed. Independent samples t-test was employed to assess differences between the treated and control groups concerning enzyme activity and MDA content. One-way analysis of variance (ANOVA) and LSD multiple comparison tests were used to determine significant differences between groups when analyzing enzyme activities and MDA levels at different time points after exposure to imidacloprid. Gene expression was analyzed by ANOVA, while normal distribution and homogeneity of variance were confirmed through Shapiro–Wilk and Levene tests, respectively. Data are expressed as mean ± standard error (SE). A significance level of p < 0.05 was considered statistically significant, while p < 0.01 was deemed statistically highly significant. All results are presented as mean ± standard error (SE) from three replications.
Results
Toxicity
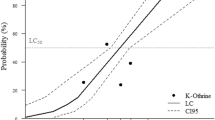
Imidacloprid exhibited lower toxicity to the larvae of P. xanthodes during shorter stress durations (up to 4 days) and higher toxicity to the larvae during longer stress durations (8 days or more). The toxicity regression equation for the 16-day period was determined to be y = − 1.619 + 0.122x (X2 = 1.360; p < 0.01). Utilizing the toxicity regression equation, the LC50 for the sublethal concentration of imidacloprid was calculated to be 13.288 mg/L, with a 95% confidence interval ranging from 11.262 to 16.435 mg/L (Table 2).
Poisoning symptoms
At the initial exposure stage, the larvae of P. xanthodes did not exhibit significant reactions to imidacloprid. However, 4 days after being exposed to imidacloprid, the larvae displayed evident signs of toxicity (Table 3). Following 8 days post-treatment, the larvae exhibited a characteristic behavior where the head was curled upward toward the ventral surface (Fig. 1). Nonetheless, the control group maintained a consistent state at each time point, resembling the condition of the larvae when initially exposed to imidacloprid (0 days).
AChE activity
At 0 days of imidacloprid exposure, there was no significant change in acetylcholinesterase activity compared to the control group (p > 0.05). However, after 1, 2, 4, 8, and 16 days of exposure to imidacloprid, acetylcholinesterase (AChE) activity was significantly inhibited (p < 0.05 and p < 0.01) compared to the control group (Fig. 2). An ANOVA demonstrated that varying durations of imidacloprid exposure significantly inhibited acetylcholinesterase activity (p < 0.05) (Table 3). The activity of AChE on day 2 showed a gradual increase compared to day 1 but was significantly inhibited again (p < 0.01) over the course of treatment, with the lowest activity observed on day 16 (Fig. 2 and Table 4). This indicates that exposure duration is a key factor influencing acetylcholinesterase activity in the larvae of P. xanthodes.
Effects of imidacloprid on the AChE activity of P. xanthodes Each bar represents the mean of three replicates, and the error bars represent the standard deviation (SD). Significant values (*p < 0.05; **p < 0.01) refer to the difference between exposed samples and the controls. U/mg protein means the number of units of enzyme activity per milligram of protein
GST activity
Following imidacloprid exposure, glutathione sulfotransferase (GST) activity showed no significant change during day 1 compared to the control (p > 0.05). However, GST activity was significantly increased after 2 days of exposure compared to controls (p < 0.01), with the lowest activity observed on day 8 (Fig. 3). ANOVA analysis revealed that different exposure times significantly affected GST activity (p < 0.05). GST exhibits a similar trend in activity to CAT. However, peak GST activity occurred at 8 days, which was 4 days later than the observed change in CAT activity (Figs. 3 and 4 and Table 4).
Effects of imidacloprid on the GST activity of P. xanthodes. Each bar represents the mean of three replicates, and the error bars represent the standard deviation (SD). Significant values (*p < 0.05; **p < 0.01) refer to the difference between exposed samples and the controls. U/mg protein means the number of units of enzyme activity per milligram of protein
Effects of imidacloprid on the antioxidant activity and MDA content of P. xanthodes. Each bar represents the mean of three replicates, and the error bars represent the standard deviation (SD). Significant values (*p < 0.05; **p < 0.01) refer to the difference between exposed samples and the controls. A Effects of imidacloprid on the CAT activity of P. xanthodes. B Effects of imidacloprid on the SOD activity of P. xanthodes. C Effects of imidacloprid on the POD activity of P. xanthodes. D Effects of imidacloprid on the MDA content of P. xanthodes. Each bar represents the mean of three replicates, and the error bars represent the standard deviation (SD). Significant values (*p < 0.05; **p < 0.01) refer to the difference between exposed samples and the controls. U/mg protein means the number of units of enzyme activity per milligram of protein, and nmol/mg protein is the amount of the substance per milligram of protein
Antioxidant enzyme activity and MDA content
The CAT activity in larvae significantly increased after 2 and 4 days of imidacloprid exposure (p < 0.01) and continued to rise significantly (p < 0.05) after 8 and 16 days of treatment compared to the control (Fig. 4A). ANOVA results demonstrated a significant effect (p < 0.05) of various exposure durations on CAT activity, which exhibited an increasing trend followed by a decrease, peaking at 4 days post-imidacloprid exposure (Table 4).
SOD activity significantly increased (p < 0. 01) at 4 days post-exposure compared to the control, while it was suppressed (p < 0.05 and p < 0.01) at other time points following exposure (Fig. 4B). ANOVA analysis indicated significant effects (p < 0.05) of different exposure durations on SOD activity. AChE activity increased over time, then decreased and was inhibited after 4 days of imidacloprid exposure (Table 4).
POD activity in larvae significantly increased (p < 0.01 and p < 0.05) after 1 day of treatment following exposure to imidacloprid (Fig. 4C). ANOVA analysis revealed that different exposure times significantly (p < 0.05) affected POD activity. After 1 day, POD activity in the treated group increased (p < 0.05) with more prolonged exposure, peaking after 16 days (Table 4).
The MDA content in larvae increased (p < 0.01 and p < 0.05) after 1 day of treatment post-imidacloprid exposure (Fig. 4D). ANOVA results indicated that different exposure times (p < 0.05) significantly influenced imidacloprid-induced MDA content. As exposure time increased, MDA content in the treated group significantly rose after 1 day of imidacloprid exposure, peaking at 16 days post-exposure (Table 4).
HSP70 expression in response to imidacloprid exposure
PxanHSP68 expression levels were significantly lower in the day 2 imidacloprid-treated group and significantly higher in the day 3 group, compared to the control group. PxanHSP70-1 expression levels were higher in both day 2 and day 3 imidacloprid-treated groups, but the difference was not significant in the day 2 group. PxanHSP70-2 expression levels were significantly lower in both day 2 and day 3 imidacloprid-treated groups compared to the control group. PxanHSP70-3 expression levels were lower in the day 2 imidacloprid-treated group and significantly higher in the day 3 group compared to the control group. PxanHSP70-4 expression levels were higher in both day 2 and day 3 imidacloprid-treated groups, though the difference in the day 2 group was not significant. PxanHSP70-5 expression levels were significantly higher in both day 2 and day 3 imidacloprid-treated groups compared to the control group (Fig. 5).
Expression profile of PxanHSP70s under imidacloprid stress. The relative expression levels were normalized with the expression levels of β-tubulin. Error bars showed the standard errors of the means of three biological replicates. Furthermore, significant differences are marked with letters (p < 0.05, one-way ANOVA). All values are mean ± SE
Discussion
Neonicotinoids present significant risks to numerous aquatic and terrestrial animals, with imidacloprid being the most extensively studied neonicotinoid thus far (Morrissey et al. 2015; Rico et al. 2018). Megaloptera larvae are sensitive to water pollution and are widely used as bioindicators for assessing freshwater ecosystem quality (Romero et al. 2021; Wen et al. 2022). Consequently, our study focused on investigating the toxicity of imidacloprid to larvae and the resulting physiological changes.
The impacts of pesticides are commonly assessed through traditional laboratory ecotoxicology studies designed to provide data for setting water quality standards and establishing safe pollutant concentrations (Walker et al. 2001). P. xanthodes larvae exposed to lower concentrations (1 mg/L) of imidacloprid exhibited a significant toxic response 16 days post-exposure, yet only a few perished after 16 days. Larvae exposed to higher concentrations (8 mg/L or more) of imidacloprid displayed a clear toxic response after 4 days of exposure, with almost half dying after 16 days. Compared to larvae of other aquatic insects, P. xanthodes larvae demonstrated remarkable resistance (Huang et al. 2021a, b; Focks et al. 2018; Cavallaro et al. 2017). This phenomenon might be associated with the size of P. xanthodes larvae or potentially linked to the route of exposure (Xie and Buchwalter 2011). In our study, after 2 days of exposure to imidacloprid, the vitality of P. xanthodes larvae was significantly reduced, impairing their ability to function normally. This phenomenon could be attributed to the possibility that imidacloprid insecticide leads to decreased neural conduction (Parkinson et al. 2020; Parkinson and Gray 2019; Wei et al. 2020). After 4 days of exposure to imidacloprid, P. xanthodes larvae continually curled their bodies, resembling the defensive curling seen in death-feigning behavior when stimulated (Humphreys and Ruxton 2018; Skelhorn 2018; Rogers and Simpson 2014). This phenomenon may be attributed to the binding of imidacloprid to nAChRs, leading to sustained conduction of nerve impulses (Topal et al. 2017; Cartereau et al. 2021). The phenomenon of a noticeable initial increase in MDA content was observed on day 2 post-imidacloprid exposure, coinciding with the onset of the decline in larval viability. This increase may be due to the damage caused by excess ROS to biological systems (Toyokuni 1999; Juan et al. 2021).
Acetylcholinesterase is an enzyme that catalyzes the hydrolysis of acetylcholine and, as such, plays a role in regulating a variety of functions in organisms, including nerve and muscle activity (Hemingway et al. 2004; Vidal-Albalat et al. 2023). Exposure to pesticides can activate AChE in organisms; therefore, AChE can serve as a biomarker for detecting pesticide residues in the environment (Sheets et al. 2016; Fulton and Key 2001; Moreira et al. 2001; Yadav et al. 2009). In this study, 2 days after treatment, AChE activity in the larvae was significantly inhibited compared to the control group. This phenomenon suggests that sublethal doses of imidacloprid may have deleterious effects on the metabolism of P. xanthodes larvae (Boily et al. 2013). However, there was a slight increase in AChE activity at 4 days of exposure compared to 2 days of exposure. This phenomenon may be due to the binding of imidacloprid to nicotinic receptors of insects (Li et al. 2017). The activity of AChE was significantly inhibited compared to the control group, and concurrently, MDA content increased at the same time point following imidacloprid exposure. This trend mirrors the reaction observed in the crayfish Procambarus clarkii. It is possible that the activation of imidacloprid induces AChE inhibition and oxidative stress (Huang et al. 2021a, b). We advocate for further investigation of the effect of imidacloprid on acetylcholinesterase in P. xanthodes larvae.
Previous studies have shown that neonicotinoids induce DNA damage, protein oxidation, and lipid peroxidation in organisms by inhibiting mitochondrial respiration and increasing reactive oxygen species (ROS) production (Xu et al. 2022; Wang et al. 2018). Antioxidant enzymes are the first line of defense against free radicals in organisms, and they work synergistically to protect cells from excess reactive oxygen species (ROS) from endogenous metabolism and the external microenvironment (Rodriguez et al. 2004; Galasso et al. 2021). Antioxidant enzymes like superoxide dismutase, catalase, glutathione transferase, and glutathione reductase are well-characterized in insects (Felton and Summers 1995; Wang et al. 2001). Previous research has shown that oxidative stress can affect these antioxidant enzymes (He et al. 2017). Mild oxidative stress stimulates enzyme activity to aid in eliminating reactive oxygen radicals, whereas severe oxidative stress disrupts the organism’s metabolic mechanisms, thereby inhibiting antioxidant enzyme activity (Siddique et al. 2007; Wang et al. 2016; Jameel et al. 2019).
In this study, we assessed the activity of several antioxidant enzymes, glutathione sulfotransferase, superoxide dismutase, catalase, and peroxidase in P. xanthodes larvae at various time points post-imidacloprid exposure. Glutathione S-transferase (GST) is a vital secondary phase enzyme involved in numerous biological processes and various detoxifying functions against toxic substances (Ranson and Hemingway 2005; Meng et al. 2023). GST is involved in insecticide resistance (Tao et al. 2022). GST breaks down insecticides into water-soluble compounds that are easily excreted, using reductive dechlorination or glutathione reactions (Enayati et al. 2005). GST active site binding, followed by conjugation with GSH, participates in detoxification, playing a significant role in insect immune responses (Kostaropoulos et al. 2001a, b). This enzyme can be used as an indicator to assess neonicotinoid toxicity (Mörtl et al. 2020). In this study, glutathione sulfotransferase (GST) activity significantly increased on day 2 post-imidacloprid exposure, continued to rise until peaking at day 8, and then slightly decreased. The observed increase in GST activity post-exposure was similar to that seen in the coding moth Cydia pomonella and Apis mellifera honey bees (Yang and Zhang 2015; Tavares et al. 2017; Dussaubat et al. 2016). Based on current toxicity studies, we found that P. xanthodes larvae exhibit extreme resistance to imidacloprid compared to other aquatic insect larvae, showing insensitivity to low pesticide concentrations. This increased resistance might be attributed to heightened GST activity following stress exposure (Yang and Zhang 2015). The trends of GST activity, POD activity, and MDA concentration changes are similar, indicating that GST may play a role in eliminating ROS (Liu et al. 2022).
Superoxide dismutase (SOD) catalyzes the disproportionation of superoxide anions to produce oxygen and hydrogen peroxide, which plays a vital role in the oxidative and antioxidant homeostasis in organisms. The results showed that SOD activity was significantly reduced on days 1, 2, 8, and 16 after treatment with imidacloprid. It suggests that imidacloprid may inhibit SOD biosynthesis in P. xanthodes larvae. This result aligns with reports of significantly decreased SOD activities in thrips and aphids following imidacloprid exposure (Li et al. 2018; Zhang et al. 2020a, b). However, SOD activity peaked on day 4 and showed a significant increase in comparison with the control group. This result aligns with findings in Galleria mellonella (Yucel and Kayis 2019). The decline in SOD activity may indicate the generation of excess ROS during early exposure, surpassing SOD’s processing capacity, and the excess O2 may inhibit SOD synthesis (Wang et al. 2016; Siddique et al. 2007). The decrease in SOD activity could be related to excess ROS, which may cause changes in enzyme synthesis, inactivation, or alter its subunit assembly (Shalini Verma 2003; Batista-Silva et al. 2019; Jameel et al. 2019).
SOD catalyzes the conversion of O2 to H2O2, and then catalase (CAT) and peroxidase (POD) are involved in further detoxification. CAT promotes the decomposition of H2O2 into oxygen molecules and water, removes hydrogen peroxide from the body, and thus protects the cells from the toxicity of H2O2, and is a key enzyme in the biodefense system (He et al. 2017). In the study, the trend of the activity of CAT continuously increased after 1 day of exposure to imidacloprid until it peaked at 4 days and then decreased. This phenomenon is similar to that of zebrafish after exposure to imidacloprid (Ge et al. 2015). Moreover, the activity of SOD also peaked at 4 days. Trends of SOD activity variation showed a similar increase and then decrease with CAT. This phenomenon may be related to the synergistic action of SOD and CAT. The antioxidant reaction catalyzed by SOD produces an excess of hydrogen peroxide. Consequently, to achieve the balanced state of hydroxyl radicals in the larvae, there is an increased stress-induced CAT activity (Zhang et al. 2013; Lv et al. 2021). After peaking, the viability of CAT decreased; however, it was still significantly higher compared to the control group. These results may be attributed to a decrease in SOD activity. Imidacloprid induces oxidative stress and alters the antioxidant defense mechanism of POD, which plays a role in resistance to lipid peroxidation (Wang et al. 2015; Ahmad et al. 2011; Hormozi et al. 2018).
POD has the dual effect of eliminating hydrogen peroxide and phenolamine toxicity. POD works in synergy with other antioxidant enzymes to scavenge excessive oxidative free radicals in the body, thus increasing the stress tolerance of the organisms (Li et al. 2013; Saleem and Afsheen 2022). In this study, POD activity continuously increased throughout the imidacloprid exposure period. This increase suggests that POD activity is stimulated when organisms are exposed to imidacloprid, acting as a defense mechanism (Fan et al. 2021; Dampc et al. 2020).
In this study, SOD activity and MDA content exhibit similar trends in their changes; this phenomenon could be related to the POD-eliminated ROS and MDA through the antioxidant defense response (Lv et al. 2022), POD may play a role in the resistance to lipid peroxidation.
Pesticide-induced oxidative stress is caused by ROS (Sule et al. 2022). MDA is one of the most important products of membrane lipid peroxidation, which can reflect the level of peroxidation of biological lipids, the level of ROS, and the degree of trauma to cell tissues (Chen et al. 2015; Zhang et al. 2020a, b). Imidacloprid can induce ROS triggering neurological and metabolic impairments in Drosophila (Martelli et al. 2020). In this study, there was no change in MDA content 1 day after exposure to imidacloprid. However, a significant increase was observed, which continued to rise after 2 days of treatment compared to the control. This phenomenon may be associated with changes in ROS and enzyme-related antioxidant responses (Wu and Liu 2012; Wang et al. 2016). ROS accumulation gradually increased MDA content in organisms with prolonged treatment (Balieira et al. 2018). Increased MDA showed that imidacloprid-induced lipid peroxidation in the larvae of P. xanthodes. ROS can cause membrane lipid peroxidation and induce many negative effects (Balieira et al. 2018; Bhattacharyya and Datta 2001; Bálint 2021). These results confirm that MDA has an indicative effect on P. xanthodes larvae under sublethal imidacloprid stress. The impacts of imidacloprid on non-target organism aqueous environments have been extensively studied. imidacloprid has been shown to inhibit enzyme activities in Eriocheir sinensis (Hong et al. 2020). Several reports indicate that oxidative stress and tissue damage are induced in zebrafish in aquatic environments (Luo et al. 2021). In particular, imidacloprid shows different toxicity to aquatic insects such as Chironomus riparius and Choroterpes (Euthralus) yixingensis and can cause oxidative stress in the individuals of those species (Njattuvetty et al. 2018; Guan et al. 2021). In our study, P. xanthodes larvae exhibited a toxic response to imidacloprid exposure, evidenced by oxidative stress and indicated by behavioral and physiological markers (Bernabò et al. 2017; Lee et al. 2006). In the experiment, the activity of MDA continuously increased during the stress period, while most of the enzyme activities tended to decrease after reaching the peak. This finding could indicate that the systems that maintain these enzymes have been damaged due to excessive ROS damage, implying that the P. xanthodes larvae will die after a certain amount of imidacloprid stress, even if the stress stops.
Wen and collaborators identified six heat shock proteins (HSPs) in the HSP70 family in P. costalis (Wen et al. 2022). In the study, the expression levels of PxanHSP68 and PxanHSP70-3 exhibited trends similar to the changes in stress duration, while PxanHSP70-1, PxanHSP70-4, and PxanHSP70-5 showed significant time-dependent expression changes. Notably, fluctuations in the expression levels of PxanHSP68, PxanHSP70-2, and PxanHSP70-5 were greatest at 1 day and 3 days of stress, compared to the unstressed state (0 days). Similar to species like the beetle Leptinotarsa decemlineata, fly louse Sogatella furcifera, and clams Corbicula fluminea, a response to imidacloprid was also observed. The relative expression levels of HSP70 varied, but the relationships between different species require further study (Dumas et al. 2019; Zhou et al. 2018; Shan et al. 2019). Overall, the expression levels of all genes were elevated, except for the reduced expression level of HSP70-2. MDA content consistently increased during the same stress time; the activities of GST, CAT, and POD had a similar trend. This might suggest that ROS damage did not lead to the misfolding of most of the genes under a short period (within 3 days) of imidacloprid stress, but on the contrary, these genes were involved in the immune response. Whereas the trend of HSP70-2 was similar to that of SOD, this decrease may signify protein misfolding due to ROS damage, which suggests some kind of synergistic effect between HSP70-2 and SOD. In conclusion, there are too few studies related to this taxon in the order of Megaloptera, and more data is required to support our hypotheses.
Imidacloprid, one of the most widely used insecticides and highly soluble in water, has led to significant residues in aquatic environments due to its widespread use. Aquatic insects are often used as indicator organisms for pollution in aquatic environments, and the stress adaptation mechanisms of physiological indicators under pesticide stress have been extensively researched. However, as an aquatic insect sensitive to water quality, the stress resistance mechanism of Megaloptera to pollutant stress has been poorly studied. This study’s toxicological characterization and physiological response analysis of P. xanthodes larvae after exposure to sublethal concentrations of imidacloprid reveal high resistance to the pesticide, with significant responses only under prolonged exposure to high concentrations.
This finding suggests that the adverse effects of environmental neonicotinoid pesticide residues on Megaloptera insects might involve a long-term process or depend on accumulation through the food chain. The impact of environmental pesticide residues on Megaloptera insects requires further study. Additionally, as Megaloptera insects are valuable for both consumption and medicine, breeding industries for these larvae exist in certain areas. However, rearing environments for larvae are generally based directly on field environments. The findings of this research can offer valuable guidance for these aquaculture industries.
References
Adak T, Kumar J, Shakil NA, Walia S (2012) Development of controlled release formulations of imidacloprid employing novel nano-ranged amphiphilic polymers. J Environ Sci Health B 47:217–225
Agatz A, Ashauer R, Brown CD (2014) Imidacloprid perturbs feeding of Gammarus pulex at environmentally relevant concentrations. J Environ Toxicol Chem 33(3):648–653
Ahmad MK, Syma S, Mahmood R (2011) Cr(VI) induces lipid peroxidation, protein oxidation and alters the activities of antioxidant enzymes in human erythrocytes. Biol Trace Elem Res. 144:426–435
Balieira and Kamila Vilas Boas (2018) Imidacloprid-induced oxidative stress in honey bees and the antioxidant action of caffeine. Apidologie. 49: 562 - 572
Bálint B (2021) Differences in the effects of sodium selenate and sodium selenite on the mortality, reproduction, lipid peroxidation and glutathione redox status of Folsomia candida Willem 1902 (Collembola). Eur J Soil Biol. n. pag.
Bass C, Denholm I, Williamson MS, Nauen R (2015) The global status of insect resistance to neonicotinoid insecticides. Pest Biochem Physiol 121:78–87
Batista-Silva W, Heinemann B, Rugen N, Nunes-Nesi A, Araújo WL, Braun HP, Hildebrandt TM (2019) The role of amino acid metabolism during abiotic stress release. Plant Cell Environ 42:1630–1644
Bernabò P, Gaglio M, Bellamoli F, Viero G, Lencioni V (2017) DNA damage and translational response during detoxification from copper exposure in a wild population of Chironomus riparius. Chemosphere 173:235–244
Bhattacharyya J, Datta AG (2001) Studies on the effects of lipopolysaccharide on lipid peroxidation of erythrocyte and its reversal by mannitol and glycerol. J Physiol Pharmacol 52:145–152
Boily M, Sarrasin B, Deblois C, Aras P, Chagnon M (2013) Acetylcholinesterase in honey bees (Apis mellifera) exposed to neonicotinoids, atrazine and glyphosate: laboratory and field experiments. Environ Sci Pollut Res Int 020:5603–5614
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55(4):611–622
Cao CQ (2014) Situation of developing and utilizing sand crawling worm in China and species definition. J Hubei Agric Sci
Cartereau A, Taillebois EL, Questel J-Y, Thany SH (2021) Mode of action of neonicotinoid insecticides imidacloprid and thiacloprid to the cockroach Pameα7 nicotinic acetylcholine receptor. Ijms 22:9880
Cavallaro MC, Morrissey CA, Headley JV, Peru KM, Liber K (2017) Comparative chronic toxicity of imidacloprid, clothianidin, and thiamethoxam to Chironomus dilutus and estimation of toxic equivalency factors. Environ Toxicol Chem 36:372–382
Chen J, Zeng L, Xia T, Li S, Yan T, Wu S, Qiu G, Liu Z (2015) Toward a biomarker of oxidative stress: a fluorescent probe for exogenous and endogenous malondialdehyde in living cells. Anal Chem 18(87):8052–8056
Chen C, Hu W, Zhang R, Jiang A, Liu C (2018) Effects of hydrogen sulfide on the surface whitening and physiological responses of fresh-cut carrots. J Sci Food Agric 98:4726–4732
Cheung CCC, Zheng GJ, Li AMY, Richardson BJ (2001) Lam PKS. Relationship between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels. Perna Viridis Aquat Toxicol 52:189–203
Cloyd RA, Bethke JA (2011) Impact of neonicotinoid insecticides on natural enemies in greenhouse and interiorscape environments. Pest Manag Sci 67:3–9
Contreras-Ramos A (2008) Notes on some neotropical alderflies (Sialidae: Megaloptera). Ann Entomol Soc Am 101:808–814
Dampc J, Kula-Maximenko M, Molon M, Durak R (2020) Enzymatic defense response of apple aphid Aphis pomi to increased temperature. Insects 11(11):436
Docile TN, Figueiró R, Portela C, Nessimian JL (2016) Macroinvertebrate diversity loss in urban streams from tropical forests. Environ Monit Assess 188:237
Dumas P, Morin MD, Boquel S, Moffat CE, Morin PJ (2019) Expression status of heat shock proteins in response to cold, heat, or insecticide exposure in the Colorado potato beetle Leptinotarsa decemlineata. Cell Stress Chaperones 24(3):539–547
Dussaubat C, Maisonnasse A, Crauser D, Tchamitchian S, Bonnet M, Cousin M, Kretzschmar A, Brunet JL, Le Conte Y (2016) Combined neonicotinoid pesticide and parasite stress alter honeybee queens’ physiology and survival. Sci Rep 31(6):31430
Enayati AA, Ranson H, Hemingway J (2005) Insect glutathione transferases and insecticide resistance. Insect Mol Biol 14:3–8
Fan W, Li G, Zhang X, Wang Y, Wang C, Xu B, Guo X, Li H (2021) The role of melatonin and tryptophan-5-hydroxylase-1 in different abiotic stressors in Apis cerana cerana. J Insect Physiol 128:104180
Felton GW, Summers CB (1995) Antioxidant systems in insects. Arch Insect Biochem Physiol 29:187–197
Flores-Céspedes F, Figueredo-Flores CI, Daza-Fernández I, Vidal-Peña F, Villafranca-Sánchez M, Fernández-Pérez M (2012) Preparation and characterization of imidacloprid lignin-polyethylene glycol matrices coated with ethylcellulose. J Agric Food Chem 60:1042–1051
Focks A, Belgers D, Boerwinkel MC, Buijse L, Roessink I, Van den Brink PJ (2018) Calibration and validation of toxicokinetic-toxicodynamic models for three neonicotinoids and some aquatic macroinvertebrates. Ecotoxicology 27:992–1007
Frew JA, Brown JT, Fitzsimmons PN, Hoffman AD, Sadilek M, Grue CE, Nichols JW (2018) Toxicokinetics of the neonicotinoid insecticide imidacloprid in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol C Toxicol Pharmacol 205:34–42
FultonKey MHPB (2001) Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ Toxicol Chem 20:37–45
Galasso M, Gambino S, Romanelli MG, Donadelli M, Scupoli MT (2021) Browsing the oldest antioxidant enzyme: catalase and its multiple regulation in cancer. Free Radic Biol Med 20(172):264–272
Ge W, Yan S, Wang J, Zhu L, Chen A, Wang J (2015) Oxidative stress and DNA damage induced by imidacloprid in zebrafish (Danio rerio). J Agric Food Chem 18(63):1856–1862
Guan JY, Zhang ZY, Cao YR, Xu XD, Storey KB, Yu DN, Zhang JY (2021) The complete mitochondrial genome of Choroterpes (Euthralus) yixingensis (Ephemeroptera: Leptophlebiidae) and its mitochondrial protein-coding gene expression under imidacloprid stress. Gene 20(800):145833
He L, He T, Farrar S, Ji L, Liu T, Ma X (2017) Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem 44:532–553
Hemingway J, Hawkes NJ, McCarroll L, Ranson H (2004) The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol 34:653–665
Hightower L (1991) Heat shock, stress proteins, chaperones and proteotoxicity. Cell 66:191–197
Hong Y, Huang Y, Wu S, Yang X, Dong Y, Xu D, Huang Z (2020) Effects of imidacloprid on the oxidative stress, detoxification and gut microbiota of Chinese mitten crab Eriocheir Sinensis. Sci Total Environ 10(729):138276
Hormozi M, Mirzaei R, Nakhaee A, Izadi S, Dehghan HJ (2018) The biochemical effects of occupational exposure to lead and cadmium on markers of oxidative stress and antioxidant enzymes activity in the blood of glazers in tile industry. Toxicol Ind Health 34:459–467
Huang A, van den Brink NW, Buijse L, Roessink I, van den Brink PJ (2021a) The toxicity and toxicokinetics of imidacloprid and a bioactive metabolite to two aquatic arthropod species. Aquat Toxicol 235:105837
Huang Y, Hong Y, Yin H, Yan G, Huang Q, Li Z, Huang Z (2021b) Imidacloprid induces locomotion impairment of the freshwater crayfish, Procambarus clarkii via neurotoxicity and oxidative stress in digestive system. Aquat Toxicol 16(238):105913
Humphreys RK, Ruxton GD (2018) A review of thanatosis (death feigning) as an anti-predator behaviour. Behav Ecol Sociobiol 72:22
Jameel M, Alam MF, Younus H, Jamal K, Siddique HR (2019) Hazardous sub-cellular effects of fipronil directly influence the organismal parameters of Spodoptera litura. Ecotoxicol Environ Saf 172:216–224
Jeschke P, Nauen R (2008) Neonicotinoids-from zero to hero in insecticide chemistry. Pest Manag Sci 64:1084–1098
Joseph B, Raj SJ (2011) Impact of pesticide toxicity on selected biomarkers in fishes. Intl J Zool Res 7(2):212
Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E (2021) The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Ijms 22:4642
Kostaropoulos I, Papadopoulos AI, Metaxakis A, Boukouvala E, Papadopoulou-Mourkidou E (2001a) The role of glutathione S-transferases in the detoxification of some organophosphorus insecticides in larvae and pupae of the yellow mealworm, Tenebrio molitor (Coleoptera: Tenebrionidae). Pest Manag Sci 57:501–508
Kostaropoulos I, Papadopoulos AI, Metaxakis A, Boukouvala E, Papadopoulou-Mourkidou E (2001b) Glutathione S-transferase in the defence against pyrethroids in insects. Insect Biochem Mol Biol 15(31):313–319
Lee SM, Lee SB, Park CH, Choi J (2006) Expression of heat shock protein and hemoglobin genes in Chironomus tentans (Diptera, Chironomidae) larvae exposed to various environmental pollutants: a potential biomarker of freshwater monitoring. Chemosphere 65(6):1074–1081
Li J, Sun H, Jin L, Cao W, Zhang J, Guo CY, Ding K, Luo C, Ye WC, Jiang RW (2013) Alleviation of podophyllotoxin toxicity using coexisting flavonoids from Dysosma versipellis. PLoS ONE 21(8):e72099
Li W, Lu Z, Li L, Yu Y, Dong S, Men X, Ye B (2018) Sublethal effects of Imidacloprid on the performance of the bird cherry-oat aphid Rhopalosiphum padi. PLoS ONE 20(13):e0204097
Li Z, Li M, He J, Zhao X, Chaimanee V, Huang WF, Nie H, Zhao Y, Su S (2017) Differential physiological effects of neonicotinoid insecticides on honey bees: a comparison between Apis mellifera and Apis cerana. Pestic Biochem Physiol 140:1–8
Li X, Wang M, Chen W, Jiang R (2019) Evaluation of combined toxicity of siduron and cadmium on earthworm (Eisenia fetida) using biomarker response index. Sci Total Environ 646:893–901
Liu X, Fu ZX, Kang ZW, Li H, Liu TX, Wang D (2022) Identification and characterization of antioxidant enzyme genes in parasitoid Aphelinus asychis (Hymenoptera: Aphelinidae) and expression profiling analysis under temperature stress. Insects 9(13):447
Liu X, Hayashi F, Yang D (2006) Systematics of the Protohermes xanthodes species-group in eastern Asia (Megaloptera: Corydalidae). Entomol Sci 9:399–409
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25(4):402–408
Luo T, Wang X, Jin Y (2021) Low concentrations of imidacloprid exposure induced gut toxicity in adult zebrafish (Danio rerio). Comp Biochem Physiol C Toxicol Pharmacol 241:108972
Lv Y, Feng Y, Lv C, Liu X (2022) Lipid peroxidation and antioxidant responses of Microcoleus vaginatus with the aid of attapulgite-based nanocomposite to wind stress. Environ Technol 20:1–9
Lv Y, Li Y, Liu X, Xu K (2021) Effect of soil sulfamethoxazole on strawberry (Fragaria ananassa): growth, health risks and silicon mitigation. Environ Pollut 286:117321
Maity S, Roy S, Chaudhury S, Bhattacharya S (2008) Antioxidant responses of the earthworm Lampito Mauritii exposed to Pb and Zn contaminated soil. Environ Pollut 151:1–7
Maloney EM, Taillebois E, Gilles N, Morrissey CA, Liber K, Servent D, Thany SH (2021) Binding properties to nicotinic acetylcholine receptors can explain differential toxicity of neonicotinoid insecticides in Chironomidae. Aquat Toxicol 230:105701
Martelli F, Zhongyuan Z, Wang J, Wong CO, Karagas NE, Roessner U, Rupasinghe T, Venkatachalam K, Perry T, Bellen HJ, Batterham P (2020) Low doses of the neonicotinoid insecticide imidacloprid induce ROS triggering neurological and metabolic impairments in Drosophila. Proc Natl Acad Sci U S A 13(117):25840–25850
Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, Sattelle DB (2001) Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci 22:573–580
Meng LW, Chen ML, Yuan GR, Zheng LS, Dou W, Peng Y, Zhang SX, Wang JJ (2023) An antenna-abundant glutathione S-transferase BdGSTd8 participates in detoxification of two organophosphorus insecticides in Bactrocera dorsalis (Hendel). J Agric Food Chem 7(71):8400–8412
Moreira SM, Coimbra J, Guilhermino L (2001) Acetylcholinesterase of Mytilus galloprovincialis LmK. Hemolymph: a suitable environmental biomarker. Bull Environ Contam Toxicol 67:0470–0475
Morrissey CA, Mineau P, Devries JH, Sanchez-Bayo F, Liess M, Cavallaro MC, Liber K (2015) Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ Int 74:291–303
Mörtl M, Vehovszky Á, Klátyik S, Takács E, Győri J, Székács A (2020) Neonicotinoids: spreading, translocation and aquatic toxicity. Int J Environ Res Public Health 18(17):2006
Martelli FM et al. (2020) Low doses of the neonicotinoid insecticide imidacloprid induce ROS triggering neurological and metabolic impairments in Drosophila. PNAS
Njattuvetty Chandran N, Fojtova D, Blahova L, Rozmankova E, Blaha L (2018) Acute and (sub)chronic toxicity of the neonicotinoid Imidacloprid on Chironomus riparius. Chemosphere 209:568–577
Parkinson RH, Gray JR (2019) Neural conduction, visual motion detection, and insect flight behaviour are disrupted by low doses of imidacloprid and its metabolites. Neurotoxicology 72:107–113
Parkinson RH, Zhang S, Gray JR (2020) Neonicotinoid and sulfoximine pesticides differentially impair insect escape behavior and motion detection. Proc Natl Acad Sci U S A 10(117):5510–5515
Pestana JL, Loureiro S, Baird DJ, Soares AM (2009) Fear and loathing in the benthos: responses of aquatic insect larvae to the pesticide Imidacloprid in the presence of chemical signals of predation risk. Aquat Toxicol 28(93):138–149
Ranson H, Hemingway J (2005) Mosquito glutathione transferases. Methods Enzymol 401:226–241
Rhee JS, Raisuddin S, Lee KW, Seo JS, Ki JS, Kim IC, Park HG, Lee JS (2009) Heat shock protein (Hsp) gene responses of the intertidal copepod Tigriopus japonicus to environmental toxicants. Comp Biochem Physiol c: Toxicol Pharmacol 149(1):104–112
Rico A, Arenas-Sánchez A, Pasqualini J, García-Astillero A, Cherta L, Nozal L, Vighi M (2018) Effects of imidacloprid and a neonicotinoid mixture on aquatic invertebrate communities under Mediterranean conditions. Aquat Toxicol 204:130–143
Rivera-Gasperín SL, Ardila-Camacho A, Contreras-Ramos A (2019) Bionomics and ecological services of Megaloptera larvae (dobsonflies, fishflies, alderflies). Insects 10(4):86
Roberts RJ, Agius C, Saliba C, Bossier P, Sung YY (2010) Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: a review. J Fish Dis 33(10):789–801
Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 36:1–9
Roessink I, Merga LB, Zweers HJ, Van den Brink PJ (2013) The neonicotinoid imidacloprid shows high chronic toxicity to mayfly nymphs. Environ Toxicol Chem 32:1096–1100
Rogers SM, Simpson SJ (2014) Thanatosis. Curr Biol 3(24):R1031–R1033
Romero GQ, Moi DA, Nash LN, Antiqueira PAP, Mormul RP, Kratina P (2021) Pervasive decline of subtropical aquatic insects over 20 years driven by water transparency, non-native fish and stoichiometric imbalance. Biol Let 17(6):20210137
Rico-Sánchez AE, Rodríguez-Romero AJ, Sedeño-Díaz JE, López-López E (2020) Assessment of seasonal and spatial variations of biochemical markers in Corydalus sp. (Megaloptera: Corydalidae), a non-conventional biomonitor, in a mountain cloud forest in Mexico. Environ Sci Pollut Res Intl 27(24):30755–30766
Saleem R, Afsheen S (2022) Analysis of antioxidants in water striders (Hemiptera: Gerridae) as bioindicator of water pollution. Braz J Biol 20(84):e258106
Sanders BM (1993) Stress proteins in aquatic organisms: an environmental perspective. Crit Rev Toxicol 23:49–75
Seifert J, Stollberg J (2005) Antagonism of a neonicotinoid insecticide imidacloprid at neuromuscular receptors. Environ Toxicol Pharmacol 20:18–21
Shalini Verma RS (2003) Dubey, Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Shan Y, Yan S, Hong X, Zha J, Qin J (2019) Effect of imidacloprid on the behavior, antioxidant system, multixenobiotic resistance, and histopathology of Asian freshwater clams (Corbicula fluminea). Aquat Toxicol 218:105333
Sharma A, Kumar V, Shahzad B, Tanveer M, Sidhu GPS, Handa N (2019) Worldwide pesticide usage and its impacts on ecosystem. SN Appl Sci 1:1446
Sheets LP, Li AA, Minnema DJ, Collier RH, Creek MR, Peffer RC (2016) A critical review of neonicotinoid insecticides for developmental neurotoxicity. Crit Rev Toxicol 46:153–190
Shi YC, Ren Y, Liu HB, Gu J, Cao CQ (2019) Analysis of main nutritional componets in hellgrammites and its anti-diuretic effect. Sci Technol Food Indust 40(05):87–92
Siddique HR, Gupta SC, Mitra K, Murthy RC, Saxena DK, Chowdhuri DK (2007) Induction of biochemical stress markers and apoptosis in transgenic Drosophila melanogaster against complex chemical mixtures: role of reactive oxygen species. Chem Biol Interact 20(169):171–188
Singh A, Leppanen C (2020) Known target and nontarget effects of the novel neonicotinoid cycloxaprid to arthropods: a systematic review. Integr Environ Assess Manag 16:831–840
Siregar P, Suryanto ME, Chen KHC, Huang JC, Chen HM, Kurnia KA et al (2021) Exploiting the freshwater shrimp Neocaridina denticulata as aquatic invertebrate model to evaluate nontargeted pesticide induced toxicity by investigating physiologic and biochemical parameters. Antioxidants 10:391
Skelhorn J (2018) Avoiding death by feigning death. Curr Biol 8(28):R1135–R1136
Soares HM, Jacob CR, Carvalho SM, Nocelli RC, Malaspina O (2015) Toxicity of imidacloprid to the stingless bee Scaptotrigona postica Latreille, 1807 (Hymenoptera: Apidae). Bull Environ Contam Toxicol 94:675–680
Sorger PK (1991) Heat shock factor and the heat shock response. Cell 65(3):363–366
Sule RO, Condon L, Gomes AV (2022) A common feature of pesticides: oxidative stress—the role of oxidative stress in pesticide-induced toxicity. Oxidative medicine and cellular longevity
Tao F, Si FL, Hong R, He X, Li XY, Qiao L, He ZB, Yan ZT, He SL, Chen B (2022) Glutathione S-transferase (GST) genes and their function associated with pyrethroid resistance in the malaria vector Anopheles sinensis. Pest Manag Sci 78:4127–4139
Tavares DA, Dussaubat C, Kretzschmar A, Carvalho SM, Silva-Zacarin ECM, Malaspina O, Bérail G, Brunet JL, Belzunces LP (2017) Exposure of larvae to thiamethoxam affects the survival and physiology of the honey bee at post-embryonic stages. Environ Pollut 229:386–393
Thompson DA, Lehmler H-J, Kolpin DW, Hladik ML, Vargo JD, Schilling KE (2020) A critical review on the potential impacts of neonicotinoid insecticide use: current knowledge of environmental fate, toxicity, and implications for human health. Environ Sci Process 22:1315–1346
Topal A, Alak G, Ozkaraca M, Yeltekin AC, Comaklı S, Acıl G (2017) Neurotoxic responses in brain tissues of rainbow trout exposed to imidacloprid pesticide: assessment of 8-hydroxy-2-deoxyguanosine activity, oxidative stress and acetylcholinesterase activity. Chemosphere 175:186–191
Toyokuni S (1999) Reactive oxygen species-induced molecular damage and its application in pathology. Pathol Int 49:91–102
Van Lexmond MB, Bonmatin JM, Goulson D, Noome DA (2015) Worldwide integrated assessment on systemic pesticides: global collapse of the entomofauna: exploring the role of systemic insecticides. Environ Sci Pollut Res Int 22:1–4
Vehovszky A, Farkas A, Acs A, Stoliar O (2015) Neonicotinoid insecticides inhibit cholinergic neurotransmission in a molluscan (Lymnaea stagnalis) nervous system. J Aquatic Toxicol 167:172–179
Vidal-Albalat A, Kindahl T, Rajeshwari R, Lindgren C, Forsgren N, Kitur S, Tengo LS, Ekström F, Kamau L, Linusson A (2023) Structure-activity relationships reveal beneficial selectivity profiles of inhibitors targeting acetylcholinesterase of disease-transmitting mosquitoes. J Med Chem 11(66):6333–6353
Vohra P, Khera KS, Sangha GK (2014) Physiological, biochemical and histological alterations induced by administration of imidacloprid in female albino rats. Pesticide Biochem Physiol 110:50–56
Walker CH, Hopkin SP, Sibly RM, Peakall DB (2001) Principles of ecotoxicology, 2nd edn. Taylor & Francis, London
Wang J, Wang J, Wang G, Zhu L, Wang J (2016) DNA damage and oxidative stress induced by imidacloprid exposure in the earthworm Eisenia fetida. Chemosphere 144:510–517
Wang J, Zhang Y, Liu R, Li X, Cui Y, Qu L (2015) Geniposide protects against acute alcohol-induced liver injury in mice via up-regulating the expression of the main antioxidant enzymes. Can J Physiol Pharmacol 93:261–267
Wang X, Anadón A, Wu Q, Qiao F, Ares I, Martínez-Larrañaga MR, Yuan Z, Martínez MA (2018) Mechanism of neonicotinoid toxicity: impact on oxidative stress and metabolism. Annu Rev Pharmacol Toxicol 6(58):471–507
Wang Y, Oberley LW, Murhammer DW (2001) Antioxidant defense systems of two lipidopteran insect cell lines. Free Radic Biol Med 1(30):1254–1262
Wei F, Wang D, Li H, Xia P, Ran Y, You J (2020) Toxicogenomics provides insights to toxicity pathways of neonicotinoids to aquatic insect. Chironomus Dilutus Environ Pollut 260:114011
Wen F, Yang J, Huang X, Huang X (2022) Analysis of differential gene expression of the aquatic insect Protohermes costalis (Walker) (Megaloptera: Corydalidae) in response to cadmium exposure. Environ Entomol 19(51):815–823
Wu H, Liu Q-Z (2012) Antioxidative responses in Galleria mellonella larvae infected with the entomopathogenic nematode Heterorhabditis sp. beicherriana. Biocontrol Sci Tech 22:601–606
Xie L, Buchwalter DB (2011) Cadmium exposure route affects antioxidant responses in the mayfly Centroptilum triangulifer. Aquat Toxicol 105:199–205
Xu X, Wang X, Yang Y, Ares I, Martínez M, Lopez-Torres B, Martínez-Larrañaga MR, Wang X, Anadón A, Martinez MA (2022) Neonicotinoids: mechanisms of systemic toxicity based on oxidative stress-mitochondrial damage. Arch Toxicol 96:1493–1520
Yadav A, Gopesh A, Pandey RS, Rai DK, Sharma B (2009) Acetylcholinesterase: a potential biochemical indicator for biomonitoring of fertilizer industry effluent toxicity in freshwater teleost, Channa striatus. Ecotoxicology 18:325–333
Yang XQ, Zhang YL (2015) Investigation of insecticide-resistance status of Cydia pomonella in Chinese populations. Bull Entomol Res 105:316–325
Yucel MS, Kayis T (2019) Imidacloprid induced alterations in oxidative stress, biochemical, genotoxic, and immunotoxic biomarkers in non-mammalian model organism Galleria mellonella L. (Lepidoptera: Pyralidae). J Environ Sci Health b 54:27–34
Zhang J, Yang Z, Zhang S, Xie Z, Han S, Wang L, Zhang B, Sun S (2020a) Investigation of endogenous malondialdehyde through fluorescent probe MDA-6 during oxidative stress. Anal Chim Acta 1116:9–15
Zhang Q, Zhu L, Wang J, Xie H, Wang J, Han Y, Yang J (2013) Oxidative stress and lipid peroxidation in the earthworm Eisenia fetida induced by low doses of fomesafen. Environ Sci Pollut Res Int 20:201–208
Zhang X, Li R, Hu C, Chen G, Xu H, Chen Z, Li Z (2020b) Population numbers and physiological response of an invasive and native thrip species following repeated exposure to imidacloprid. Front Physiol 27(11):216
Zhou C, Yang H, Wang Z, Long GY, Jin DC (2018) Comparative transcriptome analysis of Sogatella furcifera (Horváth) exposed to different insecticides. Sci Rep 8(1):8773
Acknowledgements
The authors would like to express their gratitude to Liangyong Du for his assistance with insect collection and management. Additionally, they extend their thanks to Shunhua Gui from Guizhou University for his help with the experimental site.
Author information
Authors and Affiliations
Contributions
CQC conceived the project, providing laboratory conditions and financial support. MZX oversaw the breeding and maintenance of the insects and completed the main tasks of conducting experiments, data analysis, and writing the article. MZX and YTL analyzed the data and authored the initial draft. MZX and YTL reviewed and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Vincent Doublet
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, MZ., Li, YT. & Cao, CQ. Physiological and gene expression responses of Protohermes xanthodes (Megaloptera: Corydalidae) larvae to imidacloprid. Sci Nat 111, 46 (2024). https://doi.org/10.1007/s00114-024-01932-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-024-01932-6