Abstract
The males of the Brimstone butterfly (Gonepteryx rhamni) have ultraviolet pattern on the dorsal surfaces of their wings. Using geometric morphometrics, we have analysed correlations between environmental variables (climate, productivity) and shape variability of the ultraviolet pattern and the forewing in 110 male specimens of G. rhamni collected in the Palaearctic zone. To start with, we subjected the environmental variables to principal component analysis (PCA). The first PCA axis (precipitation, temperature, latitude) significantly correlated with shape variation of the ultraviolet patterns across the Palaearctic. Additionally, we have performed two-block partial least squares (PLS) analysis to assess co-variation between intraspecific shape variation and the variation of 11 environmental variables. The first PLS axis explained 93 % of variability and represented the effect of precipitation, temperature and latitude. Along this axis, we observed a systematic increase in the relative area of ultraviolet colouration with increasing temperature and precipitation and decreasing latitude. We conclude that the shape variation of ultraviolet patterns on the forewings of male Brimstones is correlated with large-scale environmental factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The discovery that some animals perceive ultraviolet radiation goes back to Darwin’s contemporary John Lubbock, the 1st Baron Avebury (Lubbock 1882). Sensitivity to ultraviolet light has been observed in many invertebrates (Lutz and Richtmyer 1922; Lutz 1924, 1933b; DeVoe et al. 1969) and later also in vertebrates (Huth and Burkhardt 1972; Wright 1972; Silberglied 1979; Tovee 1995). Around the same time, it was also found that there exists a relationship between ultraviolet patterns in flowers and some of their pollinators (Lutz 1924). Moreover, UV-reflective surfaces with signalling and communicative functions have been found in many clades of invertebrates including butterflies (Lutz 1933a; Mazokhin-Porshnyakov 1957; Nekrutenko 1965a; Silberglied and Taylor 1978; Silberglied 1979, 1984; Eguchi and Meyer-Rochow 1983; Brunton and Majerus 1995; Brunton 1998), spiders (Heiling et al. 2003, 2005) and beetles (Pope and Hinton 1977).
As a model species for our study, we chose the Common Brimstone (Gonepteryx rhamni, Linnaeus, 1758), a Palaearctic species widely distributed from Western Europe to Eastern Asia (see Fig. 1). The genus Gonepteryx has been studied by zoologists since the early days of UV reflectance research (Mazokhin-Porshnyakov 1957). The ultraviolet wing pattern of G. rhamni was repeatedly under consideration as a trait of potential taxonomic value (Nekrutenko 1965b, 1968, 1970; Kudrna 1975). Later, the Brimstone became a popular model in studies focussing on the structural basis and physical nature of UV colouration in butterflies (Wijnen et al. 2007; Pirih et al. 2011; Wilts et al. 2011).
Previous studies of butterflies have shown an environmental dependence of different aspects of wing colouration such as the variation in colour across the latitude (Hovanitz 1944), intensity of ultraviolet reflectance (Meyer-Rochow and Järvilehto 1997), degree of melanization (Ellers and Boggs 2002; Karl et al. 2009; Fischer and Karl 2010), and seasonal polyphenism together with variability in the expression of size and composition of the eyespots (Brakefield 1987; Brakefield and French 1999; Beldade and Brakefield 2002; Brakefield et al. 2007; De Jong et al. 2010). The ultraviolet colouration in butterflies may also be influenced by temperature and the quality of food ingested during development (Kemp et al. 2006; Kemp and Rutowski 2007; Kemp 2008b). These studies, however, focussed mainly on the intensity and hue of the UV pattern without taking into account the shape and relative size of ultraviolet patches on butterfly wings.
Although a role of the size of UV patterns in sexual selection has not yet been demonstrated in the Brimstone butterfly, it has been shown that females of butterfly Bicyclus anynana select males on the basis of the size and brightness of the ultraviolet-reflecting pupils of dorsal eyespots (Robertson and Monteiro 2005). Moreover, the absence or presence of ultraviolet wing pattern may serve as isolating mechanism in New Zealand lycaenid butterflies (Meyer-Rochow 1991). The importance of UV signals during mate choice was directly evidenced in Eurema and Colias species (Papke et al. 2007; Kemp 2008a). Based on an analogy with related pierid butterflies, we suppose that male dorsal UV patterns in G. rhamni may play a role in sexual selection and that this may influence the observed variation of this trait in the natural populations of the Brimstone.
Selection pressures acting upon the formation of animal patterns are not necessarily limited to sexual selection. Morphology can also be influenced by environmental selection, as demonstrated by classical examples such as the geographic patterns of body size (Bergmann 1847) and the length of extremities in ectotherms (Allen 1877). Recent studies of animal surfaces brought further evidence pertaining to geographic patterns of plumage colours in hummingbirds (Schmitz-Ornés 2006), the elytral patterns in carabid beetles (Kleisner et al. 2012) and colour patterns in bumblebees (Williams 2007). The variation of some traits, however, cannot be fully explained by an adaptive evolutionary hypothesis (Gould and Lewontin 1979; Kleisner et al. 2012). Although particular traits may later be co-opted for various functions such as sexual signalling, their variation cannot be sufficiently explained by these new selection pressures alone (Gould and Vrba 1982; Kleisner 2008, 2011; Maran and Kleisner 2010).
Our main goal was to find possible associations between the shape of UV patterns in G. rhamni and broad-scale environmental conditions (climate, productivity). We demonstrate that environmental conditions indeed correlate with the relative size and shape of the patterns and conclude with a discussion of possible evolutionary and ecological causes of these correlations.
Material and methods
Specimens
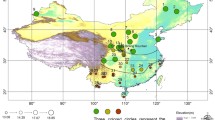
We have used a set of 110 individual male specimens: 59 observations were made in the Czech Republic and 51 observations come from the Palaearctic outside the Czech Republic. Photographs of all specimens were deposited in the entomological collections of the Natural History Museum of the University of Tartu (Estonia) and the National Museum in Prague (Czech Republic). We have recorded the geographic coordinates describing where each specimen was caught (Fig. 1).
Acquisition of photographs in the ultraviolet spectra (UV-A)
We have used a FujiFilm IS Pro digital camera which, thanks to its broad sensitivity to 330–900-nm spectrum, is suitable for UV photography (Pike 2011). The camera was equipped with an uncoated UV-transmitting lens. We used photographical filters B+W 403 and B+W BG 53. Ultraviolet band-pass filter B+W 403 (transmission range 290–410 nm with peak at 355 nm) filtered out the visible spectrum (400–700 nm) and the B+W BG 38 filter (transmission range 290–750 nm with peak at 500 nm) blocked the IR light (λ > 700 nm). To illuminate the photographed objects, we have used a UVP MRL-58 Multiple-Ray Lamp (8 W, 230 V-50 Hz, 0.16 A) equipped with mercury fluorescent lamp 8 W F8T5 long-wave 365 nm. All objects were illuminated under the angle of 45° and photographed in a standardized position (dorsal view). Based on our previous experience with the model species, the shape and size of ultraviolet pattern of G. rhamni remain the same even after a considerable change of the angle, which was also shown by Pirih et al. (2011). For all specimens, we used the following setting of the FujiFilm IS Pro camera: ISO 400, shutter time 15′ and aperture of 3.5. All images were standardized using 18 % grey card, Kodak colour separation guide and a 15-cm length scale.
Environmental and geographic correlates
As potential correlates of the UV patterns, we chose longitude, latitude and altitude. These broad-scale variables describe the spatial position of each specimen; we obtained them from locality labels on the pinned specimens. Then, we used these coordinates to assign (in ArcGIS 10.0; ESRI Inc.) to each specimen the mean annual temperature and precipitation, mean temperature in the warmest and coldest month, mean precipitation in the wettest and driest month, net primary productivity (NPP) and normalized difference vegetation index (NDVI) of the locality of the specimen. We have used these variables as predictors as they were previously demonstrated to affect insect distributions (Hawkins and Porter 2003; Battisti et al. 2005), development (Dixon et al. 2009), body size (Chown and Gaston 2010) and insect personality (Tremmel and Müller 2013). The data on temperature, precipitation and altitude came from 10 arc-min WorldClim layers (Hijmans et al. 2005). Our NPP layer came from the Postdam Institute for Climate Impact Research (Cramer et al. 1999): It represents an averaged (over 1961–1990) net production of organic compounds from atmospheric CO2. NDVI represents the amount of green vegetation cover and was downloaded from NASA Goddard DAAC. To mitigate the effect of potential geo-referencing errors, we used climatic data of a relatively coarse resolution (note that finer, 2.5 and 5 arc-min, data were also available). We made this decision in order to minimize the risk of assigning an inappropriate (spatially mismatched) climate to the specimens.
To avoid co-linearity between predictors, we performed principal component analysis (PCA) on all climatic and geographic variables, centred to zero mean and standardized to variance of 1 (package ‘stats’ in R software; R Development Core Team 2012). We used only the first two axes of this PCA for further analyses.
Landmark definitions and procrustes analysis
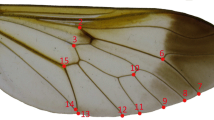
Ultraviolet digital photographs of the left forewing of each of the 110 male specimens of G. rhamni were analysed using geometric morphometrics. At each forewing, we placed 32 landmarks (including 12 semi-landmarks) using tpsDig2 software ver. 2.14 (Rohlf 2009a). Landmarks are corresponding points which can be placed on the forewing of each specimen in the set, while semi-landmarks denote curves and outlines of the forewing where proper landmarks cannot be defined (for definitions of landmark and semi-landmark locations on the butterfly forewing, see Fig. 2).
A definition of landmarks on the left forewing. Points 1–20 represent homologous anatomical landmarks found in all analysed subjects: landmark 1, wing base (the connection of anal and cubital vein); landmark 8, wing apex. The other landmarks are located at vein endings at the edge of the wing and the edge of the UV-reflecting pattern: landmarks 2 and 12, first anal vein; landmarks 3 and 13, cubitus 3; landmarks 4 and 14, cubitus 2; landmarks 5 and 15, cubitus 1; landmarks 6 and 16, media 2; landmarks 7 and 17, media 1; landmarks 9 and 18, radius 4; landmarks 10 and 19, radius 3; landmarks 11 and 20, radius 2. Points 21–32 represent semi-landmarks which serve to denote curves
All configurations of landmarks and semi-landmarks were superimposed by a generalized Procrustes analysis (GPA) performed in tpsRelw ver. 1.49 (Rohlf 2008). This procedure standardizes the size of objects and optimizes their rotation and translation until the coordinates of corresponding landmarks align as closely as possible. To reduce the dimensionality of procrustes residuals, we carried out a principal component analysis (PCA) in tpsRelw, ver. 1.49. The scores on the 10 PC axes carrying information about the wing shape were then saved and used for further analyses.
Correlations between environment and shape
To estimate the relationship between wing morphology and environmental (and geographic) variables, we have used two complementary analytical methods.
First, we applied the Permutational Multivariate Analysis of Variance using Distance Matrices (Adonis) function in the Vegan package in R (Oksanen et al. 2011) with Euclidean distance measure. We fitted a multivariate multiple regression model using Adonis, where the responses were the 10 shape PC axes which explain 90 % of shape variability of forewing and the predictors were the first two environmental PC axes. To control for shape variation due to allometry, we added wing size (computed as centroid size for each landmark configuration) as a covariate in the model. Shape changes associated with explanatory variables were visualized by thin-plate spline deformation grids available in tpsRegr, ver. 1.36 (Rohlf 2009b).
Second, we applied the two-block partial least squares (2B-PLS) method (Rohlf and Corti 2000) in order to explore covariation between the shape variables representing wing morphology and 11 environmental/geographic variables (unlike in the previous analysis, we used the original environmental/geographic variables, not PCs). Landmark configurations were transformed into partial warp scores (Rohlf et al. 1996) and analysed in tpsPLS software, ver. 1.18 (Rohlf 2006). In particular, the 2B-PLS created a pair of new variables which were linear combinations of variables within both of the original data sets (blocks). These new variables were produced so as to maximize covariation between the two original sets of variables (Rohlf and Corti 2000). Thin-plane splines were used to display the results in form of deformation grids of observed variation along the first PLS axis. These visualizations were made in tpsPLS 1.18 software (Rohlf 2006).
Geographic and environmental bias in specimen locations
There is a bias in the geographic distribution of our specimens (Fig. 1), since about half of them (59 out of 110) come from the small area of the Czech Republic, which contrasts with the rest coming from all over the Palaearctic. Such bias is commonly found in many large-scale data sets extracted from museum collections (Diniz-Filho et al. 2010). To assess whether the geographic bias affects our shape-environment correlations, we reran the 2B-PLS analysis using only specimens from outside the Czech Republic and compared them with results obtained from the complete data set.
Results
The principal component analysis of 11 environmental and geographical variables resulted in two interpretable PCA axes (Fig. 3). The first PC axis explained 46 % and the second 16 % of all variability. The first PC axis accounted mainly for the mean annual temperature, mean annual precipitation, mean precipitation in the wettest month, and latitudinal and longitudinal variation. The second axis represented variation in the NDVI and the mean precipitation in the driest month. Relative loadings of geographic and environmental variables for the first and second PC axes are presented in Table 1.
Principal component analysis of environmental and topographical variables (standardized, centred). The first and second axes explain 46 and 15.5 % of variability in environmental data respectively. The first principal component accounts mainly for annual mean temperature and precipitation, mean temperature in the warmest month (max.temperature), and the latitudinal effect (latitude), while the second axis can be interpreted as a joined effect of the normalized difference vegetation index (NDVI) and the mean precipitation in the driest month (min.precipitation). NPP net primary production. Thin-plate spline visualizations are not part of the environmental principle component analysis. They were added manually (based on the regressions of the shape data to environmental principle components) to illustrate how the wing shape changes across a range of environmental conditions
Multivariate multiple regressions (‘Adonis’ function in ‘vegan’ R package) of shape data on the two PCs extracted from the predictors (with wing centroid size as a covariate) showed that the first environmental PC axis significantly affects the shape space of the forewing (F1,106 = 15.49, p = 0.0001, R 2 = 0.12). The effect of the second PC axis was significant but it explained only a small part of variance (F1,106 = 3.48, p = 0.02, R 2 = 0.027). The effect of the centroid size was also significant (F1,106 = 3.05, p = 0.029, R 2 = 0.023).
All p values were based on 9,999 permutations. Specimens inhabiting environments with higher precipitation and temperature tend to have larger UV patches which cover most of the forewing surface.
The two-block partial least squares (2B-PLS) analysis (Rohlf and Corti 2000) focussed on covariation between the shape variables and the ecological variables. The first PLS axis accounted for 93 % of squared covariance (permutation test for 9,999 iterations, p = 0.0003), while the second PLS axis accounted only for approximately 3 % and was not statistically significant (p = 0.999). Correlations between variables and shape vectors were 0.57 (p = 0.0001) for the first PLS axis and 0.46 (p = 0.0031) for the second PLS axis. We have observed shape differences linked to the first PLS axis (Fig. 4): The association was principally with precipitation, temperature and latitude. Shape variation along the first PLS axis revealed constriction/dilation along the anterior-posterior axis of the wing. With an increase in temperature and precipitation and a decrease in latitude, the relative size of the ultraviolet pattern markedly increases at the expense of the UV non-reflective wing area (Fig. 4).
Two-block partial least squares plot for projections of Gonepteryx rhamni specimens onto ordination vectors. Deformation grids on both extremes of the x-axis show changes in shape associated with the first axis. Vectors designate estimates of standardized scores for the mean annual temperature, mean temperature in the warmest (max.temperature) and the coldest month (min.temperature), mean annual precipitation, mean precipitation in the wettest (max.precipitation) and driest month (min.precipitation), altitude, net primary production (NPP), normalized difference vegetation index (NDVI), latitude and longitude
To examine the potential confounding effect of the remaining environmental bias, we reran the 2B-PLS analysis only on specimens from outside the Czech Republic. The relationship between shape and ecological variables remained clearly significant, p < 0.001(permutation test for 9999 iterations); the first PLS axis accounted for 94 % of squared covariance. The correlation between variables and shape vectors was 0.65 (p < 0.001).
Discussion
We have demonstrated that the shape variability of ultraviolet patterns and the overall shape of the forewing correlate with the environmental conditions in which the specimens were collected, which may suggest a causal link between the environment and the observed variability. The area covered by the UV patterns on the wings decreases towards cooler and drier locations in higher latitudes, while specimens inhabiting warmer and more humid environment tend to have broader wings with UV patterns covering most of the forewing surface.
The wing area covered by the UV pattern covaries with forewing dimensions, and both are affected by the environment. The shape variation of the UV pattern and the shape variation of the whole forewing are not, however, isomorphic (see the deformation grids in Fig. 4), meaning that the area of the wing covered by a UV pattern increases at a higher rate towards lower latitudes and especially towards higher temperatures and amounts of precipitation than the UV non-reflective wing area. This suggests that the UV pattern is more environment dependent than the overall forewing shape and that in addition to the hypothesized sexual selection (Silberglied and Taylor 1978; Silberglied 1984; Brunton and Majerus 1995; Kemp 2006; Kemp and Rutowski 2007, 2011), the environmental selection, too, may contribute to the variation of the UV patterns. The role of sexual selection in formation of the UV pattern in G. rhamni, however, has not been explicitly evidenced yet.
Temperature, which has the highest correlation with the first PLS axis, seems to be an especially significant environmental factor. In ectotherms, thermoregulatory costs of maintaining the main body temperature are rather high. It has been shown, for instance, that temperature—but not the energy supply—crucially affects the expression of sexually selected UV colouration in male European green lizards (Bajer et al. 2012). Ambient temperature during larval and early pupal development affects the metabolic rate of developing butterflies (Stevens 2004), which may further affect the expression of ultraviolet patterns (Kemp et al. 2006; Kemp and Rutowski 2007; Kemp 2008b; Prudic et al. 2011). Moreover, cold treatment of pupae in Aglais urticae led to a non-expression of UV spots on the wings of the adults (Anonymous 1910). It is thus possible that other ecological variables associated with the shape of UV patterns, such as precipitation and latitude, may affect the UV patterns indirectly.
The role of ecology and nutrition of larvae in the formation of wing scale morphology responsible for the reflectance and potential signalling was thoroughly studied in Pieris butterflies. It was shown that differences in the density of pterin granules deposited within the nanostructure of scales are responsible for sexual dichromatism of many pierid species (Morehouse et al. 2007). Although different dietary regimes influence the development and phenotype of Pieris rapae, the effect seems to be restricted to larvae (Morehouse and Rutowski 2010). Feeding experiments using other butterfly species have shown that composition of larval food (both the plant species and the plant parts) significantly affects the variation in UV patterns in the European Common Blue butterfly, Polyommatus icarus (Knuttell and Fiedler 2000). In Colias eurytheme and Eurema hecabe, the quality of larval food resources also affects some properties of the ultraviolet patterns, such as brightness (Kemp et al. 2006; Kemp and Rutowski 2007; Kemp 2008b).
Another explanation is that the male UV pattern of G. rhamni indicates a whole range of mate qualities (Kemp and Rutowski 2011) including the accessibility of larval resources, the ability to acquire and assimilate these resources, thermal stability of pupal development, developmental stability and resistance to environmental perturbations, or the effectiveness of morphogenetic mechanisms responsible for the partitioning of developmental resources to UV signalling structures (Kemp 2006, 2008b; Kemp et al. 2006; Kemp and Rutowski 2007). It seems that southern specimens have access to relatively richer resources compared to the northern specimens. The costly UV pattern could be an indicator of male’s ability to assimilate and utilize these resources and can be subject to female sexual selection. Subsequently, the trade-off between the availability of environmental resources and the ability to assimilate these resources may be responsible for the latitudinal variation in the UV patterns.
Alternatively, the UV patches could be a protective adaptation against high UV exposure at high altitudes and low latitudes (Herman et al. 1999). If that were the case, UV-reflective patterns would cover larger wing areas in specimens from lower latitudes and higher altitudes (as in the case of G. rhamni), while in fact the opposite occurs in Pieris napi whose northernmost females possess brighter UV reflectivity than their southern sisters (Meyer-Rochow and Järvilehto 1997). Although our results may be seen as partially supporting this conjecture, there is a serious problem. In particular, one would need to find a good reason why such protection against the high levels of UV occurs only in males (as is the case of G. rhamni). Another possibility is that the dorsal UV patterns in males are primarily sexual patterns which were only later co-opted for a protective function. Females thus lack the adaptation simply because they have no dorsal pattern which could be used for such secondary protective function.
In order to disentangle the complex links between the development, environmental conditions, and the variation in the UV pattern, one would have to carry out breeding experiments with controlled exposure of the developing larvae to varying environmental factors. We also note that despite our efforts to account for it, our results could be affected by the uneven spread of our specimens in the environmental space and future research might benefit from a more systematic stratified random sampling. Further research could also investigate whether the comparatively smaller size of UV patterns in colder and drier environments is in any way compensated, for example, by higher intensity (or brightness) of UV reflectance. For the moment being, we tentatively conclude that the shape variation of UV patterns in male Brimstone butterflies may be due to a combination of both sexual and environmental selection.
References
Allen JA (1877) The influence of physical conditions in the genesis of species. Radic Rev 1:108–140
Anonymous (1910) Schmetterlinge. In: Meyers Grosses Konversations-Lexikon (6th edition, vol. 21). Bibliographisches Institut, Leipzig and Wien, pp 803–807
Bajer K, Molnar O, Torok J, Herczeg G (2012) Temperature, but not available energy, affects the expression of a sexually selected ultraviolet (UV) colour trait in male European green lizards. PLoS One 7:e34359. doi:10.1371/journal.pone.0034359
Battisti A, Stastny M, Netherer S, Robinet C, Schopf A, Roques A, Larsson S (2005) Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecol Appl 15:2084–2096
Beldade P, Brakefield PM (2002) The genetics and evo-devo of butterfly wing patterns. Nat Rev Genet 3:442–452. doi:10.1038/nrg818
Bergmann C (1847) Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Stud 3:595–708
Brakefield PM (1987) Tropical dry and wet season polyphenism in the butterfly Melanitis leda (Satyrinae): phenotypic plasticity and climatic correlates. Biol J Linn Soc 31:175–191. doi:10.1111/j.1095-8312.1987.tb01988.x
Brakefield PM, French V (1999) Butterfly wings: the evolution of development of colour patterns. BioEssays 21:391–401. doi:10.1002/(sici)1521-1878(199905)21:5<391::aid-bies6>3.0.co;2-q
Brakefield P, Pijpe J, Zwaan B (2007) Developmental plasticity and acclimation both contribute to adaptive responses to alternating seasons of plenty and of stress in Bicyclus butterflies. J Biosci 32:465–475. doi:10.1007/s12038-007-0046-8
Brunton CFA (1998) The evolution of ultraviolet patterns in European Colias butterflies (Lepidoptera, Pieridae): a phylogeny using mitochondrial DNA. Heredity 80:611–616. doi:10.1046/j.1365-2540.1998.00336.x
Brunton CFA, Majerus MEN (1995) Ultraviolet colors in butterflies—intraspecific or inter-specific communication. Proc R Soc B Biol Sci 260:199–204. doi:10.1098/rspb.1995.0080
Chown SL, Gaston KJ (2010) Body size variation in insects: a macroecological perspective. Biol Rev 85:139–169
Cramer W et al (1999) Comparing global models of terrestrial net primary productivity (NPP): overview and key results. Glob Chang Biol 5:1–15. doi:10.1046/j.1365-2486.1999.00009.x
De Jong MA, Kesbeke FMNH, Brakefield PM, Zwaan BJ (2010) Geographic variation in thermal plasticity of life history and wing pattern in Bicyclus anynana. Clim Res 43:91–102. doi:10.3354/cr00881
DeVoe RD, Small RJ, Zvargulis JE (1969) Spectral sensitivities of wolf spider eyes. J Gen Physiol 54:1–32. doi:10.1085/jgp.54.1.1
Diniz-Filho JAF, De Marco JP, Hawkins BA (2010) Defying the curse of ignorance: perspectives in insect macroecology and conservation biogeography. Insect Conserv Divers 3:172–179. doi:10.1111/j.1752-4598.2010.00091.x
Dixon AFG, Honěk A, Keil P, Kotela MAA, Šizling AL, Jarošík V (2009) Relationship between the minimum and maximum temperature thresholds for development in insects. Funct Ecol 23:257–264. doi:10.1111/j.1365-2435.2008.01489.x
Eguchi E, Meyer-Rochow VB (1983) Ultraviolet photography of forty-three species of lepidoptera representing ten families. Annot Zool Jpn 56:10–18
Ellers J, Boggs CL (2002) The evolution of wing color in Colias butterflies: heritability, sex linkage, and population divergence. Evolution 56:836–840
Fischer K, Karl I (2010) Exploring plastic and genetic responses to temperature variation using copper butterflies. Clim Res 43:17–30. doi:10.3354/cr00892
Giese AC (1946) Comparative sensitivity of sperm and eggs to ultraviolet radiations. Biol Bull 91:81–87
Gould SJ, Lewontin RC (1979) The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc R Soc B Biol Sci 205:581–598. doi:10.1098/rspb.1979.0086
Gould SJ, Vrba ES (1982) Exaptation; a missing term in the science of form. Paleobiology 8:4–15
Hawkins BA, Porter EE (2003) Water–energy balance and the geographic pattern of species richness of western Palearctic butterflies. Ecol Entomol 28:678–686. doi:10.1111/j.1365-2311.2003.00551.x
Heiling AM, Herberstein ME, Chittka L (2003) Pollinator attraction: crab-spiders manipulate flower signals. Nature 421:334. doi:10.1038/421334a
Heiling AM, Chittka L, Cheng K, Herberstein ME (2005) Colouration in crab spiders: substrate choice and prey attraction. J Exp Biol 208:1785–1792. doi:10.1242/jeb.01585
Herman JR, Krotkov N, Celarier E, Larko D, Labow G (1999) Distribution of UV radiation at the Earth’s surface from TOMS-measured UV-backscattered radiances. J Geophys Res Atmos 104:12059–12076. doi:10.1029/1999jd900062
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978. doi:10.1002/joc.1276
Hovanitz W (1944) The Ecological significance of the color phases of Colias chrysotheme in North America. Ecology 25:45–60
Huth HH, Burkhardt D (1972) Der spektrale Sehbereich eines Violettohr-Kolibris. Die Naturwissenschaften 59:650
Karl I, Geister TL, Fischer K (2009) Intraspecific variation in wing and pupal melanization in copper butterflies (Lepidoptera: Lycaenidae). Biol J Linn Soc 98:301–312. doi:10.1111/j.1095-8312.2009.01284.x
Kemp DJ (2006) Heightened phenotypic variation and age-based fading of ultraviolet butterfly wing coloration. Evol Ecol Res 8:515–527
Kemp DJ (2008a) Female mating biases for bright ultraviolet iridescence in the butterfly Eurema hecabe (Pieridae). Behav Ecol 19:1–8. doi:10.1093/beheco/arm094
Kemp DJ (2008b) Resource-mediated condition dependence in sexually dichromatic butterfly wing coloration. Evolution 62:2346–2358. doi:10.1111/j.1558-5646.2008.00461.x
Kemp DJ, Rutowski RL (2007) Condition dependence, quantitative genetics, and the potential signal content of iridescent ultraviolet butterfly coloration. Evolution 61:168–183. doi:10.1111/j.1558-5646.2007.00014.x
Kemp DJ, Rutowski RL (2011) The role of coloration in mate choice and sexual interactions in butterflies. In: Brockmann HJ, Roper T, Naguib M, Wynne-Edwards K, Barnard C, Mitani J (eds) Advances in the study of behavior, vol 43. Elsevier, Amsterdam, pp 55–92. doi:10.1016/B978-0-12-380896-7.00002-2
Kemp DJ, Vukusic P, Rutowski RL (2006) Stress-mediated covariance between nano-structural architecture and ultraviolet butterfly coloration. Funct Ecol 20:282–289. doi:10.1111/j.1365-2435.2006.01100.x
Kleisner K (2008) Homosemiosis, mimicry and superficial similarity: notes on the conceptualization of independent emergence of similarity in biology. Theory Biosci 127:15–21. doi:10.1007/s12064-007-0019-3
Kleisner K (2011) Perceive, Co-opt, modify, and live! Organism as a centre of experience. Biosemiotics 4:223–241
Kleisner K, Keil P, Jaroš F (2012) Biogeography of elytral ornaments in Palearctic genus Carabus: disentangling the effects of space, evolution and environment at a continental scale. Evol Ecol 26:1025–1040. doi:10.1007/s10682-011-9537-z
Knuttell H, Fiedler K (2000) On the use of ultraviolet photography and ultraviolet wing patterns in butterfly morphology and taxonomy. J Lepidopterol Soc 54:137–144
Kudrna O (1975) A revision of the genus Gonepteryx Leach (Lep., Pieridae). Entomol Gaz 26:3–37
Lubbock J (1882) Ants, bees, and wasps. A record of observations on the habits of the social Hymenoptera. D. Appleton and Co., New York
Lutz FE (1924) Apparently non-selective characters and combinations of characters, including a study of ultraviolet in relation to the flower-visiting habits of insects. Ann N Y Acad Sci 29:181–283
Lutz FE (1933a) Experiments with “stingless bees” (Trigona cressoni parastigma) concerning their ability to distinguish ultraviolet patterns. Am Mus Novit 641:1–26
Lutz FE (1933b) Invisible colors of flowers and butterflies. Nat Hist 33:565–567
Lutz FE, Richtmyer FK (1922) The reaction of Drosophila to ultraviolet. Science 55:519–519
Maran T, Kleisner K (2010) Towards an evolutionary biosemiotics: semiotic selection and semiotic co-option. Biosemiotics 3:189–200
Mazokhin-Porshnyakov GA (1957) Reflecting properties of butterfly wings and the role of ultra-violet rays in the vision of insects. Biophysics 2:285–296
Meyer-Rochow VB (1991) Differences in ultraviolet wing patterns in the New Zealand lycaenid butterflies Lycaena salustius, L. rauparaha, and L. feredayi as a likely isolating mechanism. J R Soc N Z 21:169–177. doi:10.1080/03036758.1991.10431405
Meyer-Rochow VB, Järvilehto M (1997) Ultraviolet colours in Pieris napi from northern and southern Finland: arctic females are the brightest! Naturwissenschaften 84:165–168. doi:10.1007/s001140050373
Morehouse NI, Rutowski RL (2010) Developmental responses to variable diet composition in the cabbage white butterfly, Pieris rapae: the role of nitrogen, carbohydrates and genotype. Oikos 119:636–645. doi:10.1111/j.1600-0706.2009.17866.x
Morehouse NI, Vukusic P, Rutowski R (2007) Pterin pigment granules are responsible for both broadband light scattering and wavelength selective absorption in the wing scales of pierid butterflies. Proc R Soc B Biol Sci 274:359–366
Nekrutenko YP (1965a) Gynandromorphic effect and the optical nature of hidden wing-pattern in Gonepteryx rhamni L. (Lepidoptera, Pieridae). Nature 205:417–418
Nekrutenko YP (1965b) Three cases of gynandromorphism in Gonepteryx. J Res Lepidoptera 4:103–108
Nekrutenko YP (1968) Phylogeny and geographical distribution of the genus Gonepteryx (Lepidoptera, Pieridae): An attempt of study in historical zoogeography. Naukova dumka, Kiev
Nekrutenko YP (1970) A new subspecies of Gonepteryx rhamni from Tian-shan Mountains, U.S.S.R. J Lepid Soc 34:218–220
Oksanen J et al. (2011) vegan: Community ecology package. R package version 2.0–2
Papke R, Kemp D, Rutowski R (2007) Multimodal signalling: structural ultraviolet reflectance predicts male mating success better than pheromones in the butterfly Colias eurytheme L. (Pieridae). Anim Behav 73:47–54. doi:10.1016/j.anbehav.2006.07.004
Pike T (2011) Using digital cameras to investigate animal colouration: estimating sensor sensitivity functions. Behav Ecol Sociobiol 65:849–858. doi:10.1007/s00265-010-1097-7
Pirih P, Wilts BD, Stavenga DG (2011) Spatial reflection patterns of iridescent wings of male pierid butterflies: curved scales reflect at a wider angle than flat scales. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 197:987–997. doi:10.1007/s00359-011-0661-6
Pope RD, Hinton HE (1977) A preliminary survey of ultraviolet reflectance in beetles. Biol J Linn Soc 9:331–348. doi:10.1111/j.1095-8312.1977.tb00275.x
Prudic KL, Jeon C, Cao H, Monteiro A (2011) Developmental plasticity in sexual roles of butterfly species drives mutual sexual ornamentation. Science 331:73–75. doi:10.1126/science.1197114
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Robertson KA, Monteiro A (2005) Female Bicyclus anynana butterflies choose males on the basis of their dorsal UV-reflective eyespot pupils. Proc R Soc B Biol Sci 272:1541–1546. doi:10.1098/rspb.2005.3142
Rohlf JF (2006) TpsPLS (version 1.18). Department of Ecology and Evolution, State University of New York at Stony Brook, New York
Rohlf JF (2008) tpsRelw version 1.46. Department of Ecology and Evolution, State University of New York at Stony Brook, Stony Brook
Rohlf JF (2009a) TpsDig2 (version 2.14). New York: Department of Ecology and Evolution, State University of New York at Stony Brook
Rohlf JF (2009b) TpsRegr (version 1.36). New York: Department of Ecology and Evolution, State University of New York at Stony Brook
Rohlf FJ, Corti M (2000) Use of two-block partial least-squares to study covariation in shape. Syst Biol 49:740–753. doi:10.1080/106351500750049806
Rohlf FJ, Loy A, Corti M (1996) Morphometric analysis of old world talpidae (Mammalia, Insectivora) using partial-warp scores. Syst Biol 45:344–362. doi:10.1093/sysbio/45.3.344
Schmitz-Ornés A (2006) Using colour spectral data in studies of geographic variation and taxonomy of birds: examples with two hummingbird genera, Anthracothorax and Eulampis. J Ornithol 147:495–503. doi:10.1007/s10336-006-0053-9
Silberglied RE (1979) Communication in the ultraviolet. Annu Rev Ecol Syst 10:373–398. doi:10.1146/annurev.es.10.110179.002105
Silberglied RE (1984) Visual communication and sexual selection among butterflies. In: Vane-Wright RI, Ackery PR (eds) The biology of butterflies. Academic, London, pp 207–223
Silberglied RE, Taylor OR (1978) Ultraviolet reflection and its behavioral role in courtship of sulfur butterflies Colias eurytheme and Colias philodice (Lepidoptera, Pieridae). Behav Ecol Sociobiol 3:203–243. doi:10.1007/Bf00296311
Stevens DJ (2004) Pupal development temperature alters adult phenotype in the speckled wood butterfly, Pararge aegeria. J Therm Biol 29:205–210. doi:10.1016/j.jtherbio.2004.02.005
Tovee MJ (1995) Ultra-violet photoreceptors in the animal kingdom: their distribution and function. Trends Ecol Evol 10:455–460. doi:10.1016/S0169-5347(00)89179-x
Tremmel M, Müller C (2013) Insect personality depends on environmental conditions. Behav Ecol 24:386–392
Wijnen B, Leertouwer HL, Stavenga DG (2007) Colors and pterin pigmentation of pierid butterfly wings. J Insect Physiol 53:1206–1217. doi:10.1016/j.jinsphys.2007.06.016
Williams P (2007) The distribution of bumblebee colour patterns worldwide: possible significance for thermoregulation, crypsis, and warning mimicry. Biol J Linn Soc 92:97–118. doi:10.1111/j.1095-8312.2007.00878.x
Wilts BD, Pirih P, Stavenga DG (2011) Spectral reflectance properties of iridescent pierid butterfly wings. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 197:693–702. doi:10.1007/s00359-011-0632-y
Wright AA (1972) The influence of ultraviolet radiation on the pigeon’s color discrimination. J Exp Anal Behav 17:325–337. doi:10.1901/jeab.1972.17-325
Acknowledgments
We wish to thank Jaan Luig and Pavel Chvojka for their help with providing the butterflies and David Hořák, Victor Benno Meyer-Rochow, Nathan Morehouse and two anonymous referees for their useful comments. This research was supported by the Czech Grant Agency project GACR P505/11/1459. PK was supported by EU FP7 People Programme (Marie Curie Actions; REA agreement no. 302868; project WORLDIVERSITY). PP and DS were supported by the Charles University Grant Agency project GAUK 764313.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Rights and permissions
About this article
Cite this article
Pecháček, P., Stella, D., Keil, P. et al. Environmental effects on the shape variation of male ultraviolet patterns in the Brimstone butterfly (Gonepteryx rhamni, Pieridae, Lepidoptera). Naturwissenschaften 101, 1055–1063 (2014). https://doi.org/10.1007/s00114-014-1244-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-014-1244-5