Abstract
While behavioural plasticity is considered an adaptation to fluctuating social and environmental conditions, many animals also display a high level of individual consistency in their behaviour over time or across contexts (generally termed ‘personality’). However, studies of animal personalities that include sexual behaviour, or functionally distinct but correlated traits, are relatively scarce. In this study, we tested for individual behavioural consistency in courtship and exploratory behaviour in male guppies (Poecilia reticulata) in two light environments (high vs. low light intensity). Based on previous work on guppies, we predicted that males would modify their behaviour from sneak mating tactics to courtship displays under low light conditions, but also that the rank orders of courtship effort would remain unchanged (i.e. highly sexually active individuals would display relatively high levels of courtship under both light regimes). We also tested for correlations between courtship and exploratory behaviour, predicting that males that had high display rates would also be more likely to approach a novel object. Although males showed significant consistency in their exploratory and mating behaviour over time (1 week), we found no evidence that these traits constituted a behavioural syndrome. Furthermore, in contrast to previous work, we found no overall effect of the light environment on any of the behaviours measured, although males responded to the treatment on an individual-level basis, as reflected by a significant individual-by-environment interaction. The future challenge is to investigate how individual consistency across different environmental contexts relates to male reproductive success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenotypic plasticity is a key component of fitness that allows individuals to adapt their morphology and/or behaviour to changing environmental conditions (West-Eberhard 1989). Altered phenotypes may be observed in response to a range of environmental cues. For example, early exposure to the presence of predators can induce the development of morphological defences in some species (Tollrian and Harvell 1999), while rearing density can determine male morph type (e.g. fighters) in others (reviewed in Kokko and Rankin 2006). Behavioural traits, in particular, are generally thought to be more labile than morphological traits (Relyea 2001), allowing individuals to display flexible and reversible responses to social and environmental variables such as sex ratio, resource availability and the risk of predation (Dill 1987; Kats and Dill 1998; Lima 1998).
Although the ability to modify behaviour in response to environmental change is likely to be adaptive (reviewed by West-Eberhard 1989; Via et al. 1995; Relyea 2002), there are limits to behavioural plasticity (DeWitt et al. 1998) and many species display a high level of consistency (or repeatability) in their behavioural traits over time or across contexts, generally termed ‘animal personality’ (Dall et al. 2004, 2012; Sih et al. 2004a, b; Réale et al. 2007; Sih and Bell 2008). For example, some individuals consistently display higher levels of boldness, exploratory activity or aggression than other members of the population, although the extent of individual consistency in behaviour can depend on setting (field vs. lab), timing and the type of behaviour under investigation (see Bell et al. 2009 for a meta-analysis). Furthermore, functionally distinct traits can be correlated within individuals to form a ‘behavioural syndrome’ (Bell 2005), such as the well-characterized boldness–aggression syndrome observed in sticklebacks (Gasterosteus aculeatus) (Huntingford 1976; Bell 2005) and funnel-web spiders (Agelenopsis aperta) (Riechert and Hedrick 1993). Personality traits are of evolutionary significance because they can affect fitness; in great tits (Parus major), for example, exploratory tendency is correlated with adult survival and reproductive success (Dingemanse et al. 2004; Both et al. 2005).
Few studies of animal personality have considered an individual’s consistency in sexual behaviour or whether sexually selected behaviours form part of a behavioural syndrome (reviewed by Dingemanse and Reale 2005; Sih and Bell 2008). This is surprising, given that selection for consistency is predicted for behaviours that are indicative of male quality, as these can be used to guide female mate choice or competitive interactions among males (Schuett et al. 2010). On the other hand, behavioural plasticity in sexual behaviour might be advantageous by enabling males to respond to factors such as fluctuations in female availability, the level of competition from other males or predation risks associated with mating activity (reviewed by Bretman et al. 2011). A recent study on fruit fly, Drosophila melanogaster, reported evidence for both behavioural flexibility and individual consistency (Bretman et al. 2012). However, studies of animal personalities that include sexual behaviours, and those that consider a role for sexual selection in the evolution and maintenance of personality differences, are generally lacking (Schuett et al. 2010).
The guppy (Poecilia reticulata) is a small, tropical freshwater fish that is well suited to studies of within-species behavioural variation because it exhibits extensive differences in behaviour (e.g. aggression, courtship tactics, shoaling tendency) across natural environmental gradients (Endler 1995). Male guppies use a combination of courtship (consensual) and forced (non-consensual) matings to achieve copulation (Baerends et al. 1955; Liley 1966). During courtship, males adopt a highly conspicuous S-shaped posture (termed ‘sigmoid display’) to attract receptive females. Alternatively, males can circumvent female choice by engaging in forced mating attempts (termed gonopodial thrusts), where the male approaches the female from behind and attempts to copulate without female cooperation (reviewed by Houde 1997). Individual males use both mating tactics interchangeably, but alter the relative frequency of sigmoid displays and gonopodial thrusts according to social and environmental variables such as early social experience (Guevara-Fiore 2012), recent social history (Jordan and Brooks 2012), female vigilance (Evans et al. 2002) and light intensity (Endler 1987; Reynolds et al. 1993; Archard et al. 2009; Chapman et al. 2009). For their part, female guppies choose among males on the basis of their colouration and courtship display rates (Farr 1980; Stoner and Breden 1988; Kodric-brown 1993; Houde 1997). Female guppies also prefer males that they observe are displaying bold behaviours (Godin and Dugatkin 1996), but it is unclear whether mating tactics (i.e. relative frequency of courtship displays and thrusts) and behaviours possibly linked to boldness (e.g. exploration tendency) are themselves correlated and repeatable across time or environmental contexts.
There are at least two reasons why we might expect a relationship between courtship behaviour and behaviours associated with boldness. First, individuals that are bold in one context, for example when encountering a predator, are more likely to be bold in other situations, such as exploring a novel environment or food source, behaving aggressively towards conspecifics or engaging in visually conspicuous courtship behaviours (Coleman and Wilson 1998). In male fiddler crabs, for example, bold males spend more time courting females and have higher mating success than shy males (Blackwell et al. 1999; Reaney and Backwell 2007). Second, bold male guppies (which are attractive to females, Godin and Dugatkin 1996) may be of higher quality (e.g. condition) and therefore perform increased courtship displays (which in guppies is strongly linked to male condition; Devigili et al. 2012; Rahman et al. 2013) than their more timid and lower quality counterparts.
In this study, we investigated whether male guppies show individual behavioural consistency in their mating tactics and exploratory behaviour across different light environments. We used ambient light intensity (while controlling for spectral composition) as an environmental variable because male guppies have been shown to switch to covert mating tactics (i.e. gonopodial thrusts) at high light intensities while relying predominantly on courtship displays at low light intensities (Endler 1987; Archard et al. 2009). This switch in mating tactics is because courtship under high light intensity places males at higher risk of predation (Endler 1987). Indeed, the switch in mating behaviour under different light environments occurs in the absence of predators, suggesting that light intensity is used as a cue to induce risk-sensitive behaviours (Endler 1987; Archard et al. 2009). We therefore also expected the ambient light environment (high light intensity or low light intensity) to have an effect on the tendency for males to approach a novel food source, predicting that males would be more likely to engage in risky behaviours (explore a novel object) in conditions of low light (i.e. when they are also engaging in risky courtship behaviour). We thus expected to find a positive correlation between courtship display rate and exploratory behaviour, with these behaviours being expressed at a higher frequency under low compared to high light intensity conditions. Finally, we tested for individual behavioural consistency in courtship and exploratory behaviour by determining whether inter-individual differences persisted over the period of 1 week. Given that mating behaviour is highly heritable in guppies (Evans 2010), we expected to find that behavioural differences among individuals would persist despite the changes in the light environment (i.e. behavioural rankings of the males would be maintained across the different light treatments).
Methods
Fish maintenance and experimental design
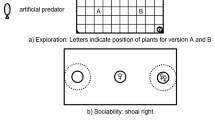
This experiment was carried out in June–July 2010. The guppies used in this experiment were descendants (∼12 generations) of wild-caught fish collected from Alligator Creek (30 km south of Townsville) in Queensland, Australia in 2006. Guppies at this location originate from Guyana, South America (c. 1910, Lindholm et al. 2005). Previous experiments on laboratory-bred guppies from this population have shown that males modify their mating strategies when exposed to variation in the ambient light spectrum in a manner similar to that observed in their native habitat (Gamble et al. 2003). Fish were transported to aquarium facilities at The University of Western Australia and maintained in mixed-sex populations at constant temperature (26 ± 0.5 °C) on a light/dark cycle of 12:12 h until required for the experiment. The observations were conducted in a series of four replicate tanks (44 × 43.5 × 30 cm high, filled to a depth of 26 cm) containing aquarium gravel, artificial plants and conditioned water. The observation tanks were located in the same room as the stock tanks so that the light/dark cycle and temperature were the same. Overhead lighting was provided by two broad-spectrum fluorescent lights (Philips Lifemax TL-D 36W/840 Cool White).
We simulated different light intensity environments in the observation tanks by covering the tanks with either a clear acetate filter (=no change in light) or a neutral density filter (0.3ND, Lee Filters™, UK), which reduces overall luminance by 50 % (400–700 nm). The filters affect all wavelengths equally so there are no changes in the spectral composition of light entering the tanks. Two observation tanks for each treatment were set up, subsequently referred to as ‘high luminance’ (clear acetate filter) and ‘low luminance’ (neutral density filter) treatment tanks. Reductions of luminance by 50 % or more have previously been shown to affect courtship behaviour in male guppies, causing an increase in visually conspicuous courtship display behaviours (Endler 1987). The tops and sides of the tanks were covered with the filters, excluding the tank ends, which were covered with black cardboard to prevent interactions among fish in adjacent tanks.
We confirmed that the neutral density filters had the desired effect by measuring the irradiance of light in each of our treatment tanks. We used a USB4000 portable spectrometer (Ocean Optics, Inc., Dunedin, FL, USA) connected to a 600-μm fibre optic and fitted with a cosine corrector (CC-3-UV) to collect light over 180° angle. These measures were performed in reference to a calibration lamp (LS-1-CAL-220, 300-1050 nm, Ocean Optics, Inc.). The probe was positioned below the surface of the water in the centre of each tank (with the lid on) facing upwards to collect down-welling light, and three readings were taken in each tank. The spectra were processed using Spectrasuite (Ocean Optics) software and were interpolated (linear interpolation at 1-nm intervals, range 400–700 nm) and converted to photon irradiance in micromole per square meter per second. These measurements confirmed that there was a difference in the total irradiance of light (integrated over range of 400–700 nm; in micromole per square meter per second) in the high luminance (tank 1 = 10.2 μmol m−2 s−1, tank 2 = 7.5 μmol m−2 s−1) versus low luminance (tank 1 = 3.0 μmol m−2 s−1, tank 2 = 1.9 μmol m−2 s−1) treatment tanks. These light intensities are similar to other studies that have used this guppy population to induce shifts in male guppy mating behaviour (luminance = 5.5–6.4 μmol m−2 s−1; Gamble et al. 2003).
Sexually mature males (n = 40; exhibiting body colouration and a fully developed gonopodium) were chosen haphazardly from our stock tanks and isolated in 2-L containers 1 week before trials commenced. The absence of female companionship for this period was designed to increase the behavioural motivation (e.g. courtship, exploration) of males. Isolated males were fed frozen Artemia nauplii daily (using a pipette) except on the morning of their trials to increase their motivation to forage during the behavioural trials. Females that were approximately matched for size by eye (approximately 25 mm in standard length) were selected randomly from a separate set of mixed-sex (4:3 ratio of females/males) stock aquaria so that they would not be familiar with the test males. We used stimulus shoals of ‘dither’ fish for both the courtship and exploratory behaviour (tendency to approach a novel food source) trials to facilitate natural behaviour in the test fish and reduce the time required for acclimatisation (Barlow 1968). We used juveniles for these stimulus shoals so that they would not be a source of sexual interest for the male but would provide social companionship and a focal point from which to approach the female (mating behaviour trial) or novel food item. On each day of the behavioural trials, dither fish were selected haphazardly from a stock of 15–20 approximately size matched juveniles.
Behavioural observations
All trials took place between 0930 and 1630 hours. Individual males received the light treatments on consecutive days; thus, on day 1, the mating and exploratory behaviour of an individual male was observed in the first light treatment (high or low luminance), while on day 2, these behaviours were recorded (at the same time of day) for the same individual in the second light treatment. The order in which the males received the light treatments was alternated so that half of the males received the low luminance treatment first while the other half began with the high luminance treatment. One week after these trials were conducted, we repeated these procedures. Importantly, the order in which males received the treatments, and the time of day at which the treatments were presented, was the same as the first week of observations.
On the afternoon prior to each behavioural trial, a stimulus shoal consisting of five (dither) juveniles was placed in each tank. These shoals were placed in a 1.5-L transparent punctured plastic bottle (to allow transmission of visual and chemical cues) and placed on one side of each observation tank. On the following morning, a non-virgin female was placed in each of the observation tanks and left for 15–20 min to acclimatise. Females that did not settle after 20 min (i.e. did not exhibit exploratory or foraging behaviour) were removed and transferred to post-treatment tanks where they played no further part in the experiment. After the female was settled, a test male was gently added to each tank; behavioural observations commenced as soon as the test male performed his first mating attempt with the female (see below).
We recorded a courtship display when the male moved in front of the female and quivered his body in a characteristic S-shaped posture (Houde 1997). Gonopodial thrusts (‘sneaks’) were recorded when the focal male approached the female from behind, swung his gonopodium forward more than 90° and attempted to forcibly inseminate the female without prior courtship (see Houde 1997). We recorded the total number of courtship displays and gonopodial thrust attempts over a 15-min period. After each mating behaviour trial, the stimulus female was gently removed with a hand net and returned to post-treatment aquaria (where she played no further part in the experiment). The bottle containing the stimulus shoal remained in the tank to provide a focal point from which males could approach a novel object.
Immediately following the observations of mating behaviour, we introduced a novel food item to the centre of the tank. This food item was specifically designed to constitute a novel stimulus for the male and consisted of a test tube bung with two thirds of its surface area covered with a paste comprising Tetramin™ fish food flake crushed in gelatine (allowing it to stick to the bung). The bung was suspended from a transparent monofilament line so that it was positioned in the centre of the tank, 6–9 cm above the gravel at the base of the tank. Although the stock population used in this study were fed intermittently (once per week) with flake food prior to the experiment, this was applied at the water surface in its ‘normal’ state and thus crushed flake attached to a submerged bung would have constituted a novel food source for the fish. Following introduction of the novel food item, we recorded the total number of times the male approached the food (within two body lengths) during a 15-min period. During preliminary trials, we noted that these approaches were similar to predator ‘inspections’ (Magurran 2005) in which the fish visually fixated on the object while slowly swimming or ‘gliding’ a fixed distance around it, before rapidly darting back to the shoal. Following the exploration behaviour trial, each male was returned to his individual holding container (labelled, for subsequent identification of individuals).
We recorded the mating and exploratory behaviour of between four and six individual males per day, during which the same shoals of stimulus fish (in the bottles) were used. At the end of each day’s behavioural trials, dither fish were returned to the juvenile stock tank and replaced with new stimulus shoals for the following day’s trials. To prevent the possible build up of chemical cues affecting the behaviour of fish in subsequent trials, the water in the observation tanks was replaced with fresh conditioned water daily. Following both observations (i.e. days 1 and 2), the males were housed individually for a period of 1 week before they took part in a repeated set of (courtship and exploration behaviour) observations, performed in dark and light environments (over consecutive days). We recorded the behaviour of a total of 40 males, each of which was observed on four separate occasions (week 1, day 1, day 2; week 2, day 1, day 2 = 160 observations).
Statistical analyses
The data (total number of displays, sneaks and exploratory approaches) were analysed using the generalised linear mixed-effect models (using the lme4 package; Bates and Maechler 2009) in the software program R, version 2.15.3 (R Development Core Team 2012). As the data were counts, we used the Poisson model (log-link) and generated likelihood values using the Laplace approximation. We entered the fixed effect of treatment (two levels: high luminance or low luminance) and five random effects: observation tank (2 levels, i.e. 2 test tanks of each treatment), week tested (2 levels), male ID (40 levels) and the interactions between male × treatment (80 levels) and week × treatment (4 levels). The inclusion of male identity allowed for repeated measures of the same male across treatments (days) and consecutive observation weeks. We tested for a week × treatment interaction to determine whether males responded differently to the treatment according to the week in which they were tested (e.g. due to habituation or familiarity with the test environment). We also looked for a significant effect of the order in which males experienced the treatments (i.e. low luminance encountered first or second) by conducting t tests (both within weeks and within treatments) on the log + 1 transformed data.
We tested the significance of each of the random effects using log likelihood ratio tests to compare the full model (five random effects, one fixed effect) to alternative models in which one of the random effects was removed. Each of the resulting models was checked for normality and homogeneity of variance by visually inspecting plots of the fitted model against the residual values. Estimates of the fixed effects, along with the associated standard error and t value, were obtained from the full models. A significant fixed effect would indicate that on average, individuals have a plastic response to the treatment, while a significant male ID-by-treatment interaction would suggest that there is inter-individual variation in the plastic response of males to the light environment. We determined whether significant male effects could be attributed to within- or among-individual phenotypic variance by running the generalised linear mixed models (GLMMs) for both random intercept models and random intercept and slope models. The former accounts for the among-individual variation in overall male responses (i.e. random intercept), while the latter incorporates among-individual variation in slopes (i.e. level of plasticity) (van de Pol and Wright 2009; Martin et al. 2011; Dingemanse and Wolf 2013). The fit of these alternative models was compared using log likelihood ratio tests, fitted with and without the random slope effect.
We tested for repeatability of male behaviour across different contexts (mating behaviour and exploration behaviour) by calculating the intraclass correlation coefficient (Lessells and Boag 1987). We first tested whether male behaviour was repeatable across consecutive weeks by calculating repeatabilities for males assigned to dark and light treatments separately. We then tested for repeatability across the different light treatments (performed on consecutive days) by conducting a separate analysis for week 1 and week 2. The data were log (+1) transformed and, after restricting the data to the period of interest, we conducted ANOVAs with male behaviour as the response variable and male identity as the fixed factor. The resulting mean square values for the within (error) and among (model) variance were used to estimate repeatability (R) and its associated standard error (Becker 1984; Lessells and Boag 1987).
Results
The GLMMs revealed that the random effect of male, and the interaction between male identity and treatment, had a significant effect on all three behaviours (Table 1; all P < 0.05). Thus, males responded to the treatments differently and on an individual-level basis. There was no difference between models fitted with and without the random slope effect, indicating that most of the variability in the data could be explained by among-individual variation in overall responses to the environment (displays: Χ 2 = 0.07, df = 2, P = 0.96; sneaks: Χ 2 = 2.16, df = 2, P = 0.34; exploratory: Χ 2 = 0.36, df = 2, P = 0.84). Light intensity (treatment) had no effect on our response variables, as indicated by the large standard errors of the estimated fixed effects (Table 1). The random effects of observation tank (median ± interquartile ranges (IQRs): tank 1 = 7 ± 10.75, tank 2 = 8 ± 10) and the week × treatment interaction had a significant effect on the number of exploratory approaches, but no effect on the other behaviours measured (Table 1; median number of approaches ± IQRs: week 1: low luminance = 9.5 ± 12, high luminance = 8.5 ± 9; week 2: low luminance = 5.5 ± 6, high luminance = 5.5 ± 10.75). There were no treatment order effects (11 t tests: P > 0.05) with the exception of the number of displays recorded on week 1 for the high luminance treatment (t 38 = 3.39, P = 0.024 after controlling for false discovery rates [FDR]); males that experienced the high luminance treatment first performed more displays (median ± IQR = 9 ± 10) than those that experienced the high luminance treatment second (median ± IQR = 1 ± 6).
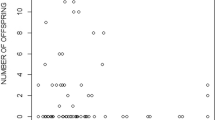
The individual behavioural consistency in male display behaviour over time is also revealed through the repeatability estimates, which showed that some male behaviours were more repeatable than others (Table 2 and Fig. 1). Specifically, the number of sigmoid displays performed by males showed the highest repeatability across both weeks and days, ranging from 0.45 to 0.73 (SE = 0.08-0.13). For all behaviours with significant repeatability, repeatability estimates were higher over short time scales (consecutive days) than longer time scales (consecutive weeks). There were no significant correlations between the mating behaviours (displays and gonopodial thrusts) and exploration behaviour after controlling for the FDR (P > 0.05; behaviours averaged over treatments; Table 3 and Fig. 1).
Individual consistency in the behaviour of male guppies (Poecilia reticulata) for the number of courtship displays (a), sneak mating attempts (b) and approaches to a novel food source (c) observed over the period of consecutive weeks (left: behaviours averaged over treatments) and consecutive days (right)
Discussion
Most studies of animal personality have focused on the aggressive–boldness syndrome, and surprisingly, few studies have considered the behavioural consistency of individual mating tactics (Dingemanse and Reale 2005; Sih and Bell 2008; Dingemanse and Wolf 2010; Schuett et al. 2010). In this study, we show that male mating behaviour (the frequency of courtship displays and gonopodial thrusts) and the frequency of approaches towards a novel object (a possible correlate of boldness) are consistent over time. However, we found no evidence that these behaviours were correlated as part of a courtship–exploration syndrome. Furthermore, in contrast to findings from previous studies (Endler 1987; Long and Houde 1989; Reynolds et al. 1993; Chapman et al. 2009), we found no overall effect of the light environment on any of the behaviours measured; instead, males responded on an individual-level basis to the change in ambient light intensity, as revealed by the significant interaction between male identity and light treatment.
Although we found evidence for personality traits associated with courtship and exploratory behaviours, the level of behavioural plasticity observed among individuals did not vary. This is in contrast to other studies that have reported individual differences in behavioural plasticity (Dingemanse et al. 2010). Differences in the way individuals manage environmental uncertainty are thought to explain personality-related differences in behavioural plasticity because of state- or frequency-dependent pay-offs associated with sampling the environment (Mathot et al. 2012). One key factor that may have impeded our ability to detect individual variation in behavioural plasticity is that our study was conducted on fish that have been born and raised in the laboratory (approximately 12 generations) and would therefore have experienced minimal environmental variation. This lack of natural variability in lighting conditions (e.g. dawn and dusk transitions) experienced by laboratory fish might also explain the lack of the overall light treatment effect. Indeed, since behavioural plasticity is costly and considered an evolutionary response to environmental variation (DeWitt et al. 1998), our findings may be a direct result of relaxed selection imposed by the laboratory environment (i.e. lack of variation in predation risk and lighting environment). The potential evolutionary loss of individual behavioural plasticity in captive environments is relevant for conservation breeding programs and requires further investigation (Mason et al. 2013).
As reported in the meta-analysis conducted by Bell et al. (2009), we found that behaviour is more likely to be repeatable over short time scales (i.e. days) than over longer intervals (i.e. 1 week). One explanation for this is that information about the environment is more likely to be reliable when it is recent, serving as a cue for behavioural prioritisation (Dingemanse and Wolf 2013) and minimising the costs associated with additional sampling (i.e. information acquisition). Consequently, we expect a stronger behavioural response to the type of environment most recently encountered. In the context of mating behaviour, similar social environments are more likely to be encountered (e.g. operational sex ratio) at short-term scales, while variation in population demographics may cause greater variation in the long term. If state-dependent effects are important, such as hunger and condition, individuals are more likely to be in the same state over short time intervals (Bell et al. 2009).
We found that some types of behaviour were more repeatable than others. Specifically, the repeatability of courtship behaviour was generally higher than that of exploratory behaviour, probably because exploration of novel objects is subject to the effects of habituation over successive encounters (hence the significant ‘week’ and ‘treatment × week’ effects). These findings are in line with other studies on the repeatability of different behaviour traits and may reflect differences in the sensitivity of particular behaviours to environmental variation (Bell et al. 2009) or the importance of maintaining courtship vigour for female mate choice. Magurran and Seghers (1990) reported that males modify their sexual behaviour according to predation risk (and population origin), but males with the highest display rates tended to maintain this high level of sexual vigour, even when a predator was present. Similarly, other studies have reported that male guppies show high repeatability of courtship displays (r = 0.62–0.94) and attempted sneak copulation attempts (r = 0.83–0.99), with differences among males being maintained across different sex ratio treatments (Magellan and Magurran 2007). Consistent with these findings, recent quantitative genetic analyses have revealed high levels of additive genetic variance and correspondingly high narrow-sense heritabilities for male sexual behaviour in guppies (Evans 2010). As courtship displays are important indicators of male quality, consistency in courtship behaviour may be the result of direct selection by females.
Sexual selection may play an important role in the evolution and maintenance of animal personality traits (Dingemanse and Reale 2005; Schuett et al. 2010). As sexual selection often promotes phenotypic differences between the sexes, divergent selection pressures may also generate sex differences in personality. For example, female convict cichlids (Cichlasoma nigrofasciatum) are more active and aggressive than males (Budaev et al. 1999). Personalities may also play a part in mate choice, through females displaying repeatability in their choice of male sexual traits (e.g. beak colour, aggression, song rate; Logue et al. 2009) or during male–male competition, where personality traits may determine a male’s position in the dominance hierarchy (Colleter and Brown 2011). Consistent with this idea, female guppies do not always agree on what constitutes an attractive male, but they nevertheless show high repeatability in individual preferences (Kodric-brown and Nicoletto 1997; Brooks and Endler 2001). In guppies, the extent to which consistent differences in male and female sexual behaviours influences fitness (e.g. Schuett et al. 2011) is currently unknown, although there is evidence that other personality traits such as boldness, activity levels of exploratory behaviour are associated with fitness (Smith and Blumstein 2010).
In contrast to previous work on guppies (Endler 1987; Long and Houde 1989; Reynolds et al. 1993), we found no change in male courtship behaviour following a shift in light intensity in the ambient environment. Gamble et al. (2003) also reported no differences in male courtship behaviour across different light environments, but in their experiment, the spectral composition of light (i.e. light availability over different parts of the spectrum) was experimentally manipulated, while the overall luminance was controlled. Interestingly, in the Gamble et al. (2003) study, the spectral composition of light (representing different times of the day or variable microenvironments) caused a shift in female sexual responsiveness, to which males responded by altering their frequency of sneak mating attempts. In our study, we did not evaluate female responsiveness and therefore were unable to control for any potential behavioural differences among stimulus females. Nevertheless, it seems that in guppies, males and females respond differentially to changes in both the spectral composition and luminance of ambient light, suggesting that spatial and temporal variation in the light environment can have important consequences for sexual selection (Gamble et al. 2003). Further work is required to determine the extent to which male and female personalities play a part in this light-mediated variation in sexual behaviour.
References
Archard GA, Cuthill IC, Partridge JC (2009) Light environment and mating behavior in Trinidadian guppies (Poecilia reticulata). Behav Ecol Sociobiol 64(2):169–182. doi:10.1007/s00265-009-0834-2
Baerends GB, Brouwer R, Waterbolk HT (1955) Ethological studies in Lebistes reticulatus (Peters). I Anal Male Courtship Pattern Behav 8:249–335
Barlow GW (1968) Dither—a way to reduce undesirable fright behaviour in ethological studies. A Tierpsychol 25:315–318
Bates D, Maechler M (2009) lme4: linear mixed-effects models using S4 classes. R package version 0.999375-32.
Becker WA (1984) A manual of quantitative genetics. Academice Enterprises, Pullman
Bell AM (2005) Behavioral differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J Evol Biol 18:464–473
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77(4):771–783. doi:10.1016/j.anbehav.2008.12.022
Blackwell PRY, Jennions MD, Christy JH, Passmore NI (1999) Female choice in the synchronously waving fiddler crab Uca annulipes. Ethology 105:415–421
Both C, Dingemanse NJ, Drent PJ, Tinbergen JM (2005) Pairs of extreme avian personalities have highest reproductive success. J Anim Ecol 74(4):667–674. doi:10.1111/j.1365-2656.2005.00962.x
Bretman A, Gage MJG, Chapman T (2011) Quick-change artists: male plastic behavioural responses to rivals. Trends Ecol Evol 26(9):467–473. doi:10.1016/j.tree.2011.05.002
Bretman A, Westmancoat JD, Gage MJG, Chapman T (2012) Individual plastic responses by males to rivals reveal mismatches between behaviour and fitness outcomes. Proc R Soc B-Biol Sci 279(1739):2868–2876. doi:10.1098/rspb.2012.0235
Brooks R, Endler JA (2001) Female guppies agree to differ: phenotypic and genetic variation in mate-choice behaviour and the consequences for sexual selection. Evolution 55:1644–1655
Budaev SV, Zworykin DD, Mochek AD (1999) Individual differences in parental care and behaviour profile in the convict cichlid: a correlational study. Anim Behav 58:195–202
Chapman BB, Morrell LJ, Krause J (2009) Plasticity in male courtship behaviour as a function of light intensity in guppies. Behav Ecol Sociobiol 63(12):1757–1763. doi:10.1007/s00265-009-0796-4
Coleman K, Wilson DS (1998) Shyness and boldness in pumpkinseed sunfish: individual differences are context-specific. Anim Behav 56:927–936. doi:10.1006/anbe.1998.0852
Colleter M, Brown C (2011) Personality traits predict hierarchy rank in male rainbowfish social groups. Anim Behav 81:1231–1237
Dall RX, Houston AI, McNamara JM (2004) The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol Lett 7:734–739. doi:10.1111/j.1461-0248.2004.00618.x
Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW (2012) An evolutionary ecology of individual differences. Ecol Lett 15(10):1189–1198. doi:10.1111/j.1461-0248.2012.01846.x
Devigili A, Kelley JL, Pilastro A, Evans JP (2012) Expression of pre- and postcopulatory traits under different dietary conditions in guppies. Behav Ecol 24:740–749. doi:10.1093/beheco/ars204
DeWitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81
Dill LM (1987) Animal decision making and its ecological consequences: the future of aquatic ecology and behaviour. Can J Zool 65:803–811
Dingemanse NJ, Reale D (2005) Natural selection and animal personality. Behaviour 142:1165–1190
Dingemanse NJ, Wolf M (2010) Recent models for adaptive personality differences: a review. Philos Trans R Soc B-Biol Sci 365(1560):3947–3958. doi:10.1098/rstb.2010.0221
Dingemanse NJ, Wolf M (2013) Between-individual differences in behavioural plasticity within populations: causes and consequences. Anim Behav 85(5):1031–1039. doi:10.1016/j.anbehav.2012.12.032
Dingemanse NJ, Both C, Drent PJ, Tinbergen JM (2004) Fitness consequences of avian personalities in a fluctuating environment. Proc R Soc B-Biol Sci 271:847–852. doi:10.1098/rspb.2004.2680
Dingemanse NJ, Kazem AJN, Reale D, Wright J (2010) Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol Evol 25:81–89
Endler JA (1987) Predation, light intensity and courtship behavior in Poecilia reticulata (Pisces: Poeciliidae). Anim Behav 35:1376–1385
Endler JA (1995) Multiple-trait coevolution and environmental gradients in guppies. TREE 10:22–29
Evans JP (2010) Quantitative genetic evidence that males trade attractiveness for ejaculate quality in guppies. Proc R Soc B-Biol Sci 277:3195–3201
Evans JP, Kelley JL, Ramnarine IW, Pilastro A (2002) Female behaviour mediates male courtship under predation risk in the guppy (Poecilia reticulata). Behav Ecol Sociobiol 52:496–502
Farr JA (1980) Social behaviour patterns as determinants of reproductive success in the guppy, Poecilia reticulata Peters (Pisces, Poeciliidae)—an experimental study of the effects of intermale competition, female choice, and sexual selection. Behaviour 74:38–91. doi:10.1163/156853980x00311
Gamble S, Lindholm AK, Endler JA, Brooks R (2003) Environmental variation and the maintenance of polymorphism: the effect of ambient light spectrum on mating behaviour and sexual selection in guppies. Ecol Lett 6(5):463–472
Godin JGJ, Dugatkin LA (1996) Female mating preference for bold males in the guppy, Poecilia reticulata. Proc Natl Acad Sci U S A 93(19):10262–10267. doi:10.1073/pnas.93.19.10262
Guevara-Fiore P (2012) Early social experience significantly affects sexual behaviour in male guppies. Anim Behav 84(1):191–195. doi:10.1016/j.anbehav.2012.04.031
Houde AE (1997) Sex, colour, and mate choice in guppies. Princeton University Press, Princeton
Huntingford FA (1976) The relationship between antipredator behaviour and aggression among conspecifics in the three-spined stickleback. Anim Behav 24:245–260
Jordan LA, Brooks RC (2012) Recent social history alters male courtship preferences. Evolution 66(1):280–287. doi:10.1111/j.1558-5646.2011.01421.x
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Kodric-brown A (1993) Female choice of multiple male criteria in guppies—interacting effects of dominance, coloration and courtship. Behav Ecol Sociobiol 32(6):415–420
Kodric-brown A, Nicoletto PF (1997) Repeatability of female choice in the guppy: response to live and videotaped males. Anim Behav 54:369–376
Kokko H, Rankin DJ (2006) Lonely hearts or sex in the city? Density-dependent effects in mating systems. Philos Trans R Soc B-Biol Sci 361(1466):319–334. doi:10.1098/rstb.2005.1784
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Liley NR (1966) Ethological isolating mechanisms in four sympatric species of poeciliid fishes. Behav (Supplement XIII) 13:1–197
Lima SL (1998) Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv Study Anim Behav 27:215–290
Lindholm AK, Breden F, Alexander HJ, Chan WK, Thakurta SG, Brooks R (2005) Invasion success and genetic diversity of introduced populations of guppies Poecilia reticulata in Australia. Mol Ecol 14:3671–3682
Logue DM, Mishra S, McCaffrey D, Ball D, Cade WH (2009) A behavioral syndrome linking courtship behavior toward males and females predicts reproductive success from a single mating in the hissing cockroach, Gromphadorhina portentosa. Behav Ecol 20(4):781–788. doi:10.1093/beheco/arp061
Long KD, Houde AE (1989) Orange spots as a visual cue for female mate choice in the guppy (Poecilia reticulata). Ethology 82:316–324
Magellan K, Magurran AE (2007) Behavioral profiles: individual consistency in male mating behaviour under varying sex ratios. Anim Behav 74:1545–1550
Magurran AE (2005) Evolutionary ecology: the Trinidadian guppy. Oxford University Press, Oxford
Magurran AE, Seghers BH (1990) Risk sensitve courtship in the guppy (Poecilia reticulata). Behaviour 112:194–201
Martin JGA, Nussey DH, Wilson AJ, Réale D (2011) Measuring individual differences in reaction norms in field and experimental studies: a power analysis of random regression models. Methods Ecol Evol 2(4):362–374. doi:10.1111/j.2041-210X.2010.00084.x
Mason G, Burn CC, Dallaire JA, Kroshko J, Kinkaid HM, Jeschke JM (2013) Plastic animals in cages: behavioural flexibility and responses to captivity. Anim Behav 85(5):1113–1126. doi:10.1016/j.anbehav.2013.02.002
Mathot KJ, Wright J, Kempenaers B, Dingemanse NJ (2012) Adaptive strategies for managing uncertainty may explain personality-related differences in behavioural plasticity. Oikos 121:1009–1020
R Development Core Team (2012) A language and environment for statistical computing. R Foundation for Statistical Computing, Austria
Rahman MM, Kelley JL, Evans JP (2013) Condition-dependent expression of pre- and postcopulatory sexual traits in guppies. Ecol Evol 3(7):2197–2213. doi:10.1002/ece3.632
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82(2):291–318. doi:10.1111/j.1469-185X.2007.00010.x
Reaney LT, Backwell PRY (2007) Risk-taking behavior predicts aggression and mating success in a fiddler crab. Behav Ecol 18(3):521–525. doi:10.1093/beheco/arm014
Relyea RA (2001) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82(2):523–540. doi:10.1890/0012-9658(2001)082[0523:mabpol]2.0.co;2
Relyea RA (2002) Costs of phenotypic plasticity. Am Nat 159(3):272–282. doi:10.1086/338540
Reynolds JD, Gross MD, Coombs MJ (1993) Environmental conditions and male morphology determine alternative mating behaviour in Trinidadian guppies. Anim Behav 45(1):145–152
Riechert SE, Hedrick AV (1993) A test for correlations among fitness-linked behavioral traits in the spider Agelenopsis aperta (Araneae, Agelenidae). Anim Behav 46(4):669–675. doi:10.1006/anbe.1993.1243
Schuett W, Tregenza T, Dall SRX (2010) Sexual selection and animal personality. Biol Rev 85:217–246
Schuett W, Dall SRX, Royle NJ (2011) Pairs of zebra finches with similar ‘personalities’ make better parents. Anim Behav 81(3):609–618. doi:10.1016/j.anbehav.2010.12.006
Sih A, Bell AM (2008) Insights for behavioral ecology from behavioral syndromes. Adv Study Behav 38:227–281
Sih A, Bell A, Johnson JC (2004a) Behavioral syndromes: an ecological and evolutionary overview. TREE 19:372–378
Sih A, Bell A, Johnson JC, Ziemba R (2004b) Behavioral syndromes: an integrative overview. Q Rev Biol 79:241–277
Smith BR, Blumstein DT (2010) Behavioral types as predictors of survival in Trinidadian guppies (Poecilia reticulata). Behav Ecol 21(5):919–926. doi:10.1093/beheco/arq084
Stoner G, Breden F (1988) Phenotypic differentiation in female preference related to geographic variation in male predation risk in the Trinidad guppy (Poecilia reticulata). Behav Ecol Sociobiol 22(4):285–291. doi:10.1007/bf00299844
Tollrian R, Harvell D (1999) The ecology and evolution of inducible defences. Princeton University Press, Princeton
van de Pol M, Wright J (2009) A simple method for distinguishing within- versus between-subject effects using mixed models. Anim Behav 77(3):753–758. doi:10.1016/j.anbehav.2008.11.006
Via S, Gomulkiewicz R, De Jong G, Scheiner SM, Schlichting CD, Van Tienderen PH (1995) Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol Evol 10(5):212–217. doi:10.1016/S0169-5347(00)89061-8
West-Eberhard MJ (1989) Phenotypic plasticity and the origins of diversity. Ann Rev Ecol Syst 20:249–278
Acknowledgments
We are grateful to Cameron Duggin for assistance with maintenance and fish care and Joe Tomkins and Tom Pizzari for discussion. We also thank Ben Chapman and the anonymous reviewers whose comments greatly improved this paper. We also acknowledge the Universities of Western Australia and Oxford, and the Australian Research Council, for financial support.
Ethical standards
These experiments were conducted according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and were approved by the Animal Ethics Committee of The University of Western Australia (approval number: RA/3/100/513).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Rights and permissions
About this article
Cite this article
Kelley, J.L., Phillips, S.C. & Evans, J.P. Individual consistency in exploratory behaviour and mating tactics in male guppies. Naturwissenschaften 100, 965–974 (2013). https://doi.org/10.1007/s00114-013-1097-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-013-1097-3