Abstract
Atherosclerosis (AS) is a chronic inflammatory vascular disease that occurs in the intima of large and medium-sized arteries with the immune system’s involvement. It is a common pathological basis for high morbidity and mortality of cardiovascular diseases. Abnormal proliferation of apoptotic cells and necrotic cells leads to AS plaque expansion, necrotic core formation, and rupture. In the early stage of AS, macrophages exert an efferocytosis effect to engulf and degrade apoptotic, dead, damaged, or senescent cells by efferocytosis, thus enabling the regulation of the organism. In the early stage of AS, macrophages rely on this effect to slow down the process of AS. However, in the advanced stage of AS, the efferocytosis of macrophages within the plaque is impaired, which leads to the inability of macrophages to promptly remove the apoptotic cells (ACs) from the organism promptly, causing exacerbation of AS. Moreover, upregulation of CD47 expression in AS plaques also protects ACs from phagocytosis by macrophages, resulting in a large amount of residual ACs in the plaque, further expanding the necrotic core. In this review, we discussed the molecular mechanisms involved in the process of efferocytosis and how efferocytosis is impaired and regulated during AS, hoping to provide new insights for treating AS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases (CADs) have become a serious threat to human health. AS is a chronic disease of the arterial wall and the pathological basis of most CADs. The pathological basis of AS is abnormal lipid metabolism, characterized by lipid accumulation in the arteries and plaque formation. However, a large amount of lipid deposition in macrophages can form foam cells. It has been shown that the removal of foam cells must be accomplished by efferocytosis [1]. Efferocytosis is a programmed method of removing apoptotic or senescent cells [2]. It also plays an irreplaceable role in reducing inflammation and repairing damage caused by inflammation [3]. In addition, it has been shown that macrophages can effectively remove foam cells through efferocytosis, attenuating the local inflammatory response and secondary cell necrosis in the early stages of AS, thereby delaying the progression of plaques [4]. With the development of AS, the effect of efferocytosis is weakens, leading to a significant reduction in both the speed and effectiveness of cell clearance. Schrijvers et al. presented the most robust evidence for impaired efferocytosis during human AS formation, showing that the proportion of free versus phagocytosed ACs was 19 times higher in human atherosclerotic plaques than in human tonsils [5]. In the past decade, several studies have discovered the mechanisms of efferocytosis, and some therapeutic directions have been proposed for the study of the mechanism, such as targeting to promote the transformation of macrophages to the M2 phenotype or using CD47 antibody. In this paper, we summarize the latest research status of the mechanism and the cause of injury in the hope of providing new targets for the treatments for CADs.
Definition of efferocytosis
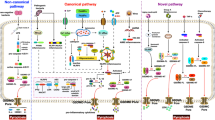
The human body undergoes a massive cellular turnover daily, with around 200 to 300 billion cells being replaced. Although a large number of cells go through apoptosis, it is quite challenging to detect them in the body tissues. This is because the process of efferocytosis is highly efficient in removing apoptotic cells. Efferocytosis is the last stage of apoptosis, and its primary function is to stop inflammation and promote degenerative processes [6]. In contrast, most types of phagocytosis, like Fc receptor-mediated phagocytosis, trigger an immune response. The survival of multicellular organisms must maintain a balance between cell death and efficient clearance of dead cells. However, suppose the body is incapable of efferocytosis. In that case, it can disrupt tolerance to autoantigens and secondary necrosis, exacerbating chronic inflammatory and autoimmune diseases such as CADs, rheumatoid arthritis, and systemic lupus erythematosus [7]. There are three distinct categories of phagocytes involved in the process of efferocytosis - professional, non-professional, and specialized. Efferocytosis is mainly performed by macrophages in highly phagocytic “professional” phagocytes, and to a lesser extent by other highly phagocytic “professional” phagocytes, such as monocytes and dendritic cells [3]. Also, non-professional phagocytes such as endothelial cells, fibroblasts, and smooth muscle cells can contribute to efferocytosis [8]. Non-professional phagocytes have lower phagocytosis efficiencies compared with professional phagocytes. However, they are still important in promoting efferocytosis in tissues where macrophages are scarce or inaccessible to ACs [9]. Additionally, Lu et al. also discovered that non-specialized phagocytes should be the first line of defense against necrotic cell-induced local inflammation by phagocytosing necrotic cells [10]. Specialized phagocytes have both phagocytic and non-phagocytic functions that are specific to the tissues they serve. For example, in the testis, Sertoli cells are specialized phagocytes that can clear apoptotic germ cells continuously. Efferocytosis is a multi-step, complex, and strictly regulated process influenced by factors such as the phagocyte-to-AC ratio, phagocyte type, and stimulating and signaling molecules [11]. Furthermore, efferocytosis can be simply summarized as follows: ACs release “Find me” signals that attract macrophages. These macrophages bind to and absorb ACs, leading to digestion and degradation. The process can be broken down into three steps: the “Find me” stage, the “Eat me” stage, and the endocytosis stage. All three phases involve signaling molecules that also impact efferocytosis.
“Find me” stage
First, ACs release “Find me” signals such as CX3C-chemokine ligand 1 (CX3CL1) and lyso-phosphatidylcholine (LPC) (Table 1), which attract macrophages through chemotaxis, recruiting and guiding their migration to ACs. Simultaneously, “Find me” signals regulate the cytoskeleton of phagocytes and amplify the expression of phagocytosis receptors and digestive mechanisms to prepare for the uptake of ACs [12, 13]. In addition, to achieve specific chemotaxis of phagocytes such as monocytes and macrophages, ACs release “keep out” signals, like lactoferrin (LF) and Annexin-A1 (ANXA1), prohibiting the recruitment of other inflammatory cells, including granulocytes [14]. CX3CL1 is a membrane protein expressed predominantly in macrophages, dendritic cells, endothelial cells, and neurons. In apoptosis, CX3CL1 will be cleaved by disintegrin and metalloproteinases [15]. The secreted CX3CL1 then associates with CX3C motif chemokine receptor 1 (CX3CR1) on macrophages and microglia, which attracts phagocytes to AC sites [16]. LPC, one of the earliest recognized signals, can be synthesized by phosphatidylcholine via caspase-3-activated calcium-independent phospholipase A2 [17], which will subsequently be released by ATP-binding cassette transporter A1 (ABCA1). LPC recognizes and binds to the target G-protein-coupled receptor G2A, thereby initiating migration and engulfment of ACs [18].
“Eat me” stage
ACs exhibit extramembrane structural changes, leading to changes in external lipids or carbohydrates. For instance, phosphatidylserine (PS), which is present exclusively on the surface of living cell membranes, ectopically binds to the extracellular surface of ACs through a cysteine-aspartate protease cascade-dependent reaction as an “Eat me” signal (Table 1) and binds to macrophage surface proteins, including stabilin 1, T cell immunoglobulin mucin receptor 1 (TIM1), and Adhesion G protein-coupled receptor B1 (ADGRB1) and activates macrophage [30]. This can distinguish ACs from other healthy neighboring cells. Moreover, it is interesting that live cells exposed to PS differ from ACs. Therefore, stimulation of live cells does not induce phagocytosis [30]. The presence of the “Eat me” signals guarantees specific recognition of ACs, and the same can be said for calreticulin (CALR), oxidized low-density lipoprotein (ox-LDL), ICAM-3, and so on. Additionally, macrophages can enhance efferocytosis by combining with bridging molecules that mediate binding between PS on ACs and receptors on macrophages. For example, growth arrest-specific protein 6 (Gas6) and protein S link externalized PS on ACs to TAM receptor tyrosine kinases (TAMs), including TYRO3, AXL, and MerTK [47]. Another bridging molecule, MFG-E8, connects PS on ACs to integrins αVβ3 and αVβ5 [48]. Low-density lipoprotein receptor-related protein (LRP) can interact with PS via the bridging molecule β-2-glycoprotein 1 to promote macrophage phagocytosis of ACs [49]. Significantly, not all ACs are exposed to the same “eat me” signal, and a subset of them with PS seem to be sufficiently recognized by specific receptors on phagocytes.
While healthy cells occasionally exhibit “Eat me” signals, the body has a mechanism to prevent them from being engulfed. Healthy cells display “Don’t eat me” signals, like CD47, CD31, and CD24, that bind to phagocyte receptors, thus avoiding phagocytosis [50]. As an example, CD47 and CD24 can respectively bind to receptor signaling regulatory protein-alpha (SIRPα) and Siglec-10 in macrophages and inhibit efferocytosis [51, 52]. Antibody-dependent cellular phagocytosis is also negatively regulated by the CD47-SIRP axis. Meanwhile, the phagocytic pathway can also be hindered by inhibiting enzymes required for the phagocytic process, like SH2-containing protein tyrosine phosphatase-1 (SHP-1) and SHP2. Alternatively, signaling by LILRB1 inhibits the activation of endocytosis [3].
Endocytosis stage
Macrophage binding to “Eat me” signals directly or indirectly via bridging molecules activates RHO family proteins, such as the Rho subfamily (RhoA and RhoB) and the Rac subfamily (Rac1, Rac2, etc.), which promote the triggering of dynamic actin remodeling and the formation of phagosomes through changes in membrane morphology to mediate internalization of ACs [53]. Among RHO family proteins, the collaboration of Rac1 and RhoA enables the endocytosis process to occur rapidly and accurately. Rac1 located near the binding site can facilitate the polymerization of G-actin into F-actin, followed by the formation of phagocytic cups for endocytosis [50]. It has recently been shown that macrophages can take up amino acids from ACs during efferocytosis and activate Rac1 to enhance efferocytosis through amino acid metabolism [54]. In contrast, RhoA stimulates and activates the retraction of phagocytic cups towards the macrophage, terminating endocytosis [55]. After successful phagocytosis of ACs, macrophages accumulate large amounts of proteins, nucleotides, and lipids, thereby contributing to the energy burden of macrophages. Therefore, macrophages undergo multiple rounds of efferocytosis to prevent tissue necrosis and establish a microenvironment conducive for wound repair [56]. This allows the macrophages to clear a variety of ACs in a short time, which is known as continuous efferocytosis [57]. Interestingly, continuous efferocytosis, distinct from single efferocytosis, relies on the processing of apoptotic cell-derived cargo [57]. This process becomes particularly important in cases where the number of dead cells exceeds the number of macrophages, such as in many injuries in vivo (Fig. 1) [56].
The process of efferocytosis. ACs, apoptotic cells; ox-LDL, oxidized low-density lipoprotein; PS, phosphatidylserine; ATP, adenosine triphosphate; UTP, uridine triphosphate; LPC, lyso-phosphatidylcholine; Mø, macrophages; Gas6, growth arrest-specific protein 6; M1, classically activated macrophages; M2, alternatively activated macrophages
Molecules related to efferocytosis in AS
Mer tyrosine kinase (MerTK)
MerTK is expressed in macrophages and plays an important role in maintaining efferocytosis and preventing the development of atherosclerotic lesions. Activation of MerTK increases the synthesis and expression of the bridging molecule Gas6, which mediates the phagocytosis of ACs, thereby increasing the clearance of ACs [58]. A recent study showed that MerTK expression is also sensitive to constitutive putrescine synthesis, as deletion of ornithine decarboxylase ODC reduced MerTK expression and prevented AS elimination [59]. Also, during AS, multiple pro-inflammatory stimuli activate a disintegrin and metalloprotease 17 (ADAM17), and then ADAM17 cleaves MerTK and impairs efferocytosis [60]. It has been found that in LDLR−/− mice, MerTK-dependent efferocytosis increases the production of specialized pro-resolving mediators and results in smaller necrotic cores and thickened fibrous caps in the plaques [61]. The area of necrotic plaques in AS increased in MerTK−/−/ApoE−/− mice, and the reduced efferocytosis capacity, accumulation of ACs, increased inflammation, and larger necrotic core of as plaques in MerTK−/−/LDLR−/− mice [61]. Consistently, loss of MerTK function, either by direct deletion or by replacement of endogenous MerTK with a form containing an inactive kinase structural domain, has deleterious effects on AS induced by defective efferocytosis.
LDL receptor-related protein 1 (LRP1)
LRP1 receptor is present in macrophages and vascular smooth muscle cells. It promotes the regression of atherosclerotic plaques by promoting efferocytosis process and promoting the anti-inflammatory macrophage phenotype M2 [62]. LRP1 synergizes with its co-receptor CRT to enhance phagocytosis of ACs by acting on PS, which in turn ligates the complement factor C1q. After transplantation of LDLR−/− mice into the bone marrow of LRP1 deficient mice, the efferocytic capacity was decreased, the residual ACs in the lesion increased, the necrotic core area increased, and the formation of AS plaques was accelerated [63]. Furthermore, deficiency of bone marrow-derived macrophage LRP1 leads to significant inhibition of efferocytosis, accumulation of macrophages apoptosis and, enlargement of necrotic core [62]. These results indicate that LRP1 plays a vital role in regulating the balance between apoptosis and efferocytosis in macrophage, which is crucial for developing anti-AS. Nevertheless, it has been shown that the LRP1 receptor leads to the formation of foam cells by enhancing the uptake of LDL by macrophages, which play a key role in the development and progression of AS [64]. Moreover, Mueller et al. found that deleting macrophage LRP1 negates the blockade of CD47, hindering efferocytosis. In contrast, loss of LRP1 enhances plaque regression [65]. The two studies mentioned above suggest a more complex mechanism by which LRP1 affects efferocytosis. Further research is necessary to determine the relationship among LRP1, efferocytosis and AS.
Scavenger receptor class B type I (SR-BI)
SR-BI is a scavenger receptor responsible for the uptake of cholesteryl esters by liver and steroidogenic tissues playing a crucial role in regulating the endothelial transport of low-density lipoproteins in an AS mouse model [66]. It has been found that SR-BI can directly connect to PS, leading to phosphorylation of Src kinase, phosphorylation and subsequent activation of phosphoinositide 3-kinase (PI3K) and Rac1 proteins and downstream signals, thereby facilitating AC clearance and reducing intra-plaque inflammation [62]. In contrast, macrophage-specific knockdown of SR-BI exhibited impaired efferocytosis function in both mice and isolated cells. It could significantly cause upregulation of the expression of inflammatory factors, including interleukin-6 (IL-6), IL-1β, and tumor necrosis factor α (TNF-α), as well as downregulation of the expression of anti-inflammatory factors such as transforming growth factor β (TGF-β) [33, 67]. In addition, the deletion of SR-BI in macrophages from ApoE−/− mice accelerated the progression of AS, and an accumulation of ACs, an enlargement of the necrotic core, and a thinning of the fibrous cap were observed at the lesion. Pharmacological activation of Rac1 in vitro can correct the impaired efferocytosis caused by defective SR-BI in macrophages, suggesting that impaired SR-BI signaling may affect other efferocytosis pathways. Indirectly confirming the above possibility, a study found that macrophages with SR-BI deletion showed increased expression of the inflammatory factor high mobility group box 1 and could further decrease the activity of Rac1, resulting in reduced efferocytosis [68].
Extracellular signal-regulated kinase 5 (ERK5)
ERK5 is one of the mitogen-activated protein kinase families and one of the major molecules involved in the regulation of efferocytosis. ERK5 can not only enhance the expression of “Find me” and “Eat me” signaling molecules but also regulate the expression of various phagocytic receptors and bridging molecules on the macrophage membrane, including MerTK, C1q, and Gas6, thus enhancing the efferocytosis of macrophages [69]. It has been demonstrated that the upregulation of C1qA expression by activating ERK5 increases the efferocytosis in macrophages loaded with ox-LDL [46]. Additionally, the activation of ERK5 also promotes the transformation of macrophages into an M2 anti-inflammatory repair phenotype, thereby enhancing efferocytosis, reducing the inflammatory response, and limiting the development of AS [46].
Cluster of differentiation 47 (CD47)
CD47 is a supramolecular complex consisting of specific integrins, G proteins, and cholesterol. It serves as a crucial “Don’t eat me” signaling molecule widely found on cell membranes. In the development of AS, CD47 also plays an essential role by downregulating its expression, redistributing CALR, and activating LRP1 via the CALR-LRP1 signaling pathway, resulting in increased efferocytosis [65, 70]. However, the expression of CD47 in atherosclerotic plaques remains unaltered, and it facilitates SHP-1 phosphorylation by binding to SIRP-α, inactivating myosin assembly, therefore preventing cytoskeletal rearrangement around phagosomes and sparing ACs from macrophage phagocytosis, resulting in a large number of residual ACs in the plaques, which further enlarges the necrotic core [71]. Kojima et al. showed that the administration of CD47 antibody in the AS mice model inhibited SHP-1 phosphorylation and blocked the CD47 signaling axis downstream of SIRP-α resulting in a significant decrease in AS and a reduction in the number of macrophage-independent free apoptotic bodies (indicative of the extent of damage to the vesicular process) and necrotic core within the plaques [34]. There is increasing evidence that certain atherosclerotic lncRNAs are involved in lipid homeostasis, such as cholesterol uptake modified lipoproteins uptake, and reverse cholesterol transport, which affects AS progression [72, 73]. Myocardial infarction associated transcript (MIAT) is a highly conserved mammalian lncRNA. In addition, it MIAT was found to be significantly elevated in serum with symptoms of vulnerable atherosclerotic plaques and in macrophages with necrotic cores in a mouse model of advanced AS [74]. MIAT knockdown promoted the clearance of ACs by macrophages, attenuated AS progression, reduced necrotic core size, and increased plaque stability in vivo. The mechanistic research suggests that MIAT competitively binds miR-149-5p to act as a decoy, blocking its interaction with CD47, increasing ox-LDL-induced of CD47 upregulation, and promoting defective efferocytosis and plaque vulnerability [74].
Milk fat globule epidermal growth factor 8 (MFGE8)
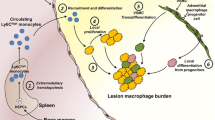
MFGE8 is one of the major glycoproteins of the milk fat globular membranes [75]. MFGE8 recognizes PS through its C-terminus and binds to αvβ3 and αvβ5 receptors on phagocytes [48], acting as an adhesion and signaling molecule involved in critical cellular processes such as phagocytosis, migration and proliferation. Additionally. MFGE8 also directly binds to TG2, promoting cholesterol reversal and preventing plaque progression. LDLR−/− mice lacking TG2 also showed increased plaque area and expanded necrotic core [76]. It has been proven that in a mouse model of AS, lack of MFGE8 expression in bone marrow-derived cells causes a massive accumulation of apoptotic debris within lipid lesions [44]. Moreover, in the absence of MFGE8, dendritic cell-dependent alterations in natural regulatory T-cell function show impaired regulatory immune responses, all of which can accelerate plaque formation and AS lesions (Fig. 2) [44].
Binding sites of macrophages and ACs during efferocytosis. ↓, inhibitory effect; ACs, apoptotic cells; PS, phosphatidylserine; C1q, complement component 1q; LRP1, LDL receptor-related protein 1; ADAM17, a disintegrin and metalloprotease 17; SR-BI, scavenger receptor class B type I; Gas6, growth arrest-specific protein 6; MerTK, Mer tyrosine kinase; CD47, cluster of differentiation 47; SIRPα, signal regulatory protein; SHP-1, SH2-containing protein tyrosine phosphatase-1
Efferocytosis damage and regulatory mechanism during AS process
Studies have shown that in the early stage of AS, macrophages undergo a robust efferocytic process, effectively restricting the progression of AS plaques. However, with the development of the disease, macrophages show a decline in efferocytic capacity, leading to the accumulation of AC apoptotic bodies in plaques, secondary necrosis formation, and the formation of “inflammatory nuclei” in vessels [5, 77, 78].
Impaired clearance of ACs
CD47, the “Don’'t eat me” signal on ACs, is up-regulated with lesion progression in a TNF-α dependent manner during lesion progression, preventing efficient clearance of ACs and decreasing efferocytosis capacity. However, administration of a CD47-blocking antibody in ApoE−/− mice improves lesion efferocytosis and reduces necrotic core areas [34]. Furthermore, ACs within lesions exhibit decreased levels of surface “Eat me” signaling on their surface. Kojima et al. found that TNF-α induces a sustained upregulation of CD47 expression in human AS [34]. It has been confirmed that the deletion of cyclin dependent kinase inhibitor 2B (Cdkn2B) gene at the risk site of chromosome 9p21 in atherosclerotic plaques leads to the down-regulation of CALR expression and resistance to macrophage internalization, which enlarges the necrotic core at the lesion and impairs the average clearance of ACs [31]. Therefore, there is ongoing debate regarding the potential suppression of atherosclerotic lesions by inhibiting the expression of this gene and thereby promoting efferocytosis.
Lack of bridging molecules
When phagocytes and ACs bind indirectly, the bridging molecule plays a crucial role in connecting the two entities. Thus, the absence of the bridging molecule leads to impaired clearance of ACs. The inflammatory response environment within the plaque affects the expression levels of signaling molecules related to efferocytosis. The toll-like receptor signaling related to inflammatory response will downregulate the expression level of MFGE8 and inhibit the activities of “Eat me” receptor ligands MerTK and LRP1, thereby impairing the ability of efferocytosis [79]. In addition, Bhatia et al. found that AS plaques exhibited reduced C1q expression since lesions in C1qA−/−/LDLR−/− mice were nearly 3 times larger than those in LDLR−/− mice [45].
Impaired phagocytosis of macrophages
According to the macrophage phenotype and molecular surface markers, macrophages in AS can be divided into proinflammatory M1-type macrophages and anti-inflammatory M2-type macrophages. The M2-type macrophages were found to possess a higher phagocytosis capacity and play a role in addressing inflammation within plaques [80]. However, monocytes are more likely to differentiate into M1-type macrophages under inflammatory conditions, decreasing the number of M2-type macrophages and thus reducing the efferocytic capability of macrophages. M2 macrophages are polarized by Th2 cytokines such as IL-4 and IL-13, with subsequent secretion of anti-inflammatory cytokines such as IL-10 and TGF-β which are utilized to inhibit inflammatory cell recruitment [81]. In addition, M2 macrophages express high levels of arginase 1 and increase collagen secretion, promoting tissue repair [82]. Besides, inflammatory factors and proteases produced by M1-type macrophages, such as interleukin-12 (IL-12), monocyte chemotaxis protein-1 (MCP-1), ADAM-17, and TNF-α, can weaken macrophage recognition receptors [83,84,85]. Expression of various matrix metalloproteinases is elevated in atherosclerotic plaques, and ADAM17 has been identified as the cause of MerTK and LRP1 deficiency [61, 84].
Disruption of the regulatory mechanisms
Doran et al. found that CaMKII γ-deficient macrophages in atherosclerotic lesions exhibit increased expression of the transcription factor ATF6. ATF6 induces liver X receptor-alpha (LXR-α), and increased MerTK expression and efferocytosis in CaMKII γ-deficient macrophages is dependent on LXR-α [86]. Besides, Brophy et al. found that ox-LDL treatment increased the ubiquitination of LRP1, which subsequently binds to epsin and is internalized from the cell surface, suggesting that epsin promotes LRP1 ubiquitin-dependent internalization and downregulation and decreases efferocytosis [87].
After phagocytosis of ACs or lipids, macrophages metabolize them by increasing cholesterol esterification, cholesterol efflux, and the activation of PI-3 kinase/Akt and NF-κB pathways [88]. For example, lysosomal acid lipase (LIPA) not only hydrolyzes fatty substances on CD36 scavenger receptor-mediated endocytosis and promotes mitochondrial fatty acid oxidation but also hydrolyzes cholesteryl esters at the site of fusion of lipid droplets with lysosomes and support free cholesterol efflux [89]. In addition, defective efferocytosis is a sign of inadequate resolution of inflammation, which is mediated by specialized pro-decomposing lipid mediators (SPMs) derived from omega-3 fatty acids or arachidonic acid, as well as associated protein and signaling molecules. SPM production is dependent on the cellular environment. Under inflammatory condition, 5-lipoxygenase (5-LOX) in macrophages is phosphorylated, leading to its translocation to the nuclear membrane and interaction with FLAP (5-LOX activating protein) to promote leukotriene B4 (LTB4) synthesis. In a non-inflammatory condition, 5-LOX remains non-phosphorylated and localizes to the cytoplasm to promote LXA4 (lipoprotein A4) production [58]. Signaling through the MerTK receptor increases 5-LOX dephosphorylation and increases LXA4 production [90]. Continuous efferocytosis is regulated by efferocytosis-activated SLC and other molecules. For example, SLC2A1 induces aerobic glycolysis and inhibits oxidative phosphorylation, contributing to continuous efferocytosis. Morioka et al. found that knockdown of SLC2A1 with siRNA reduces uptake into ACs, which is rescued by siRNA-resistant SLC2A1 [91].
Regulation of efferocytosis by miRNAs
There is growing evidence that non-coding RNAs are associated with efferocytosis and are involved in epigenetic regulation by modulating gene expression. Defects in efferocytosis may result from dysregulation of the expression and function of microRNAs (miRNAs), non-coding RNAs involved in the post-transcriptional regulation of gene expression. miR-155 has been shown to inhibit macrophage-mediated efferocytosis and enhance foam cell aggregation in atherosclerotic lesions by suppressing Bcl6 expression [92]. Bcl6 is a potent transcriptional inhibitor highly expressed in advanced atherosclerotic lesions. It may indirectly block RhoA activation; thus, inhibition of Bcl6 by miR155 contributes to the overactivation of RhoA, which negatively affects cytoskeletal remodeling in macrophages and impairs efferocytosis.
It was found that a macrophage-specific lncRNA MAARS (macrophage-associated atherosclerosis lncRNA sequence) expression in the aortic intima-media increased by 270-fold with the progression of AS and decreased by 60% with regression [93]. Moreover, deletion of MAARS in macrophages significantly reduced apoptotic markers and enhanced macrophage-mediated vesiculation. Overexpression and knockdown studies confirmed that MAARS is a key regulator of macrophage apoptosis and increased vesiculation in vitro [93].
Future directions and challenges
Although numerous medications are available to lower cholesterol levels, AS remains the leading cause of death worldwide. Furthermore, conventional treatments rarely focus on efferocytosis. A variety of efferocytosis receptors such as MerTK and LRP1 are associated with anti-AS, and by downregulating and upregulating the expression of certain signaling molecules, it may be possible to achieve the enhancement of macrophage efferocytosis at the lesion and thus inhibit the development of AS. In addition, statins, as one of the commonly used drugs for the treatment of AS, are associated not only with the ability to reduce LDL levels but also to enhance macrophage efferocytosis to achieve anti-AS effects [94]. Guanxinkang decoction, a traditional Chinese medicinal drug, upregulated the expression of efferocytosis-associated molecules such as AXL, MerTK, TYRO3, and MFGE8 and increased phagocytosis of ox-LDL-induced RAW264.7 cells, which suggests that Guanxinkang ameliorates AS by enhancing efferocytosis [94]. Isoflurane enhances the clearance of ACs by mouse macrophages through the AMPK-ADAM17-Mer signaling pathway, promoting inflammation recovery by enhancing macrophage efferocytosis [95]. Bories et al. found that nagilactone B, a specific agonist of LXR, promotes macrophage M2 polarization and vesiculation and reduces plaque formation and necrotic core area, suggesting that targeting macrophage polarization to modulators of the M2 phenotype may be a promising therapeutic strategy for AS [96].
However, there are still many shortcomings in the current studies on the impairment of efferocytosis during AS. For example, the role of most efferocytosis-related signaling molecules in AS has not been elucidated and whether there are specific signaling molecules involved in the process of AS needs further exploration and research. Additionally, a recent study reported that CD47-deficient mice were predicted to protect against atherosclerosis, but in fact, some mice had increased lesion formation due to increased lymphocyte activation [97]. Moreover, efferocytosis is prevalent in all tissues and organs of the body and involves highly tissue-specific PS receptors and AC load. Therefore, an obvious challenge in pro-efferocytotic therapy is to target the right receptor type, while it also carries the risk of off-target [98]. In summary, with further relevant research, new therapeutic approaches targeting the activation and enhancement of efferocytosis will continue to emerge, hopefully bringing light to the treatment of AS.
Availability of data and material
Not applicable.
References
Ley K, Miller YI, Hedrick CC (2011) Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol 31(7):1506–1516
Golforoush P, Yellon DM, Davidson SM (2020) Mouse models of atherosclerosis and their suitability for the study of myocardial infarction. Basic Res Cardiol 115(6). https://doi.org/10.1007/s00395-020-00829-5
Boada-Romero E et al (2020) The clearance of dead cells by efferocytosis. Nat Rev Mol Cell Biol 21(7):398–414
Rahman MS, Woollard K (2017) Atherosclerosis. Adv Exp Med Biol 1003:121–144
Schrijvers DM et al (2005) Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol 25(6):1256–1261
Penberthy KK, Lysiak JJ, Ravichandran KS (2018) RethinkingPhagocytes: Clues from the Retina and Testes. Trends Cell Biol 28(4):317–327
Evans AL et al (2017) Antagonistic coevolution of MER tyrosine kinase expression and function. Mol Biol Evol 34(7):1613–1628
Parnaik R, Raff MC, Scholes J (2000) Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr Biol 10(14):857–860
Arandjelovic S, Ravichandran KS (2015) Phagocytosis of apoptotic cells in homeostasis. Nat Immunol 16(9):907–917
Lu J et al (2019) Efficient engulfment of necroptotic and pyroptotic cells by nonprofessional and professional phagocytes. Cell Discov 5:39
Morioka S, Maueroder C, Ravichandran KS (2019) Living on the edge: efferocytosis at the interface of homeostasis and pathology. Immunity 50(5):1149–1162
Medina CB, Ravichandran KS (2016) Do not let death do us part: “find-me” signals in communication between dying cells and the phagocytes. Cell Death Differ 23(6):979–989
Medina CB et al (2020) Metabolites released from apoptotic cells act as tissue messengers. Nature 580(7801):130–135
Bournazou I et al (2009) Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J Clin Invest 119(1):20–32
Lee M et al (2018) Tissue-specific role of CX(3)CR1 expressing immune cells and their relationships with human disease. Immune Netw 18(1):e5
Truman LA et al (2008) CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood 112(13):5026–5036
Lauber K et al (2003) Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113(6):717–730
Peter C et al (2012) Release of lysophospholipid “find-me” signals during apoptosis requires the ATP-binding cassette transporter A1. Autoimmunity 45(8):568–573
Apostolakis S, Spandidos D (2013) Chemokines and atherosclerosis: focus on the CX3CL1/CX3CR1 pathway. Acta Pharmacol Sin 34(10):1251–1256
Gu Y et al (2015) Defective apical extrusion signaling contributes to aggressive tumor hallmarks. Elife 4:e04069
Matsumoto T, Kobayashi T, Kamata K (2007) Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr Med Chem 14(30):3209–3220
Ferrari D et al (2015) Purinergic signaling in atherosclerosis. Trends Mol Med 21(3):184–192
Zhao X, Kruzel M, Aronowski J (2021) Lactoferrin and hematoma detoxification after intracerebral hemorrhage. Biochem Cell Biol 99(1):97–101
Chen C et al (2023) The role of lactoferrin in atherosclerosis. Biometals 36(3):509–519
da Rocha GHO et al (2019) Control of expression and activity of peroxisome proliferated-activated receptor gamma by Annexin A1 on microglia during efferocytosis. Cell Biochem Funct 37(7):560–568
Li YZ et al (2022) Annexin A protein family in atherosclerosis. Clin Chim Acta 531:406–417
Frasch SC et al (2011) Signaling via macrophage G2A enhances efferocytosis of dying neutrophils by augmentation of Rac activity. J Biol Chem 286(14):12108–12122
Cui X et al (2021) The G2A receptor deficiency aggravates atherosclerosis in rats by regulating macrophages and lipid metabolism. Front Physiol 12:659211
Barnawi J et al (2017) Reduced DNA methylation of sphingosine-1 phosphate receptor 5 in alveolar macrophages in COPD: a potential link to failed efferocytosis. Respirology 22(2):315–321
Birge RB et al (2016) Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ 23(6):962–978
Kojima Y et al (2019) Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J Clin Invest 129(5):2164
Khatana C et al (2020) Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxid Med Cell Longev 2020:5245308
Tao H et al (2015) Macrophage SR-BI mediates efferocytosis via Src/PI3K/Rac1 signaling and reduces atherosclerotic lesion necrosis. J Lipid Res 56(8):1449–1460
Kojima Y et al (2016) CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536(7614):86–90
Caligiuri G (2020) CD31 as a therapeutic target in atherosclerosis. Circ Res 126(9):1178–1189
Manta CP et al (2022) Targeting of scavenger receptors stabilin-1 and stabilin-2 ameliorates atherosclerosis by a plasma proteome switch mediating monocyte/macrophage suppression. Circulation 146(23):1783–1799
Lee W et al (2018) Macrophagic stabilin-1 restored disruption of vascular integrity caused by sepsis. Thromb Haemost 118(10):1776–1789
Foks AC et al (2016) Blockade of Tim-1 and Tim-4 enhances atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 36(3):456–465
Foks AC et al (2013) T-cell immunoglobulin and mucin domain 3 acts as a negative regulator of atherosclerosis. Arterioscler Thromb Vasc Biol 33(11):2558–2565
Mueller PA et al (2018) Deletion of macrophage low-density lipoprotein receptor-related protein 1 (LRP1) accelerates atherosclerosis regression and increases C-C chemokine receptor type 7 (CCR7) expression in plaque macrophages. Circulation 138(17):1850–1863
Cai B, Kasikara C (2020) TAM receptors and their ligand-mediated activation: role in atherosclerosis. Int Rev Cell Mol Biol 357:21–33
Tian K et al (2020) CD36 in atherosclerosis: pathophysiological mechanisms and therapeutic implications. Curr Atheroscler Rep 22(10):59
Hurtado B et al (2011) Expression of the vitamin K-dependent proteins GAS6 and protein S and the TAM receptor tyrosine kinases in human atherosclerotic carotid plaques. Thromb Haemost 105(5):873–882
Ait-Oufella H et al (2007) Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 115(16):2168–2177
Bhatia VK et al (2007) Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol 170(1):416–426
Heo KS et al (2014) ERK5 activation in macrophages promotes efferocytosis and inhibits atherosclerosis. Circulation 130(2):180–191
McShane L et al (2019) TAM receptors in cardiovascular disease. Cardiovasc Res 115(8):1286–1295
Hanayama R et al (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417(6885):182–187
Maiti SN et al (2008) Beta-2-glycoprotein 1-dependent macrophage uptake of apoptotic cells. Binding to lipoprotein receptor-related protein receptor family members. J Biol Chem 283(7):3761–6
Nakaya M et al (2008) Spatiotemporal activation of Rac1 for engulfment of apoptotic cells. Proc Natl Acad Sci USA 105(27):9198–9203
Barkal AA et al (2019) CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 572(7769):392–396
Oldenborg PA et al (2000) Role of CD47 as a marker of self on red blood cells. Science 288(5473):2051–2054
Miki H, Suetsugu S, Takenawa T (1998) WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J 17(23):6932–6941
Yurdagul A Jr et al (2020) Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell Metab 31(3):518–533
Kim SY et al (2017) Coordinated balance of Rac1 and RhoA plays key roles in determining phagocytic appetite. PLoS ONE 12(4):e0174603
Yurdagul A Jr (2021) Metabolic consequences of efferocytosis and its impact on atherosclerosis. Immunometabolism 3(2). https://doi.org/10.20900/immunometab20210017
Kumar D, Pandit R, Yurdagul A Jr (2023) Mechanisms of continual efferocytosis by macrophages and its role in mitigating atherosclerosis. Immunometabolism (Cobham) 5(1):e00017
Cai B et al (2016) MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc Natl Acad Sci USA 113(23):6526–6531
Yurdagul A Jr et al (2021) ODC (ornithine decarboxylase)-dependent putrescine synthesis maintains MerTK (MER tyrosine-protein kinase) expression to drive resolution. Arterioscler Thromb Vasc Biol 41(3):e144–e159
Thorp E et al (2011) Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cdelta, and p38 mitogen-activated protein kinase (MAPK). J Biol Chem 286(38):33335–33344
Cai B et al (2017) MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Invest 127(2):564–568
Yancey PG et al (2010) Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arterioscler Thromb Vasc Biol 30(4):787–795
Yancey PG et al (2011) Low-density lipoprotein receptor-related protein 1 prevents early atherosclerosis by limiting lesional apoptosis and inflammatory Ly-6Chigh monocytosis: evidence that the effects are not apolipoprotein E dependent. Circulation 124(4):454–464
Chen J et al (2021) The dual role of low-density lipoprotein receptor-related protein 1 in atherosclerosis. Front Cardiovasc Med 8:682389
Mueller PA et al (2022) Macrophage LRP1 (low-density lipoprotein receptor-related protein 1) is required for the effect of CD47 blockade on efferocytosis and atherogenesis-brief report. Arterioscler Thromb Vasc Biol 42(1):e1–e9
Huang L et al (2019) SR-B1 drives endothelial cell LDL transcytosis via DOCK4 to promote atherosclerosis. Nature 569(7757):565–569
Yu P et al (2018) PDZK1 in leukocytes protects against cellular apoptosis and necrotic core development in atherosclerotic plaques in high fat diet fed ldl receptor deficient mice. Atherosclerosis 276:171–181
Saddar S et al (2013) Scavenger receptor class B type I is a plasma membrane cholesterol sensor. Circ Res 112(1):140–151
Tajbakhsh A et al (2018) Efferocytosis in atherosclerotic lesions: malfunctioning regulatory pathways and control mechanisms. Pharmacol Ther 188:12–25
Henson PM (2017) Cell removal: efferocytosis. Annu Rev Cell Dev Biol 33:127–144
Barclay AN, Van den Berg TK (2014) The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol 32:25–50
Kumar S et al (2019) Role of flow-sensitive microRNAs and long noncoding RNAs in vascular dysfunction and atherosclerosis. Vascul Pharmacol 114:76–92
Sallam T, Sandhu J, Tontonoz P (2018) Long noncoding RNA discovery in cardiovascular disease: decoding form to function. Circ Res 122(1):155–166
Ye ZM et al (2019) LncRNA MIAT sponges miR-149-5p to inhibit efferocytosis in advanced atherosclerosis through CD47 upregulation. Cell Death Dis 10(2):138
Wang P (2014) MFG-E8 and inflammation. Springer, Netherlands
Toth B et al (2009) Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J Immunol 182(4):2084–2092
Roy P, Orecchioni M, Ley K (2022) How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat Rev Immunol 22(4):251–265
Chang MK et al (1999) Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA 96(11):6353–6358
Polykratis A et al (2012) Conditional targeting of tumor necrosis factor receptor-associated factor 6 reveals opposing functions of Toll-like receptor signaling in endothelial and myeloid cells in a mouse model of atherosclerosis. Circulation 126(14):1739–1751
Martinez FO, Helming L, Gordon S (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 27:451–483
Shapouri-Moghaddam A et al (2018) Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 233(9):6425–6440
Moore KJ, Sheedy FJ, Fisher EA (2013) Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13(10):709–721
Ma X (2001) TNF-alpha and IL-12: a balancing act in macrophage functioning. Microbes Infect 3(2):121–129
Xie Y et al (2022) Novel insight on the role of macrophages in atherosclerosis: focus on polarization, apoptosis and efferocytosis. Int Immunopharmacol 113(Pt A):109260
Ji X et al (2020) Sphingosine 1-phosphate/microRNA-1249-5p/MCP-1 axis is involved in macrophage-associated inflammation in fatty liver injury in mice. Eur J Immunol 50(11):1746–1756
Doran AC et al (2017) CAMKIIgamma suppresses an efferocytosis pathway in macrophages and promotes atherosclerotic plaque necrosis. J Clin Invest 127(11):4075–4089
Brophy ML et al (2019) Myeloid-specific deletion of epsins 1 and 2 reduces atherosclerosis by preventing LRP-1 downregulation. Circ Res 124(4):e6–e19
Cui D et al (2007) Pivotal advance: macrophages become resistant to cholesterol-induced death after phagocytosis of apoptotic cells. J Leukoc Biol 82(5):1040–1050
Viaud M et al (2018) Lysosomal cholesterol hydrolysis couples efferocytosis to anti-inflammatory oxysterol production. Circ Res 122(10):1369–1384
Cai B et al (2018) MerTK signaling in macrophages promotes the synthesis of inflammation resolution mediators by suppressing CaMKII activity. Sci Signal 11(549). https://doi.org/10.20900/immunometab20210017
Morioka S et al (2018) Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature 563(7733):714–718
Wei Y et al (2015) Regulation of Csf1r and Bcl6 in macrophages mediates the stage-specific effects of microRNA-155 on atherosclerosis. Arterioscler Thromb Vasc Biol 35(4):796–803
Simion V et al (2020) A macrophage-specific lncRNA regulates apoptosis and atherosclerosis by tethering HuR in the nucleus. Nat Commun 11(1):6135
Zhang Y et al (2021) Guanxinkang decoction attenuates the inflammation in atherosclerosis by regulating efferocytosis and MAPKs signaling pathway in LDLR(-/-) mice and RAW264.7 cells. Front Pharmacol 12:731769
Du X et al (2017) Isoflurane promotes phagocytosis of apoptotic neutrophils through AMPK-mediated ADAM17/Mer signaling. PLoS ONE 12(7):e0180213
Bories G et al (2013) Liver X receptor activation stimulates iron export in human alternative macrophages. Circ Res 113(11):1196–1205
Engelbertsen D et al (2019) Increased lymphocyte activation and atherosclerosis in CD47-deficient mice. Sci Rep 9(1):10608
Engelen SE et al (2022) Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nat Rev Cardiol 19(8):522–542
Funding
This work was supported by the Scientific Research Project of Hunan Provincial Department of Education (23A0338) and the Project of the Hunan Provincial Health Committee (D202302048902) and the College Student Innovation and Entrepreneurship Training Program of Hunan Province (S202210555279, S202310555323, S202310555104, and S202310555321), University of South China, China.
Author information
Authors and Affiliations
Contributions
Li-Xia Shu designed the review, prepared the figures, and wrote the review. Xin Guo prepared the table, consulted the literature, and co-wrote the review. Liu-li Cao revised the review. Zong-Bao Wang and Shu-Zhi Wang supervised the project, provided scientific direction, and revised the review.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shu, LX., Cao, Ll., Guo, X. et al. Mechanism of efferocytosis in atherosclerosis. J Mol Med 102, 831–840 (2024). https://doi.org/10.1007/s00109-024-02439-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-024-02439-3