Abstract
Polyurethane coatings were prepared by the "prepolymer mixing" method in two steps, i.e. synthesis of prepolymer and further the reaction of the obtained prepolymer with branched polyester. The synthesis of the urethane prepolymer in the reaction of aliphatic diisocyanates [2.4.4-trimethyl-1.6-hexamethylene diisocyanate (TMDI) and 3-isocyanato-3.5.5-trimethylcyclohexyl isocyanate (IPDI) and trimethylolpropane (TMP)] in xylene were carried out in presence of stannous 2-ethyl hexanoate. Next, the obtained prepolymer was exposed to the reaction with branched polyester polyols. This reaction was carried out in presence of the catalysts: DABCO, TEA and stannous 2-ethyl hexanoate. In the synthesis of polyurethanes the aliphatic diisocyanates with linear (TMDI) and cyclic (IPDI) structures were used. The polyurethanes obtained using these diisocyanates hadn't oxidized. Oxidation is disadvantageous particularly in case of the coating, containing aromatic diisocyanates. The changes in the quality are monitored by determining some properties of the cured coating, such as hardness, flexibility and scratch resistance.
Zusammenfassung
Polyurethan-Anstriche wurden mittels der Prepolymer-Misch-Methode in zwei Schritten vorbereitet und zwar die Synthese des Prepolymer und die weitere Reaktion des Prepolymers mit verzweigtem Polyester. Die Synthese des Urethan-Prepolymers aus aliphatischen Diisocyanaten wurde in Gegenwart von Zinn-2-ethylhexonat durchgeführt. Als nächstes wurde das erhaltene Prepolymer mit verzweigtem Polyester gekoppelt. Diese Reaktion wurde mittels der Katalysatoren: DABCO, TEA und Zinn-2-ethylhexonat durchgeführt. Bei der Synthese von Polyurethan wurden aliphatischen Dissocyanate mit linearen (TMDI) und zyklischen (IPDI) Strukturen verwendet. Die Polyurethane, die mittels dieser Diisocyanate gewonnen wurden, waren nicht oxidiert. Die Oxidation ist ungünstig, vor allem im Falle eines Anstrichs, der aromatische Diisocyanate enthält. Die Veränderungen in der Qualität wurden beobachtet durch Messen einiger Eigenschaften des Anstrichs, wie Härte, Flexibilität und Kratzfestigkeit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polyurethanes are a versatile class of polymers with a variety of applications, because their properties can easily be tailored by varying the components from which they are constructed: rigid diols, flexible polyols and polyisocyanates (Lai et al. 1995a). Presently, polyurethanes are used as elastomers, foams, adhesives and coating components with the object of finished wood surface.
Diisocyanate oligomers are widely used, because they are highly reactive and slightly toxic owing to low vapour pressure at ambient temperature. Diisocyanate trimers (Wejchan-Judek et al. 2001) or oligomers are used in the synthesis of polyurethane coatings and adhesives. These materials exhibit a high fire and a high thermal resistance. In the synthesis of polyurethanes generally the aromatic diisocyanates are used. The polyurethanes obtained with these diisocyanates are easily oxidized and this causes the colour change of the products. This oxidation is disadvantageous particularly in the case of coating. Therefore the aliphatic diisocyanates with linear or cyclic structures are used. However, the reactivity of these isocyanates in the presence of amino catalysts and stannous organic compounds is much lower in the synthesis of polyurethanes.
Usually, the synthesis of polyurethanes is carried out in two stages. The first stage is the synthesis of a prepolymer containing free isocyanate groups and in the second stage there are reactions of chain extension or crosslinking of the polymer chains.
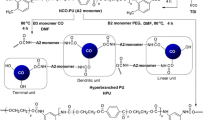
This paper reports the investigation of the effect of amino catalysts such as: 1.4-diazabicyclo[2.2.2]octane (DABCO), triethylamine (TEA) and stannous 2-ethyl hexanoate on the synthesis of urethane oligomers with isophorone diisocyanate (3-isocyanato-3.5.5-trimethylcyclohexyl isocyanate) (IPDI) or trimethyl-hexamethylene diisocyanate (2.4.4-trimethyl-1.6-hexamethylene diisocyanate) (TMDI) and trimethylolpropane (TMP) in xylene solution as well as investigation of the reaction of the obtained prepolymers with the branched polyester polyols. If higher functional isocyanates and/or polyols are used, a branched or even crosslinked polymer is formed (Brock et al. 2001). In the synthesis the aliphatic diisocyanates: IPDI (I) and TMDI (II) were used (Fig. 1).
From among the available aliphatic diisocyanates, only IPDI contains the first and the second order isocyanate groups. These groups show different reactivity in relation to hydroxyl groups in TMP (Lai et al. 1995b). The first order isocyanate groups are more reactive than the second order ones. This enables an easy use of the prepolymer method in the synthesis of polyurethanes as shown in Fig. 2, where in the first step of the reaction adduct of diisocyanate with trimethylolpropane was obtained (Eqn. 1). Then the obtained prepolymer was reacted with branched polyester polyol (Eqn. 2).
Moreover, IPDI contains methyl groups in the ring that facilitate solubility in many solvents. IPDI proves stability at increased temperatures and low concentration of monomer radicals in the crosslinking of polyurethanes. The reaction of the obtained prepolymers with the branched polyesters was carried out in presence of the above-mentioned catalysts.
The effect of wood species, wood moisture content and ratio of polyurethane coating components on the quality of finished wood surfaces is thus expressed by the adhesive strength of the coating (Jaic et al. 1997). The changes in quality are monitored by determining some properties of the cured coating, such as hardness, flexibility and scratch resistance.
2 Experimental
2.1 Synthesis of prepolymer
To xylene (160.2 g) TMP (26.8 g, 0.3 mole) was added and stirred intensely at temperature of 75°C, until TMP was dissolved. It was heated in an argon atmosphere. Then stannous 2-ethyl hexanoate (0.5%/100 cm3 a solution of TMP and IPDI) was added. The reaction mixture was heated at 80–90°C. IPDI (133.2 g, 0.9 mole) was added dropwise during about 5 h. The increased temperature allowed a total reaction of TMP with diisocyanates. The obtained prepolymer was poured into a dry, dark bottle. The bottle was air proof and kept at surrounding temperature. At this temperature the prepolymer was a colourless and viscous liquid. It was characterized by good stability for three months thanks to the lower reactivity of the second order isocyanate groups.
Under the same conditions the synthesis of the prepolymer containing TMP and TMDI was carried out. 40.2 g (0.3 mole) TMP and 189.3 g (0.9 mole) TMDI in 123.4 g xylene in the presence of stannous 2-ethyl hexanoate (0.5%/100 cm3 a solution of TMP and TMDI) were used.
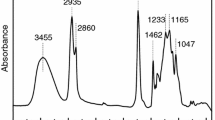
The obtained prepolymer did not contain free isocyanate groups. Also, IR spectrum did not reveal bands at 1550 cm−1 and 1740 cm−1 responsible for urea groups.
2.2 Synthesis of polyurethane
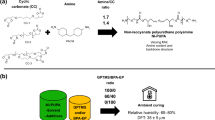
The reactions of the obtained prepolymers with the branched polyester polyols: Poles 50/23 (HV 45-55, MW 2300), Poles 80/14 (HV 53-60, MW 1400) and Poles 220/T (HV 240-280, MW 1200-1600) in the molar ratio of NCO groups in the prepolymer to OH groups in polyesters 1.2:1 were carried out. MW is designated weight—average molecular weight. The ratio of polyols and isocyanate components was calculated from the hydroxyl value (HV).
These reactions were carried out at surrounding temperature in the presence of the following catalysts: DABCO, TEA and stannous 2-ethyl hexanoate (0.5% in relation to a mass of the prepolymer and polyesters). These catalysts were also used in combinations.
The progress of the reaction was observed by examining the absorption band of isocyanate groups in the range of 2270 cm−1 and the rate of polymer crosslinking in the presence of researched catalysts.
3 Results and discussion
In the reaction of the prepolymers with IPDI or TMDI and Poles 50/23 in the presence of DABCO, TEA and stannous 2-ethyl hexanoate no homogenous solutions were obtained, even when heated to 60°C. The obtained solutions exhibited the tendency to gelatinize for 24 h. Based on the IR spectrum of the obtained polymer the absence of the isocyanate groups could not be stated.
The solutions of the polymers obtained from Poles 220/T were applied on wood and underwent crosslinking for 1 h. After 24 h they showed characteristic properties of a hardened coating. In contrast to the above described polymers, the polymers obtained from Poles 80/14 were characterised by a considerable stickiness after 3 h. In the case of the coatings obtained from Poles 50/23 the time of crosslinking was lengthened to 24 h.
Summing up, the reactivity of the studied polyester polyols in the reaction of the prepolymers with IPDI or TMDI was ranged in decreasing order as follows: Poles 220/T, Poles 80/14 and Poles 50/23.
The physic-mechanical researches concerning bending strength, hardness, flexibility and scratch resistance confirmed, that in case of the coatings obtained from Poles 80/14 and Poles 220/T, the DABCO and TEA system was the best.
As far as the light resistance of the coatings is concerned, the addition of these catalysts has a negative influence. In this respect, the stannous 2-ethyl hexanoate is the best catalyst.
References
Brock T, Groteklaes M, Mischke P (2001) Polyurethane coating systems. European Coatings Journal (1–2):110–111
Jaic M, Zivanovic R (1997) The influence of the ratio of the polyurethane coating components on the quality of finished wood surface. Holz als Roh- und Werkstoff 55(5):319–322
Lai YC, Quinn ET (1995a) Syntheses and characterization of UV-curable polisiloxane based polyurethane prepolymers. J Polym Sci Part A 33:1783–1793
Lai YC, Quinn ET, Valint PL (1995b) Control of hard segment size in polyurethane formation. J Polym Sci Part A 33:1767–1772
Wejchan-Judek M, Polus I, Doczekalska B, Pertek H (2001) Trimerization of 3-isocyanatomethyl-3.5.5-trimethylcyclohexyl isocyanate (IPDI). Polimery 46(2):131–132
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polus, I. Synthesis of polyurethane coating components with IPDI and TMDI. Holz Roh Werkst 61, 238–240 (2003). https://doi.org/10.1007/s00107-003-0390-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-003-0390-9