Abstract
Purpose
Infected wounds, such as diabetic foot infections, are mostly polymicrobial and microorganisms have high resistance rates to antimicrobials. Infected wounds in diabetic patients have high cost, morbidity, and mortality rates. Based on these facts, there is a need for supportive localized treatment options such as platelet-rich plasma (PRP) implementations. Demonstrating the in vitro antimicrobial effect, our aim was to lead up to clinical trials of localized PRP implementations in infected wounds such as diabetic foot infections. In this study, we aimed to demonstrate the in vitro antibacterial activity of PRP against methicilin-resistant Staphylococcus aureus (MRSA) and three more multi-drug resistant bacteria species that are important and hard-to-treat in wound infections.
Materials and methods
In vitro antimicrobial activity of autologous PRP, platelet-poor plasma (PPP), and phosphate-buffered saline (PBS) on methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus spp., extended spectrum beta lactamase producing Klebsiella pneumoniae, and carbapenem-resistant Pseudomonas aeruginosa was compared by assessment of bacterial growth on agar plates and antimicrobial susceptibility test results.
Results
When compared to control group, PRP and PPP significantly suppressed bacterial growth of MRSA, K. pneumoniae, and P. aeruginosa at 1st, 2nd, 5th, and 10th hours of incubation (p < 0.05). VRE was the only bacteria that PRP and PPP showed limited activity against. When compared to PPP, PRP showed higher activity against MRSA, K. pneumoniae, and P. aeruginosa. However, the differences between PRP and PPP were statistically significant only against MRSA and P. aeruginosa at the first hour of incubation.
Conclusions
Emerging PRP and other platelet-derived products seem to be promising alternative tools besides antibiotic treatment, debridement, negative pressure wound therapy, hyperbaric oxygen therapy, and other treatment options for treating diabetic foot infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antibiotics have broken a new ground to cure infections in the 20th century. However, antibiotic resistance is the leading factor to lose the gains of post-antibiotics era, and it is related to increase in mortality, morbidity, length of stay in the hospital, and hospital costs [1]. In addition, there are not enough emerging antimicrobial drugs to solve the antibiotic resistance problem in near future.
Infected wounds, such as diabetic foot infections, are one of the most serious infections to treat because of the polymicrobiality of infections and high resistance of microorganisms. Diabetic patients also have peripheral arteriopathy as a result of hypercoagulability, inflammation, endothelial dysfunction, and vascular smooth muscle cell dysfunction [2]. Thus, peripheral blood cells, such as erythrocytes, thrombocytes, leukocytes, and also systemic treatment options cannot reach deep enough to the infected site [3].
In view of these reasons, surgical procedures, such as localized debridement, negative pressure vacuum implementations, intra-lesional epidermal growth factor (EGF), and platelet-rich plasma (PRP) implementations, remain under investigation as a supportive therapy options [2]. As a result of the improvements in new molecular procedures in the last decade, the central role of platelets in host defense against microorganisms has been revealed much more clearly. Platelets release antimicrobial molecules by engaging bacterial pathogens specifically, rapidly and directly or indirectly, and form the adaptive immunity [4]. PRP promotes tissue regeneration, enhance collagen synthesis, and trigger angiogenesis and immune responses by releasing growth factors and cytokines [5].
We had already revealed the efficacy of PRP on methicillin-resistant Staphylococcus aureus (MRSA)-related surgical wound in an animal model [6]. In this study, we aimed to demonstrate the in vitro antibacterial activity of PRP against MRSA and three more multi-drug-resistant (MDR) bacteria species that are important and hard-to-treat in wound infections.
Materials and methods
The study was approved by the Institutional Ethics Committee of Haydarpasa Numune Training and Research Hospital on March 13, 2017 (HNEAH-KAEK 2017/KK25). In the study group, the ten volunteers served as donors to obtain blood samples to manufacture PRP had no infection and history of medication in last 10 days. The study was performed on MRSA, vancomycin-resistant Enterococcus spp. (VRE), extended spectrum beta lactamase (ESBL) producing Klebsiella pneumoniae, and carbapenem-resistant Pseudomonas aeruginosa, which are all MDR bacteria. The strains were isolated from deep tissue specimens of different patients with diabetic foot infection. The isolates were identified by VITEK-2 automated identification system (bioMérieux, France). All the strains were cultured in skim milk and were stocked up at − 70 °C. The isolates were revived by subculturing on 5% sheep blood agar (Salubris, Turkey) at 35 °C for 24 h. Two suspensions were prepared from the growth on 5% sheep blood agar (Salubris, Turkey) in Trypticase Soy Broth (Merck, Germany) equivalent to McFarland 0.5 standard (1.5 × 108 CFU/ml). One of these suspensions was diluted to 105 for studying in the PRP groups. The other suspension was used for the antimicrobial susceptibility test.
In vitro antimicrobial susceptibility testing
Antibiotic susceptibility tests were performed with the Kirby–Bauer disc-diffusion method in compliance with The European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines. Microbial suspensions of four isolates corresponding with concentration of 0.5 McFarland were completely distributed on the surface of Mueller–Hinton agar (Merck, Germany) plates.
Cefoxitin, ceftriaxone, meropenem, and vancomycin are surrogate markers for the detection of methicillin resistance, extended spectrum beta lactamase resistance, carbapenem resistance, and vancomycin resistance, respectively (The EUCAST guideline on detection of resistance mechanisms v 2.0). Standard 6 mm antibiotic discs of cefoxitin (30 µg), ceftriaxone (30 µg), meropenem (10 µg), or vancomycin (5 µg) alone and the ones coated with PRP or platelet-poor plasma (PPP) were placed on the agar plates using separate micropipettes for MRSA, ESBL producing K. pneumonia, carbapenem-resistant P. aeruginosa and VRE, respectively. Via instillation technique of drops by sterile pipettes, the empty discs coated with one of those: 10 µl of PRP, 10 µl of PPP, and 10 µl of PBS as control with 2 µl of autologous thrombin were also placed on the same agar plates. The agar plates were incubated at 37 °C. Antimicrobial activity was assessed at 24 h of incubation by measuring the zones of inhibition; the values are presented using the median.
Preparation of PRP and autologous thrombin
PRP was obtained through 54 ml of blood from brachial vein of each of ten volunteers with a 60-ml syringe in Magellan PRP™ kit, which contained 6 ml anticoagulant citrate dextrose solution adenine (ACD-A). We obtained 3 ml of PRP and 20 ml of platelet-poor plasma (PPP) at the end of the procedure. The number of white blood cells (WBC) and platelets in PRP and PPP for each volunteer was analyzed by the complete blood cell analyzer (Cell Dyn Sapphire, Abbott Diagnostics, USA). Then, the results were compared with the whole blood results of the same volunteer.
We also put 9 ml of whole blood of the donors into Vacuette® (BD Vacutainer, Plymouth, UK) serum clot activation tube, and then, we added and 1 ml of 10% calcium gluconate (Calcium Picken Flk., Adeka, Turkey) into these tubes. The tubes were kept at room temperature for 10 min until clotting. Then, the tubes were centrifuged at 3000g for 3 min. The top layer (supernatant) is considered thrombin and is transferred to a new tube.
Study groups
We had a total of three groups: two study groups (PRP and PPP), and phosphate-buffered saline (PBS) as a control group. For each study group, we had four different bacteria, as MRSA, VRE, and ESBL producing K. pneumoniae and carbapenem-resistant P. aeruginosa. Thus, 12 different groups were formed to be investigated.
PRP groups
For the PRP groups, having regulated colony counts (1 × 105) of 100 µl of each bacteria were put into the sample tubes. Then, 700 µl of liquid medium, 160 µl of PRP, and then 40 µl of thrombin were added into these tubes. Finally, we had 1 × 104 CFU/ml of bacteria concentration for each group (Table 1). These tubes were placed in an incubator and at their time on an agitator (Thermolyne Maxi Mix III Type 65800, Thermo Fischer Scientific, USA) which runs on 200 rpm at 37 °C. Then, after vortexing the tubes at 1, 2, 5, and 10 h of incubation, 10 µl of the mixture in four different groups were coated with 90 µl of sterile saline in a sterile Petri plate by using a sterile pipette (10–100 µl, AHN, Germany). After this step, the mixtures inoculated on 5% sheep blood agar (Salubris, Turkey). Colonies were counted with the aid of a magnifying glass after 24 h incubation at 37 °C. Every counted colony on agar plaque was marked by a loop to avoid remarking. Colony counts over 1000 were accepted as 1000.The bacterial colony count is presented as mean (standard deviation value of log-transformed bacterial colony counts).
PPP- and PBS-control groups
The same processes as above for PRP groups were followed for PPP- and PBS-control groups using PPP or PBS instead of PRP (Table 1).
In vitro susceptibility testing
In vitro susceptibility tests were determined by the Kirby–Bauer disc-diffusion method on Mueller–Hinton agar (Merck, Germany). First, agar plates were coated with one of the four bacterial strains: MRSA, ESBL-positive K. pneumoniae, carbapenem-resistant P. aeruginosa, and VRE. Then, we placed standard 6 mm empty discs coated with one of those: 10 µl of PRP, 10 µl of PPP, and 10 µl of PBS as control with 2 µl of autologous thrombin. In addition, we placed standard 6 mm antibiotic discs of cefoxitin, ceftriaxone, meropenem, or vancomycin alone and the ones coated with PRP or PPP using separate micropipettes. The agar plates were incubated at 37 °C. Antimicrobial activity was assessed at 24 h of incubation by measuring the zones of inhibition.
Time-kill assays
Time-kill assays were conducted with plasma preparations (PRP and PPP) suspended in 0.9% saline. PBS, suspended in 0.9% saline without any plasma preparations (PRP and PPP) addition, was used as control group. Bacterial growth in PBS (control) at 0th, 1st, 2nd, 5th, and 10th hours was considered as reference growth rate (100%). Bacterial growth rate of PRP and PPP groups in time-kill assays was analyzed with comparing to the concurrent bacterial growth rate in PBS (control) group. The peak point of effectiveness for each study group against each bacterium was described as the hours of assessment in time-kill assay that have maximum rate of suppression of bacterial growth. Time-kill assays were performed as described by Yang et al. [7].
Statistical analysis
Statistical analysis was performed using SPSS for Windows in 22.0 (IBM, Armonk, NY, USA). Descriptive statistics were given as mean and standard deviation. MRSA, VRE, ESBL producing K. pneumoniae, and carbapenem-resistant P. aeruginosa growth percentages in PRP and PPP groups were determined by comparing with the control group. Kolmogorov–Smirnov test was used to analyze normality for colony count and growth percentages. Mann–Whitney U and Wilcoxon tests were used for hypothesis testing. Statistical significance was defined as 0.95 (α = 0.05) for the study.
Results
Demographic characteristics
The volunteers were all men and the mean age of was 32 ± 5. According to complete blood count analysis results, the mean WBC and platelet count was 6.56 ± 1.5 × 103, and 221.3 ± 59 × 103 µl, respectively.
WBC and platelet counts in PRP and PPP groups
For the PRP group, the mean number of WBC and platelets was 52.9 ± 31.5 × 103 and 2056.6 ± 494.5 × 103 µl, respectively. We had eightfold increase in WBC and 9.3-fold increase in platelet counts compared to whole blood results of volunteers.
For the PPP group, the mean number of WBC and platelets was 1.47 ± 0.5 × 103 and 85.3 ± 35 × 103 µl, respectively. We had 4.5 times and 2.6 times decreases in WBC and platelet counts respectively, compared to volunteers.
Colony counts on agar plates
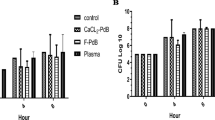
For MRSA, mean values of colony count in PRP group were 5.8, 4.4, 19.6, and 217.9 × 104; in PPP group 39.1, 19.4, 18.4, and 343.8 × 104; in PBS (control) group 95, 223, 660.9 × 104 and > 1000 at 1, 2, 5, and 10 h, respectively (Table 2). The antimicrobial effect of PRP reached its peak point at second hour of incubation, but the peak point for PPP was at fifth hour of incubation, and it continued for both PRP and PPP groups until tenth hour of incubation (Fig. 1). However, the decrease in effectiveness was more rapid in PPP than PRP after 5 h. When compared to control group, the peak point of effectiveness for PRP and PPP inhibited nearly 90% of MRSA growth rate.
Relationship between growth rate curve and time; colony counts on 5% sheep blood agar (Salubris, Turkey) of MRSA. Asterisk: plaque agar images of one of the volunteers as an example are on the right side. The difference in concurrent colony counts on plaques shows significantly higher antimicrobial activity of platelet-rich plasma (PRP) compared to platelet-poor plasma (PPP) and phosphate-buffered saline (PBS-control) groups. Bacterial growth rate of PRP and PPP groups in time-kill assays was analyzed with comparing to the concurrent bacterial growth rate in PBS (control) group. Bacterial growth in PBS (control) at 0th, 1st, 2nd, 5th, and 10th hours was considered as reference growth rate (100%). The antimicrobial effects of both PRP and PPP against MRSA are significant (up to 95% inhibitor effect in PRP group compared to control group)
For K. pneumoniae, mean values of colony count in PRP group were 66.1, 27.9, 73.3, and 642.8 × 104; in PPP group 69.4, 60.7, 107.8, and 605 × 104; in PBS (control) group 97.2, 307.6, 763.6, and 923.1 × 104 at 1, 2, 5, and 10 h, respectively (Table 2). The antimicrobial effect of PRP reached its peak point at second hour of incubation, but the peak point for PPP was at fifth hour of incubation, and it continued for both PRP and PPP until 10 h of incubation (Fig. 2). However, the decrease in effectiveness was similar in PPP and PRP after 5 h. When compared to control group, the peak point of effectiveness for PRP and PPP inhibited around 90% of Klebsiella pneumoniae growth rate.
Relationship between growth rate curve and time; colony counts on 5% sheep blood agar (Salubris, Turkey) of Klebsiella pneumoniae. Asterisk: Plaque agar images of one of the volunteers as an example are on the right side. The difference in concurrent colony counts on plaques in PRP group (especially 5th hour) shows significantly higher antimicrobial activity compared to platelet-poor plasma (PPP) and phosphate-buffered saline (PBS-control) groups. Bacterial growth rate of PRP and PPP groups in time-kill assays was analyzed with comparing to the concurrent bacterial growth rate in PBS (control) group. Bacterial growth in PBS (control) at 0th, 1st, 2nd, 5th, and 10th hours was considered as reference growth rate (100%). The antimicrobial effects of both PRP and PPP against ESBL (+) Klebsiella pneumoniae are significant (up to 90% inhibitor effect in PRP group compared to control group)
For P. aeruginosa, mean values of colony count in PRP group were 18.5, 6.7, 10, and 20 × 104; in PPP group 31.6, 10.2, 13.5, and 40.3 × 104; in PBS (control) group 43.1, 44, 85.7, and 505.5 × 104 at 1, 2, 5, and 10 h, respectively (Table 2). The antimicrobial effect of PRP had not reached its peak point yet at the tenth hour of incubation, but the peak point for PPP was at fifth hour of incubation (Fig. 3). The effectiveness of PPP reached a plateau between fifth and tenth hours. When compared to control group, the rate of effectiveness (decrease in bacterial growth rate) at tenth hour for PRP and PPP was about 80% (Fig. 3).
Relationship between growth rate curve and time, colony counts on 5% sheep blood agar (Salubris, Turkey) of Pseudomona aeruginosa. Asterisk: Plaque agar images of one of the volunteers as an example are on the right side. The difference in concurrent colony counts on plaques in both PRP and PPP groups shows significantly higher antimicrobial activity compared to phosphate buffer saline (PBS-control) group. Bacterial growth rate of PRP and PPP groups in time-kill assays was analyzed with comparing to the concurrent bacterial growth rate in PBS (control) group. Bacterial growth in PBS (control) at 0th, 1st, 2nd, 5th, and 10th hours was considered as reference growth rate (100%). The antimicrobial effects of both PRP and PPP against carbapenem-resistant Pseudomonas aeruginosa are significant (up to 80% inhibitor effect in PRP group compared to control group)
For VRE, mean values of colony count in PRP group were 22.9, 43.4, 178.2, and 784.2 × 104; in PPP group 24.5, 41.1, 124.3, and 719.8 × 104; in PBS (control) group 22.1, 60.4, 218.4, and 739.1 × 104 at 1, 2, 5, and 10 h, respectively (Table 2). Even though the Fig. 4 points out that the peak point for VRE in PRP and PPP group was at 2nd and 5th hours of incubation; these antimicrobial effects were very limited. When we look at the Table 2, PRP had no statistically different antimicrobial effect.
Relationship between growth rate curve and time; colony counts on 5% sheep blood agar (Salubris, Turkey) of vancomycin-resistant Enterococcus spp. (VRE). Asterisk: Plaque agar images of one of the volunteers as an example are on the right side. No significant difference in colony counts on platelet-rich plasma (PRP), platelet-poor plasma (PPP), and phosphate-buffered saline (PBS-control) groups. Bacterial growth rate of PRP and PPP groups in time-kill assays was analyzed with comparing to the concurrent bacterial growth rate in PBS-control group. Bacterial growth in PBS (control) at 0th, 1st, 2nd, 5th, and 10th hours was considered as reference growth rate (100%). The antimicrobial effects of both PRP and PPP against VRE are very limited (maximum ~ 40% inhibitor effect compared to control group)
When compared to the control group, MRSA, K. pneumoniae, and P. aeruginosa growth suppression capabilities of PRP and PPP were statistically significantly higher at 1, 2, 5, and 10 h (p < 0.05). VRE was the only bacteria that PRP and PPP showed limited activity against, and only PPP showed statistically significant activity at second and fifth hours against VRE compared to control group (Table 2).
When compared to PPP, in general, PRP showed higher activity against MRSA, K. pneumoniae, and P. aeruginosa. However, the differences in activities were not statistically significant, except for against MRSA and P. aeruginosa at 1st hour of incubation. The activity of PRP and PPP against VRE was similar (p > 0.05) (Table 2).
In vitro susceptibility results
The baseline susceptibility test results for various antibiogram discs, PRP plus thrombin-coated blank discs, PPP plus thrombin-coated blank discs, and PBS plus thrombin-coated blank discs against four different bacteria are shown in Table 3.
Median value of inhibition zones against MRSA was 6 mm in diameter for PRP, PPP, and PBS. Although inhibition zone was 15 mm for cefoxitin, it was 16 mm cefoxitin plus PRP (Table 3). Median value of inhibition zones against K. pneumonia and P. aeruginosa was 8 mm in diameter for PRP disc, and they were 6 mm for PPP- and PBS-coated discs (Table 3; Fig. 5a, b). Median value of inhibition zones against VRE was 8 mm in diameter for PRP disc and 6 mm for PPP- and PBS-coated discs. No increases in diameter were revealed for PRP coated ceftriaxone, meropenem, and vancomycin disc for the rest of the microorganisms (Table 3).
Images showing the inhibition zones on K. pneumonia (a) and P. aeruginosa (b). Asterisk: in vitro susceptibility tests were determined by the Kirby–Bauer disc-diffusion method on Mueller–Hinton agar (Merck, Germany). Disc no 3 showing 2 mm increase in inhibition zone, and no additional increase in inhibition zone in disc no 2 at a. Disc no 8 showing 2 mm increase in inhibition zone, and no additional increase in inhibition zone in disc no 7 at b
Discussion
Chronic infected wounds, such as diabetic foot infections (DFI), are one of the most important causes of morbidity and mortality in diabetic patients. Because of that DFI’s are mostly polymicrobial and complicated infections, alternative treatment methods are needed [8, 9].
In association with the preparation procedure, platelets are the main component in PRP. Platelets have interaction with microorganism besides their hemostatic functions [4]. It is revealed that invertebrates and early vertebrates have one cell type which is called hemocyte. This cell has both hemostatic functions and host defense functions. However, types and functions of cells got more sophisticated during the evolution process in mammals. Platelets in mammals called as immature hemocytes or guardian cells, because they still have functions against microorganisms in host defense [10].
Studies about the use of PRP for wound healing have increased dramatically over the last decade. However, there are controversial results of the studies about the effect of PRP on diabetic wounds. Steed et al. [11] and Wieman et al. [12] concluded that PRP had significant clinical results on wound healing among diabetic patients, while Hemecourt et al. [13], Smiell et al. [14], and Robson et al. [15] had not. It is concluded that the main reason of this may be the platelet counts in PRP or inadequate activation of platelets, so that various amounts of chemokine, kinocidin, or anti-inflammatory cytokines, which are the most important elements on wound healing process, may occur. Amable et al. [16] tested 15 different conditions including relative centrifugal force (RCF), centrifugation time, and temperature to revealed proper procedure to have optimal platelet yield. The result of the study revealed that they had 0.6–5.2-fold increase in platelet counts depending on the conditions above meaning that one of the most important issues about the effect of PRP is the preparation process to obtain optimal platelet counts. In our study, we used Magellan® PRP fully automated system to have standardization and we had 9.3-fold increase in platelet counts compared to whole blood.
There are some studies published in the literature about antimicrobial effect of PRP that showed the bacteriostatic and/or bactericidal effects of platelets on microorganisms. Jago and Jacox [17], Weksler [18], Kahn et al. [19], Czuprynski and Balish [20], and Miragliotta et al. [21] revealed the antibacterial effect on various microorganism like Bacillus, Staphylococcus, Listeria, and Salmonella; however, there are only a few studies about resistant microorganism which are capable of surviving for a long time and hard to treat.
Our study results revealed that PRP and PPP had antimicrobial effect on three resistant microorganisms as MRSA, ESBL-positive K. pneumoniae, and carbapenem-resistant P. aeruginosa, while they had no significant effect on VRE. When we consider the results as a whole, antimicrobial effect of PRP was more effective than PPP; however, it was statistically significant only in MRSA and P. aeruginosa group and only in the first hours of the study. According to a study results by Li [22] and Li [23], PRP had up to 100-fold reduction of in vitro MRSA growth compared to PPP and PBS. In addition, in another study having both in vitro and in vivo parts, Li et al. showed that PRP significantly inhibited the growth of methicillin-sensitive and methicillin-resistant Staphylococcus aureus, Group A Streptococcus, and N. gonorrhoeae within the first few hours. According to the results of this study, PRP had no significant antimicrobial effect against E. coli and Pseudomonas. In contrast, we revealed better antimicrobial effect of PRP against P. aeruginosa in our study, although it was a carbapenem-resistant isolate. In the in vivo part of this study of an implant-associated spinal infection rabbit model, they also revealed that PRP treatment had significant effect on reduction of bacterial colonies in bone samples and thereby better bone healing at post-operative weeks 2 and 3 [22, 23].
Mariani et al. [24] showed that PRP had significantly inhibited the growth of five different nosocomial bacteria: E. coli, S. aureus, P. aeruginosa, K. pneumonia, and E. faecalis. Another result of this study was that the antimicrobial effect of PRP decreased depending on the increased concentration of bacteria. These isolates, unlike the ones in our study, were all susceptible to antimicrobials. In this study, PRP was prepared by manual method and the median platelet count was 290 × 103/µl. Antibacterial effect in this study lasted for up to 2 h of incubation. In our study, PRP was collected by a fully automated device which is licensed to obtain platelet yield seven times greater than baseline concentration of donor platelet count in microliters [25, 26]. The median value of platelet counts in PRP in our study was 2208 × 103/µl., and the antibacterial effect lasted for up to 10 h of incubation. Although the bacteria that we used in our study were multi-drug-resistant isolates, we had longer antimicrobial effect when compared with Mariani’s study. This result indicates that higher platelet counts in PRP solutions may be associated with the duration of the antimicrobial effect.
One of the unresolved questions in our study is the role of leukocytes in PRP on antimicrobial effect. Yang et al. [7] and Drago et al. [27] revealed that platelets rather than leukocytes are the main component for antimicrobial effect, but leukocytes are responsible for maintenance of the antimicrobial effect. In addition, Anitua et al. [28] showed that additional leukocytes in PRP did not increase the antimicrobial effect on Staphylococcal strains.
Moojen et al. [29] revealed that antimicrobial effect of PRP is thanks to platelets rather than leukocytes by showing PRP without thrombin did not have antimicrobial effect. PRP needs to be activated by thrombin to develop antibacterial effect. Thrombin activates only platelets, but it has no effect on leukocytes in PRP. One of the important clinical studies searching in vitro antibacterial effect of platelet-derived products from patients with diabetic ulcers was made by Chen et al. [30]. They searched the antibacterial effects of PRP and PPP without activation by thrombin, autologous platelet-rich gel (APG) activated by thrombin, APG-APO (APG combined with apocynin; apocynin inactivates leukocytes), and PBS as a control group. They revealed that APG and APG-APO groups showed a rapid and significant decrease compared to PBS group in the first 4 h. The APG and APG-APO groups were significantly more effective than PRP and PPP groups, meaning that activation of platelets by thrombin is essential. In addition, they showed that there was no difference between the APG and APG-APO groups, meaning that inactivation of leukocytes had no influence on antimicrobial effect.
Although there are not definitive recommendations or determined methods how to do it, we also performed in vitro susceptibility tests for PRP discs determined by the Kirby–Bauer disc-diffusion method on Mueller–Hinton agar. Results in our study revealed 2 mm increase in diameter for PRP coated empty antibiogram discs against K. pneumoniae, P. aeruginosa, and VRE. For MRSA, although PRP showed higher activity considering both mean colony counts on blood sheep agars and time-kill curve analysis; it showed no increase in inhibition zones on Mueller–Hinton agar. Contradictorily, for VRE, although PRP showed very limited activity considering both mean colony counts on blood sheep agars and time-kill curve analysis, it showed 2 mm increase in inhibition zones on Mueller–Hinton agar. We had no reasonable explanation to clarify this contradiction according to the results in our study. According to study results of Bielecki et al. [31] that show the susceptibility zones on Mueller–Hinton agar, platelet-rich gel showed antimicrobial activity against S. aureus and E. coli but not against K. pneumoniae, E. faecalis, and P. aeruginosa. Inhibition zones produced by platelet-rich gel were between 6 and 24 mm (mean 9.83 mm) in diameter. We find no other study in the literature about inhibition zones of PRP, so there is a need for further in vitro studies to determine standard methods and susceptibility criteria for PRP.
In recent years, there is an increasing interest for the clinical use of various autologous platelet-derived products on variety of medical fields such as orthopedics, plastic surgery, dentistry, cosmetics, and dermatology. Growth factors such as PDGF, TGF-β, EGF, VEGF, IGF-1, FGF, HGF, and various anti-inflammatory peptides released by the activated platelets are responsible for the wound healing [27]. Because of the fact that there are not randomized and controlled clinical studies, there is not a consensus for the use of platelet-derived products on infected wounds.
In mice wound model by Yang et al. [32], results indicated that PRP significantly decreased the wound size and enhanced angiogenesis compared to control groups. Another study made in particularly infected wounds on rabbits by Cetinkaya et al. [6] revealed that PRP significantly reduced the inflammatory response and achieved wound healing on MRSA-related surgical-site infections.
In a case presented with an infected amputation size wound by multi-drug-resistant Acinetobacter baumannii/haemolyticus of a diabetic patient and which remain unresolved by various prolonged antibiotic treatments, Sun et al. [33] successfully eradicated the infection and achieved wound closure using only autologous platelet-rich gel and negative pressure wound therapy.
Clinical results and effectiveness of PRP vary from study to study and contradictive results are presented about the effectiveness of PRP among a particular microorganism. Differences in study design such as preparation of PRP, differences in platelets counts, platelets whether activated or not, yield of bacteria, and antibiotic resistance profile of bacteria may be the cause of the different results. However, all the in vitro and in vivo studies reveal that there is not a contraindication for the use of PRP on infected wounds.
Conclusions
Considering the trend on diabetes and antibiotic resistance rates, treating chronic infected wounds is going to continue to be one of the most important and the most difficult fields in medicine in the future. Emerging PRP and other platelet-derived products seem to be a promising alternative tool besides antibiotic treatment, debridement, and negative pressure wound therapy, hyperbaric oxygen therapy, and other treatment options for treating diabetic foot infections. However, there is a need for further randomized-controlled clinical studies.
References
Friedman ND, Temkin E, Carmeli Y. The negative impact of antibiotic resistance. Clin Microbiol Infect. 2016;22(5):416–22.
Huysman E, Mathieu C. Diabetes and peripheral vascular disease. Acta Chir Belg. 2009;109(5):587–94.
Lipsky BA. International consensus group on diagnosing, treating the infected diabetic foot. A report from the international consensus on diagnosing and treating the infected diabetic foot. Diabetes Metab Res Rev. 2004;20(Suppl 1):S68-77.
Yeaman MR. Platelets in defense against bacterial pathogens. Cell Mol Life Sci. 2010;67(4):525–44.
Piccin A, Di Pierro AM, Canzian L, Primerano M, Corvetta D, Negri G, et al. Platelet gel: a new therapeutic tool with great potential. Blood Transfus. 2017;15(4):333–40.
Cetinkaya RA, Yilmaz S, Unlu A, Petrone P, Marini C, Karabulut E, et al. The efficacy of platelet-rich plasma gel in MRSA-related surgical wound infection treatment: an experimental study in an animal model. Eur J Trauma Emerg Surg. 2017.
Yang LC, Hu SW, Yan M, Yang JJ, Tsou SH, Lin YY. Antimicrobial activity of platelet-rich plasma and other plasma preparations against periodontal pathogens. J Periodontol. 2015;86(2):310–8.
Grigoropoulou P, Eleftheriadou I, Jude EB, Tentolouris N. Diabetic foot infections: an update in diagnosis and management. Curr Diab Rep. 2017;17(1):3.
Kosinski MA, Lipsky BA. Current medical management of diabetic foot infections. Expert Rev Anti Infect Ther. 2010;8(11):1293–305.
Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol. 2014;12(6):426–37.
Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study Group. J Vasc Surg. 1995;21(1):71–8; discussion 9–81.
Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21(5):822–7.
Hemecour P. Sodium carboxymethylcellulose aqueous-based gel vs. becaplermin gel in patients with nonhealing lower extremity diabetic ulcers. Wounds. 1998;10:69–75.
Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen. 1999;7(5):335–46.
Robson M. Integrating the results of phase IV (postmarketing) clinical trial with four previous trials reinforces the position that Regranex (becaplermin) gel 0.01% is an effective adjunct to the treatment of diabetic foot ulcers. J Appl Res. 2005(5):35–45.
Amable PR, Carias RB, Teixeira MV, da Cruz Pacheco I, Correa do Amaral RJ, Granjeiro JM, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4(3):67.
Jago R, Jacox RF. Cellular source and charcter of a heatstable bactericidal property associated with rabbit and rat platelets. J Exp Med. 1961;113:701–11.
Weksler BB, Nachman RL. Rabbit platelet bactericidal protein. J Exp Med. 1971;134(5):1114–30.
Kahn RA, Flinton LJ. The relationship between platelets and bacteria. Blood. 1974;44(5):715–21.
Czuprynski CJ, Balish E. Interaction of rat platelets with Listeria monocytogenes. Infect Immun. 1981;33(1):103–8.
Miragliotta G, Lafata M, Jirillo E. Anti-bacterial activity mediated by human platelets. Agents Actions. 1988;25(3–4):401–6.
Li H, Li B. PRP as a new approach to prevent infection: preparation and in vitro antimicrobial properties of PRP. J Vis Exp. 2013(74):e50351.
Li H, Hamza T, Tidwell JE, Clovis N, Li B. Unique antimicrobial effects of platelet-rich plasma and its efficacy as a prophylaxis to prevent implant-associated spinal infection. Adv Healthc Mater. 2013;2(9):1277–84.
Mariani E, Filardo G, Canella V, Berlingeri A, Bielli A, Cattini L, et al. Platelet-rich plasma affects bacterial growth in vitro. Cytotherapy. 2014;16(9):1294–304.
Fitzpatrick J, Bulsara MK, McCrory PR, Richardson MD, Zheng MH. Analysis of platelet-rich plasma extraction: variations in platelet and blood components between 4 common commercial kits. Orthop J Sports Med. 2017;5(1):2325967116675272.
Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J Cutan Aesthet Surg. 2014;7(4):189–97.
Drago L, Bortolin M, Vassena C, Taschieri S, Del Fabbro M. Antimicrobial activity of pure platelet-rich plasma against microorganisms isolated from oral cavity. BMC Microbiol. 2013;13:47.
Anitua E, Alonso R, Girbau C, Aguirre JJ, Muruzabal F, Orive G. Antibacterial effect of plasma rich in growth factors (PRGF(R)-Endoret(R)) against Staphylococcus aureus and Staphylococcus epidermidis strains. Clin Exp Dermatol. 2012;37(6):652–7.
Moojen DJ, Everts PA, Schure RM, Overdevest EP, van Zundert A, Knape JT, et al. Antimicrobial activity of platelet-leukocyte gel against Staphylococcus aureus. J Orthop Res. 2008;26(3):404–10.
Chen L, Wang C, Liu H, Liu G, Ran X. Antibacterial effect of autologous platelet-rich gel derived from subjects with diabetic dermal ulcers in vitro. J Diabetes Res. 2013;2013:269527.
Bielecki TM, Gazdzik TS, Arendt J, Szczepanski T, Krol W, Wielkoszynski T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Jt Surg Br. 2007;89(3):417–20.
Yang HS, Shin J, Bhang SH, Shin JY, Park J, Im GI, et al. Enhanced skin wound healing by a sustained release of growth factors contained in platelet-rich plasma. Exp Mol Med. 2011;43(11):622–9.
Sun S, Wang C, Chen D, Cen S, Lv X, Wen X, et al. Combating superbug without antibiotic on a postamputation wound in a patient with diabetic foot. Int J Low Extrem Wounds. 2016;15(1):74–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Riza Aytac Cetinkaya, Ercan Yenilmez, Patrizio Petrone, Soner Yılmaz, Bayhan Bektore, Berksan Simsek, Tugba Kula Atik, Mustafa Ozyurt, and Aytekin Ünlü declare that they have not conflict of interest.
Informed consent
The study was approved by the Institutional Ethics Committee of Haydarpasa Numune Training and Research Hospital on March 13, 2017.
Rights and permissions
About this article
Cite this article
Çetinkaya, R.A., Yenilmez, E., Petrone, P. et al. Platelet-rich plasma as an additional therapeutic option for infected wounds with multi-drug resistant bacteria: in vitro antibacterial activity study. Eur J Trauma Emerg Surg 45, 555–565 (2019). https://doi.org/10.1007/s00068-018-0957-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-018-0957-0