Abstract
Purpose
There is a risk of misinterpreting the clinical signs of acute compartment syndrome of the lower limb resulting in delayed fasciotomy. Up to date, the diagnosis of compartment syndrome is based on clinical assessment and of invasive needle pressure measurement in uncertain cases. Close monitoring is necessary for early recognition of raising compartment pressures. Clinical assessment of muscle firmness by the physician’s palpation alone is unreliable. Thus, a device objectifying this assessment would be beneficial. The purpose of this study was to determine the feasibility of muscle compartment elasticity measurements by a novel and non-invasive device using pressure-related ultrasound.
Methods
In a cadaveric model, the anterior tibial compartment was prepared to simulate raising intra-compartmental pressures (0–80 mmHg) by saline infusion. Standard invasive pressure monitoring was compared with a novel method to determine tissue elasticity. Changing cross-sectional view in B-mode ultrasound was exerted to measure the compartment depth before and after physician’s probe compression of 100 mmHg. Compartment displacement (∆d) was measured and related to the corresponding compartmental pressure (Spearman correlation coefficient). Delta (mm) of the control group at 10 mmHg compartment pressure was compared with measured data at rising compartmental pressures of 30, 50, and 70 mmHg using the Wilcoxon rank-sum test. The intra-observer reliability (κ) was additionally calculated.
Results
Fresh and never frozen lower human limbs (n = 6) were used. The average displacement measured in the anterior tibial compartment was 2.7 mm (0.3–6.7 mm). A concordant consistent correlation between the compartmental displacement and the intra-compartmental pressure occurred. The Spearman coefficient (r s = 0.979) showed a significant correlation between the rising pressure and the decreasing tissue displacement visualized by ultrasound. The intra-observer value kappa showed reliable values (κ 10 = 0.73, κ 30 = 0.80, and κ 70 = 0.79).
Conclusions
We introduce a new method of ultrasound imaging enhanced with probe pressure measurement to determine changes of the visco-elastic behavior of isolated muscle compartments. Pressure-related ultrasound could be a reliable tool to determine the correlation between the measured compartmental displacement and the increasing intra-compartmental pressure. Its accuracy revealed promising results. This technique may help the physician to objectify the clinical assessment of compartment elasticity, mainly indicated in cases of unconscious patients and imminent pathology. Further clinical studies and improvements of this technique are required to prove its accuracy and reliability in cases of compartment syndrome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pressure increase within the fascial layers is an important and probably the most impartial parameter, accelerating the pathophysiology of an acute compartment syndrome (ACS) [1]. Timely diagnosis of compartment syndrome is mainly based on clinical examination including patient’s history and physical examination. In this context, the assessment of soft tissue firmness and the examination of muscle tissue elasticity represent important clinical findings for early identification of ACS [2]. However, belittlement or misinterpretation of clinical examination may provoke a devastating delay of fasciotomy, which is the most relevant cause of a poor outcome after ACS [3–6]. Furthermore, health-related quality of life is reported to be decreased after compartment syndrome in cases, which required skin graft and had a prolonged closure time [7]. Therefore, adjunct invasive compartmental pressure measurement is used to improve the accuracy of clinical diagnosis and to specify the indication of fasciotomy. Nevertheless, also the combination of clinical findings [8] and invasive pressure measurements [9] may be misleading for decision making because of a low reliability particularly in specific subsets of patients. In this context, a high reliability of three simple clinical parameters (pain, swelling, and loss of function) for diagnosis of ACS has been described in orientated and cooperative patients. However, when the cooperation of patient is limited, the probability of early diagnosis declines significantly [10]. In unconscious patients, the only clinical finding that does not change is the firmness of the muscle compartment. Therefore, in the early period of the syndrome, serial physical examinations in these patients by manual palpation are required to determine and to unmask critical pressure elevations [11]. But the needle compartment pressure measurement itself is susceptible for measuring errors due to weak users’ reliability [11].
Therefore, a simple, non-invasive, low cost, and reliable screening tool to objectify clinical assessment of palpation in uncertain cases of elevated muscle compartment pressures may help to improve diagnostic accuracy and may reduce the sequelae of delayed fasciotomy. The aim of this study was to test the use of an investigational non-invasive, pressure-related ultrasound device to assess the stiffness of the affected muscle compartment. We hypothesize that a high correlation between an increase of the compartmental pressure and a decrease of compartmental elasticity measured by the technique introduced in this study exists. If there is sufficient reliability and accuracy, this enhanced ultrasound determination of elevated compartment pressures may improve early diagnosis of ACS.
Materials and methods
Compartment model
Fresh and never frozen lower limbs were used in our cadaveric model. All specimens sustained the time of death within 48 h prior the investigation, to avoid exceeded autolysis at the time of measuring the compartments. The body mass index (BMI) of any torso resulted in the normal values within 20–25. The temperature of the extremities at the point of measurement was kept between 60 °F (15.5 °C) and 77 °F (25 °C). Specimens with previous lower leg surgery, skin damage, fracture or other operations of the lower leg were excluded. The anterior tibial compartment was utilized to simulate raising intra-compartmental pressures (ICP). In this pressure-controlled model, saline (0.9 % sodium chloride solution) was infused via a 2-mm cannula, which was placed precisely in the middle of the anterior tibial compartment. The needle tip position was verified by B-mode ultrasound. The pressure increase was performed stepwise until the required ICP was reached.
Intra-compartmental pressure measurement
The resulting intra-compartmental pressure induced by the infused solution was monitored by invasive pressure measurement using a Codman intracranial pressure monitor (Serial number: LE 10351, Codman & Shortleff, Raynham, MA, USA). The pressure measurement device was calibrated at barometric pressure. This piezoelectric microsensor catheter was placed in the muscle belly as described in the manufacturer’s manual guidelines for measurements. Again the sensor tip positioning was verified with ultrasound imaging.
Pressure-related ultrasound device
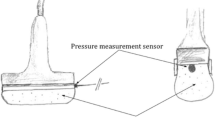
Brightness-mode (B-mode) cross-sectional ultrasound view of the muscle compartment (Aloka SSD 1700, Dynaview II, Tokyo, Japan) was used to determine the compartment depth of the muscle belly. These images were measured with and without the physician’s manual compression of the US probe. The extrinsic pressure of 100 mmHg was determined using a water-filled probe head connected to a pressure sensor device (Fig. 1). The pressure sensor was placed in the cavity of the probe head and was calibrated at barometric pressure. The pressure transducer within the probe head determined the exerted pressures on the lower limb. Ultrasound images at 0 and 100 mmHg of probe pressure were taken and the difference of the compartment depth (∆d) was measured using the software of the ultrasound unit. Linear compartment displacement in mm (∆d), measured in the cross-sectional ultrasound view, was assessed and related to the increasing compartment pressure every 5 mmHg (6.66 hPa). The absolute values were related to the compartment depth and illustrated as relative values to eliminate differences between the individual compartment sizes.
Groups
The control group was represented by the delta measurements at compartment pressures of 10 mmHg. Accordingly, measurements at 30 mmHg (group I), 50 mmHg (group II), and 70 mmHg intra-compartmental pressures (group III) were assessed.
Statistics
All values were assumed to be arithmetic mean and ranges. The correlation was determined using Spearman rank correlation analysis (r s). The intra-rater agreement (κ) was calculated at an ICP of 10 mmHg, 30 mmHg, and 70 mmHg with ten measurements each. A kappa value of 1 indicates a perfect level of agreement. A value of 0 traditionally indicates that the level of agreement is equal to that of chance. Intermediate values are categorized as follows: ≤0.2, slight agreement; 0.21–0.4, fair; 0.41–0.6, moderate; 0.61–0.8, good; ≥0.81, very good agreement. Differences in soft tissue movement at increased ICP were compared with a rank-sum test for paired samples (Wilcoxon test). The probability level was set at p < 0.05. Statistical analyses were performed using MedCalc, version 11.3 (MedCalc Software, Belgium).
Results
Compartmental diameter
In this cadaveric model, six lower limbs of different specimens (two male, four female, age 73–84 years) were included. Measurement of the compartment diameter showed an individual range from 19.3 up to 36.3 mm. The elastic properties at low pressures (10 mmHg) were measured with the described pressure-related ultrasound technique. Hereby, we determined the control group in each series (n = 6). Then the ICP of the anterior tibial compartment was raised subsequently and monitored. The volume of saline solution needed to achieve an ICP of 80 mmHg ranged individually between 59.5 and 95 ml (mean volume = 75.5 ml).
Compartmental elasticity
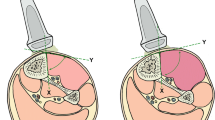
There was an increase of the compartment diameter recognized by rising pressures up to 80.6 % of its original size (Table 1). A concordant consistent correlation between the compartmental displacement and the intra-compartmental pressure occurred (Fig. 2). Linear tissue displacement in the cross-sectional view, illustrated with ∆d, ranged in the control group from 13.0 up to 27.5 %. In cases of elevated ICP ∆d it ranged from 4.8 to 19.2 % in group I, from 2.0 to 11.9 % in group II, and from 1.2 to 5.7 % in group III (Fig. 3).
Statistical values
The difference between the control and the elevated compartment pressure of group I showed no statistical differences (p = 0.063). With rising pressures (group II and III), the compartment elasticity (∆d) showed a further decrease, leading to significant differences (group II: p = 0.031 and group III: p = 0.027). The correlation, determined with the Spearman correlation coefficient revealed values of r s = 0.979 (absolute values) and 0.998 (relative values), and showed statistically high correlations. The reliability of the observer (kappa), κ 10 mmHg = 0.73, κ 30 mmHg = 0.80, and κ 70 mmHg = 0.79, revealed good values.
Discussion
Palpation, the historically most disseminated as well as easiest way to determine soft tissue firmness, is one of the most important clinical investigations to diagnose compartment syndromes [8, 12]. But isolated manual estimation of elevated compartment elasticity has been proven to be a poor parameter particularly in unconscious patients [12]. Hence, there is a need for a simple, low cost, and reliable tool objectifying the clinical findings [13]. Invasive pressure measurement is considered to be the gold standard method to objectify elevated intra-compartmental pressures. But its reliability is weak and therefore counts as an adjunct implement [11]. Therefore, our study aimed to evaluate the usefulness of ultrasound for diagnosis of compartment syndrome.
Our main findings are:
-
Depth of muscle compartments in B-mode ultrasound changes at different intra-compartmental probe pressures (∆d).
-
The compartment depth (∆d) correlates with rising intra-compartmental pressures.
-
Delta (mm) changes significantly with pathological compartment pressures compared to physiological levels.
The idea to investigate the visco-elastic properties of muscle tissue has already been followed in different studies, although some authors reported about difficulties due to the anisotropic behavior of muscle tissue [14, 15]. Murayama et al. [16] have shown that muscle stiffness and muscle volume of the biceps brachii muscle increased following eccentric exercise. They concluded that muscle stiffness after muscle damage may be a consequence of accumulation of intra-compartmental water caused by muscle swelling. Arokoski et al. [17] showed that soft tissue hardness of the forearm increased during venous occlusion with 60 mmHg. They stated that the increase of soft tissue stiffness is associated with a rise of the internal pressure of soft tissue due to an increase in blood volume. Steinberg and Gelberman observed a strong positive correlation between stiffness and interstitial intra-compartmental pressure in patients suspected of developing limb compartment syndrome [18]. Steinberg also demonstrated in a model of increased interstitial pressure by inflating a tourniquet cuff in healthy volunteers that muscle stiffness depends on the degree of interstitial pressure within the muscle compartment [19]. Therefore, a safe and reliable determination of the elastic properties of muscle compartment may improve the accuracy of the clinical finding and may help to objectify the physician’s palpation. In our study, we demonstrate a simple enhancement of ultrasound to measure decreased elastic properties of the anterior tibial compartment in case of compartment syndrome. We tested the principle of pressure-related ultrasound to determine the correlation between the ICP, the intra-compartmental volume, and its elasticity, evaluated by the compartmental depth measurement. Our results revealed a strong positive correlation between stiffness decrease and the ICP increase. This technique compromises several advantages. The use of relative values may allow discrimination of a percentage of compartment motion or displacement, which suggests an elevated risk or even reveals as a manifest acute compartment syndrome. In cases of uncertain compartment firmness the comparison with the contra-lateral limb might be helpful. Furthermore, ultrasound imaging is a low cost, non-invasive and viable tool in clinical practice. The selective measurement of the fascial displacement excludes the dermal and subcutaneous tissue. This may reduce misinterpretation in obese patients. Also subcutaneous hematoma could be identified (e.g., decollement) and excluded from measurements. This visualizing technique allows determining the elasticity of different compartments. Even the deep dorsal compartment of the lower limb could be interpreted.
In the past, several non-invasive measurement tools for diagnosis of ACS were introduced and showed promising results. Probably the most competitive technique was published by Steinberg et al. [18, 19]. They introduced a novel and non-invasive measurement of muscle compartment elasticity. Their technique revealed the correlation between the firmness of the muscle tissue and the intra-compartmental pressure. This was the first reliable tool objectifying the muscle elasticity. However, in contrast to our technique, this method does not visualize the compartments and, therefore, lacks the ability to discriminate between the different compartments and hematoma. Imaging and estimation of tissue elasticity by ultrasound have developed over the last two decades. Elastography, estimating the visco-elastic properties of soft tissue, is able to visualize areas with different hardness within the tissue by imaging [20, 21]. Hence, this might be helpful to outline hematoma and edema within the muscle tissue in cases of acute compartment syndrome. However, the pathophysiology of a compartment syndrome implicates a widely distributed change of visco-elastic behavior of the muscle with edema. Gennisson et al. investigated the relationship between muscle hardness using transient elastography technique and its activity level. They showed linear relationship between transverse shear moduli and the corresponding isometric muscle activity values [22]. This may allow observation of the visco-elastic properties changing in rising ICP. Kimura et al. [23] demonstrated that tissue stiffness by palpation of the body surface is highly correlated with the cross-sectional area. This cross-sectional area apparently is associated with the intra-compartmental pressure, within soft tissue. Therefore, internal pressure within soft tissue contributes to tissue stiffness, which is amenable to assessment from the body surface. Furthermore, they stated that the changes in soft tissue stiffness palpated from the body surface reflect changes in the fascia or intramuscular tissue volume rather than in dermal–subcutaneous tissue. Gershuni et al. [24] demonstrated a strong correlation between intra-compartmental pressure and compartmental volume, as well as the cross-sectional area, within the leg compartments following exercise and determined this by ultrasound. These investigations underline our idea, to visualize muscle compartment displacement correlated to extrinsic pressure to determine the muscle tissue elasticity.
There are some important limitations of this investigation. We present a feasibility study using a cadaveric model. But the pathophysiology of acute compartment syndrome, simulated by saline infusion, does not reflect the muscle edema expected in compartment syndromes. The homogeneity of the intra-compartmental pressure distribution in case of ACS is not simulated properly. But the over-all elasticity of a single isolated compartment was determined to test the basic principle of pressure-related ultrasound, used in our study. The number of cases does not allow general proposition regarding its reliability and effectiveness, despite results being promising. In addition, the technical setup is complicated and susceptible to impairment. The tightness of the probe head filled with water, the reduced penetration depth of the ultrasound, and difficulty calibrating the measurements all have to be improved before clinical implementation is feasible. Our model is not capable of proving the clinical reliability or utility. To answer this, we need data showing that the displacement depends preliminary on the intra-compartmental pressure. Furthermore, it is unclear whether other factors such as compartment diameter, fracture hematoma, and individual surrounding tissue composition do not confound these measurements. In summary, a human subject model is required.
Evidence of an improved accuracy in measuring the compartment elasticity is difficult to achieve, as the demanded clinical relevance can neither be proven by an in vitro model nor by a cadaver study simulating rising compartmental pressures. They all approximate the pathophysiology of compartment syndromes. However, the main purpose of our investigation was to test the feasibility of a simple but potentially useful technique, which may have important clinical impact after further improvements.
Whether the probe head pressure of 100 mmHg is useful or should be adapted, has to be shown in further studies. This technique of pressure-related ultrasound shows convincing advantages, but it has to show an appropriate level of accuracy, reliability, and practicability before introduction to daily clinical trauma care. The clinical relevance has to be shown in a prospective clinical trial comparing the clinical signs, the values of the invasive and non-invasive measurements.
Conclusion
Our data suggest that pressure-related ultrasound of single compartments might be suitable for early detection of intra-compartmental pressure elevation. However, improvements in pressure-related ultrasound imaging might help to develop a reliable tool for early detection of elevated compartment pressures. Further clinical studies are required to prove its validity and reliability in clinical scenario. The impact of the individual compartment size, volume, muscle fat content, and general data as age, sex, and BMI has to be considered in these investigations.
References
Mubarak SJ, Hargens AR. Acute compartment syndromes. Surg Clin North Am. 1983;63:539–65.
Heckman MM, Whiteside TE Jr, Grewe SR, et al. Histologic determination of the ischemic threshold of muscle in the canine compartment syndrome model. J Orthop Trauma. 1993;7:199–210.
Finkelstein J, Hunter G, Hu R, et al. Lower limb compartment syndrome: course after delayed fasciotomy. J Trauma. 1996;40:342–4.
McQueen MM, Christie J, Court-Brown CM. Acute compartment syndrome in tibial diaphyseal fractures. J Bone Joint Surg Br. 1996;78:95–8.
Rorabeck CH. The treatment of compartment syndrome of the leg. J Bone Joint Surg Br. 1984;66:93–7.
Oprel PP, Eversdijk MG, Vlot J, et al. The acute compartment syndrome of the lower leg: a difficult diagnosis? Open Orthop J. 2010;4:115–9.
Giannoudis PV, Nicolopoulos C, Dinopoulos H, et al. The impact of lower leg compartment syndrome on health related quality of life. Injury. 2002;33(2):117–21.
Shuler FD, Dietz MJ. Physicians’ ability to manually detect isolated elevations in leg intracompartmental Pressure. J Bone Joint Surg Am. 2010;92:361–7.
Prayson MJ, Chen JL, Hampers D, et al. Baseline compartment pressure measurements in isolated lower extremity fractures without clinical compartment syndrome. J Trauma. 2006;60:1037–40.
Ulmer T. The clinical diagnosis of compartment syndrome of the lower leg: are clinical findings predictive of the disorder? J Orthop Trauma. 2002;16:572–7.
Shadgan B, Menon M., O’Brien PJ, et al. Diagnostic techniques in acute compartment syndrome of the leg. J Orthop Trauma. 2008; 22:581–587.
Al-Dadah OQ, Darrah C, Cooper A, et al. Continuous compartment pressure monitoring vs. clinical monitoring in tibial diaphyseal fractures. Injury. 2008;39:1204–9.
Shadgan B, Menon M, Sanders D, et al. Current thinking about acute compartment syndrome of the lower extremity. Can J Surg. 2010;53:329–34.
Ramana YV, Krishna GG, Khadeer MA. Shear velocity of muscle tissue. J Biomed Eng. 1980;2:211–5.
Aaron R, Huang M, Shiffman CA. Anisotropy of human muscle via non-invasive impedance measurements. Phys Med Biol. 1997;42:1245–62.
Murayama G, Nosaka K, Yoneda T, et al. Changes in stiffness of the human elbow flexor muscles after eccentric exercise. Eur J Appl Physiol. 2000;82:361–7.
Arokoski JPA, Srakka J, Ojala T, et al. Feasibility of the use of a novel soft tissue stiffness meter. Physiol Meas. 2005;26:215–28.
Steinberg BD, Gelberman RH. Evaluation of limb compartments with suspected increased interstitial pressure. A non-invasive method for determining quantitative stiffness. Clin Orthop Relat Res. 1994;300:248–53.
Steinberg BD. Evaluation of limb compartments with increased interstitial pressure. An improved noninvasive method for determining quantitative hardness. J Biomech. 2005;38:1629–35.
Ophir J, C’espedes I, Ponnekanti H, et al. Elastography: a quantitative method for imaging the elasticity of biological tissue. Ultrason Imaging. 1991;13:111–34.
C’espedes I, Ophir J, Ponnekanti H, et al. Elastography: elasticity imaging using ultrasound with application to muscle and breast in vivo. Ultrason Imaging. 1993;15:73–88.
Gennisson JL, Cornu C, Cathline S, et al. Human muscle hardness assessment during incremental isometric contraction using transient elastography. J Biomech. 2005;38:1543–50.
Kimura K, Watanabe Y, Umeda M, et al. Quantitative analysis of the relation between soft tissue stiffness palpated from the body surface and tissue hemodynamic in the human forearm. Physiol Meas. 2007;28:1495–505.
Gershuni DH, Gosink BB, Hargens AR, et al. Ultrasound evaluation of the anterior musculofascial compartment of the leg following exercise. Clin Orthop Rel Res. 1982;167:185–90.
Acknowledgments
This investigation and research project was supported by the START-Program of the faculty of medicine, RWTH Aachen University, Germany.
Conflict of interest
R. M. Sellei, S. J. Hingmann, C. Weber, S. Jeromin, F. Zimmermann, J. Turner, F. Hildebrand and H.-C. Pape disclose all possible conflicts of interest in the manuscript, including financial, consultant, institutional and other relationships that might lead to bias or a conflict of interest.
Ethical standards
This study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The authors gave their informed consent prior to their inclusion in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sellei, R.M., Hingmann, S.J., Weber, C. et al. Assessment of elevated compartment pressures by pressure-related ultrasound: a cadaveric model. Eur J Trauma Emerg Surg 41, 639–645 (2015). https://doi.org/10.1007/s00068-014-0449-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-014-0449-9